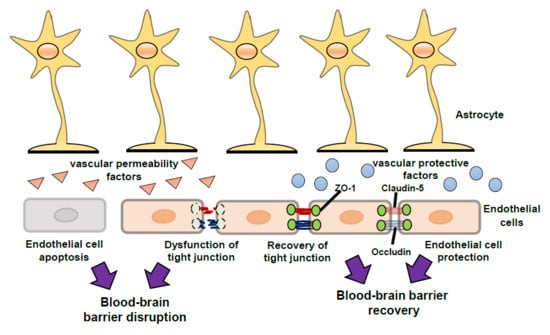
Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage
Abstract
:1. Introduction
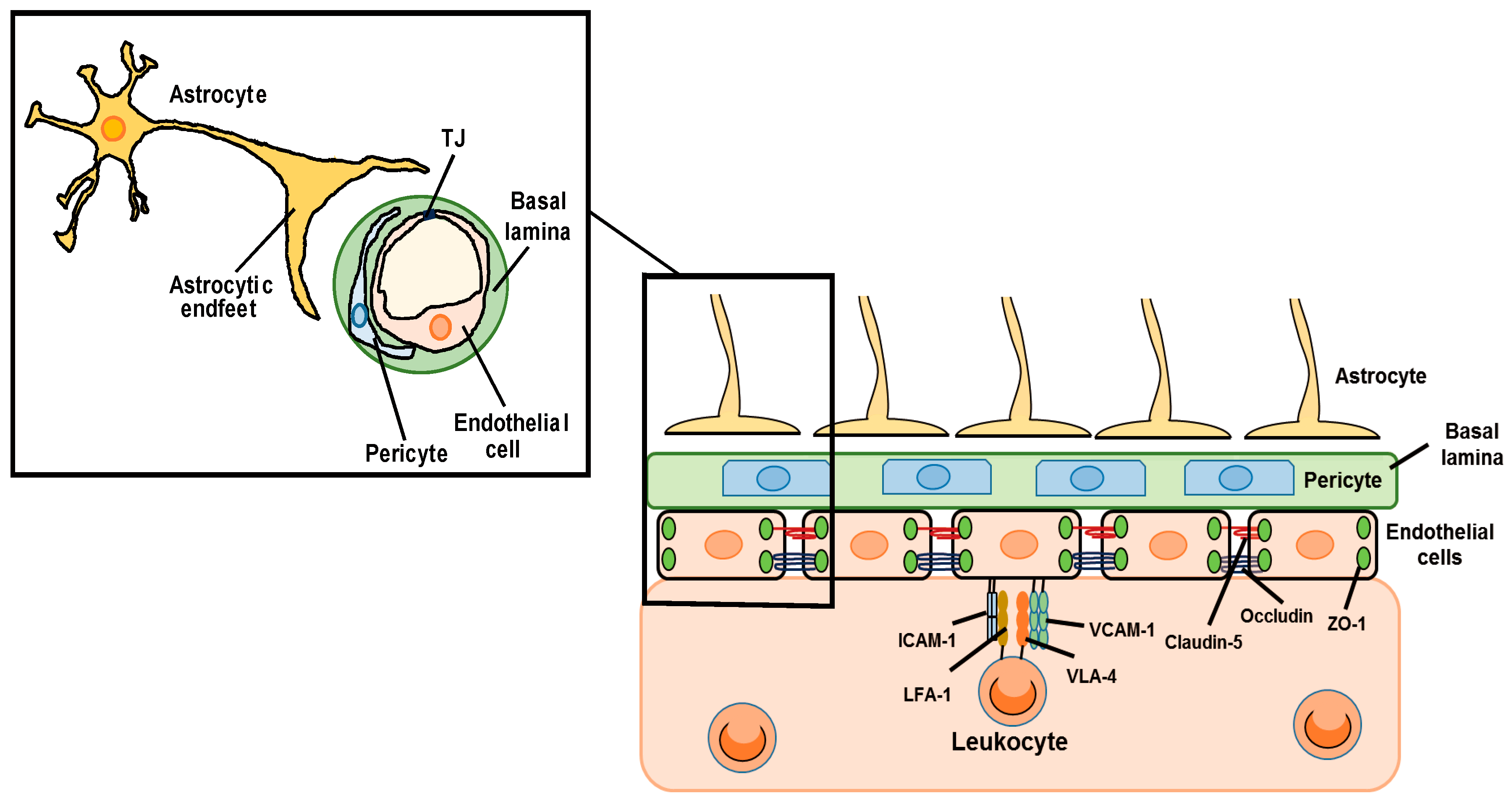
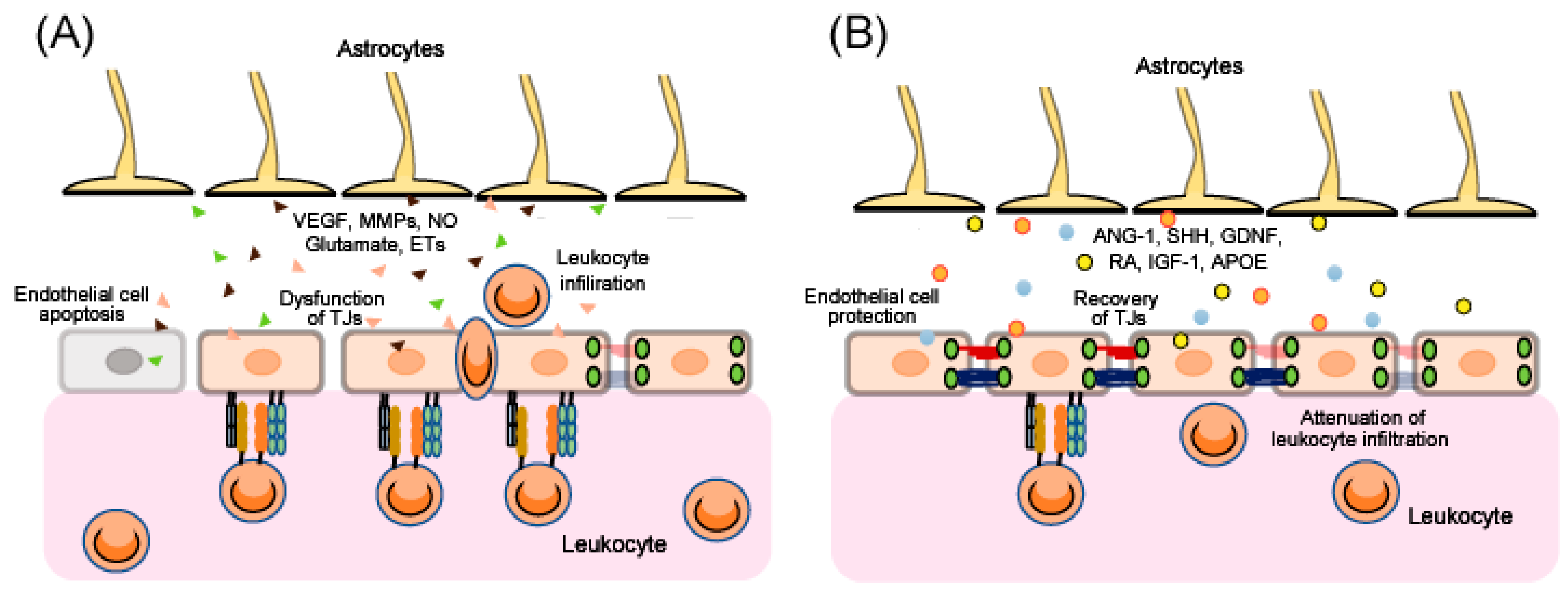
2. The Pathogenesis of BBB Disruption
3. Regulation of BBB Function by Astrocyte-Derived Factors
3.1. The Vascular Permeability Factors
3.1.1. Vascular Endothelial Growth Factor
3.1.2. Matrix Metalloproteinases
3.1.3. Nitric Oxide
3.1.4. Glutamate
3.1.5. Endothelins
3.2. The Vascular Protective Factors
3.2.1. Angiopoietin-1
3.2.2. Sonic Hedgehog
3.2.3. Glial-Derived Neurotrophic Factor
3.2.4. Retinoic Acid
3.2.5. Insulin-Like Growth Factor-1
3.2.6. Apolipoprotein E
4. Astrocytic Molecules as Candidates for Therapeutic Strategies to Protect BBB
4.1. Estrogen Receptors
4.2. Endothelin Receptor Type B
4.3. Cannabinoid Receptors
4.4. MicroRNAs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ANG-1 | angiopoietin-1 |
| BBB | blood-brain barrier |
| CAMs | cell adhesion molecules |
| CCI | controlled cortical impact |
| CB | cannabinoid |
| cGMP | cyclic guanosine monophosphate |
| CLN | claudin |
| CNS | central nervous system |
| ECM | extracellular matrix |
| ERs | estrogen receptors |
| ETs | endothelins |
| ETA | endothelin receptor type A |
| ETB | endothelin receptor type B |
| FPI | fluid percussion injury |
| GDNF | glial-derived neurotrophic factor |
| HUVECs | human umbilical vascular endothelial cells |
| ICAM-1 | intercellular adhesion molecule-1 |
| IGF-1 | insulin-like growth factor-1 |
| KO | knock-out |
| LFA-1 | lymphocyte function-associated antigen 1 |
| miRNAs | microRNAs |
| MMPs | matrix metalloproteinases |
| NMDA | N-methyl-D-aspartate |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| OCLN | occludin |
| PTCH1 | Patched-1 |
| RA | retinoic acid |
| RALDH | retinaldehyde dehydrogenase |
| RARs | retinoic acid receptors |
| SCI | spinal cord injury |
| SHH | sonic hedgehog |
| TBI | traumatic brain injury |
| TJ | tight junction |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
| VEGFR-1 | vascular endothelial growth factor receptor-1 |
| VEGFR-2 | vascular endothelial growth factor receptor-2 |
| VLA-4 | very late antigen-4 |
| ZO | zonula occluden |
References
- Wevers, N.R.; de Vries, H.E. Morphogens and blood-brain barrier function in health and disease. Tissue Barriers 2015, 4, e1090524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almutairi, M.M.; Gong, C.; Xu, Y.G.; Chang, Y.; Shi, H. Factors controlling permeability of the blood-brain barrier. Cell. Mol. Life Sci. 2016, 73, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.F.; Mohanta, S.K.; Frontczak-Baniewicz, M.; Swinny, J.D.; Zablocka, B.; Gorecki, D.C. Absence of glial alpha-dystrobrevin causes abnormalities of the blood–brain barrier and progressive brain edema. J. Biol. Chem. 2012, 287, 41374–41385. [Google Scholar] [CrossRef]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef]
- Muller, W.A. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am. J. Pathol. 2014, 184, 886–896. [Google Scholar] [CrossRef]
- Hay, J.R.; Johnson, V.E.; Young, A.M.; Smith, D.H.; Stewart, W. Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar]
- Arba, F.; Leigh, R.; Inzitari, D.; Warach, S.J.; Luby, M.; Lees, K.R. STIR/VISTA imaging collaboration. Blood-brain barrier leakage increases with small vessel disease in acute ischemic stroke. Neurology 2017, 89, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Van de Haar, H.J.; Burgmans, S.; Jansen, J.F.; van Osch, M.J.; van Buchem, M.A.; Muller, M.; Hofman, P.A.; Verhey, F.R.; Backes, W.H. Blood-brain barrier leakage in patients with early Alzheimer Disease. Radiology 2016, 281, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.P.; Simonsen, H.; Frederiksen, J.L.; Rostrup, E.; Larsson, H.B. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin. 2013, 4, 182–189. [Google Scholar] [CrossRef]
- Gray, M.T.; Woulfe, J.M. Striatal blood-brain barrier permeability in Parkinson’s disease. J. Cereb. Blood Flow Metab. 2015, 35, 747–750. [Google Scholar] [CrossRef]
- Michinaga, S.; Kimura, A.; Hatanaka, S.; Minami, S.; Asano, A.; Ikushima, Y.; Matsui, S.; Toriyama, Y.; Fujii, M.; Koyama, Y. Delayed Administration of BQ788, an ETB antagonist, after experimental traumatic brain injury promotes recovery of blood-brain barrier function and a reduction of cerebral edema in mice. J. Neurotrauma 2018, 35, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Begum, G.; Song, S.; Wang, S.; Zhao, H.; Bhuiyan, M.I.H.; Li, E.; Nepomuceno, R.; Ye, Q.; Sun, M.; Calderon, M.J.; Stolz, D.B.; et al. Selective knockout of astrocytic Na+ /H+ exchanger isoform 1 reduces astrogliosis, BBB damage, infarction, and improves neurological function after ischemic stroke. Glia 2018, 66, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.D.; Yao, N.W.; Guo, J.H.; Shen, C.C.; Lee, H.T.; Chiu, Y.P.; Ji, H.R.; Chen, X.; Chen, C.C.; Chang, C. Inhibition of astrocytic activity alleviates sequela in acute stages of intracerebral hemorrhage. Oncotarget 2017, 8, 94850–94861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapouly, C.; Tadesse Argaw, A.; Horng, S.; Castro, K.; Zhang, J.; Asp, L.; Loo, H.; Laitman, B.M.; Mariani, J.N.; Straus Farber, R.; et al. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain 2015, 138, 1548–1567. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Bu, F.; Min, J.W.; Munshi, Y.; Howe, M.D.; Liu, L.; Koellhoffer, E.C.; Qi, L.; McCullough, L.D.; Li, J. Inhibition of calcium/calmodulin-dependent protein kinase (CaMKK) exacerbates impairment of endothelial cell and blood-brain barrier after stroke. Eur. J. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.X.; Jing, Y.; Liu, Y.L.; Xu, Z.M.; Yuan, F.; Wang, M.L.; Geng, Z.; Tian, H.L. Inhibition of transient receptor potential vanilloid 1 attenuates blood-brain barrier disruption after traumatic brain injury. in mice. J. Neurotrauma 2018. [Google Scholar] [CrossRef] [PubMed]
- Main, B.S.; Villapol, S.; Sloley, S.S.; Barton, D.J.; Parsadanian, M.; Agbaegbu, C.; Stefos, K.; McCann, M.S.; Washington, P.M.; Rodriguez, O.C.; et al. Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol. Neurodegener. 2018, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; He, Q.W.; Li, Q.; Chen, X.L.; Baral, S.; Jin, H.J.; Zhu, Y.Y.; Li, M.; Xia, Y.P.; Mao, L.; et al. MicroRNA-150 regulates blood-brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J. 2016, 30, 2097–2107. [Google Scholar] [CrossRef]
- Sun, J.; Yu, L.; Huang, S.; Lai, X.; Milner, R.; Li, L. Vascular expression of angiopoietin1, α5β1 integrin and tight junction proteins is tightly regulated during vascular remodeling in the post-ischemic brain. Neuroscience 2017, 362, 248–256. [Google Scholar] [CrossRef]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.S.; Fang, H.L.; Chen, Y.; Liang, S.S.; Zhu, Z.G.; Zeng, Q.Y.; Li, J.; Xu, H.Q.; Shao, B.; He, J.C.; et al. Idazoxan reduces blood-brain barrier damage during experimental autoimmune encephalomyelitis in mouse. Eur. J. Pharmacol. 2014, 736, 70–76. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.H.; Park, S.W.; Brooks, N.; Lang, B.T.; Vemuganti, R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008, 1244, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Rancan, M.; Otto, V.; Hans, V.H.; Gerlach, I.; Jork, R.; Trentz, O.; Kossmann, T.; Morganti-Kossmann, M.C. Upregulation of ICAM-1 and MCP-1 but not of MIP-2 and sensorimotor deficit in response to traumatic axonal injury in rats. J. Neurosci. Res. 2001, 63, 438–446. [Google Scholar] [CrossRef]
- Williams, A.J.; Wei, H.H.; Dave, J.R.; Tortella, F.C. Acute and delayed neuroinflammatory response following experimental penetrating ballistic brain injury in the rat. J. Neuroinflamm. 2007, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Fee, D.; Crumbaugh, A.; Jacques, T.; Herdrich, B.; Sewell, D.; Auerbach, D.; Piaskowski, S.; Hart, M.N.; Sandor, M.; Fabry, Z. Activated/effector CD4+ T cells exacerbate acute damage in the central nervous system following traumatic injury. J. Neuroimmunol. 2003, 36, 54–66. [Google Scholar] [CrossRef]
- Harting, M.T.; Jimenez, F.; Adams, S.D.; Mercer, D.W.; Cox, C.S., Jr. Acute, regional inflammatory response after traumatic brain injury: Implications for cellular therapy. Surgery 2008, 144, 803–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenne, E.; Erlandsson, A.; Lindbom, L.; Hillered, L.; Clausen, F. Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J. Neuroinflamm. 2012, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhind, S.G.; Crnko, N.T.; Baker, A.J.; Morrison, L.J.; Shek, P.N.; Scarpelini, S.; Rizoli, S.B. Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. J. Neuroinflamm. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, J.M.; Downie, S.A.; Lyman, W.D.; Berman, J.W. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J. Immunol. 1998, 161, 6896–6903. [Google Scholar] [PubMed]
- Guo, M.F.; Meng, J.; Li, Y.H.; Yu, J.Z.; Liu, C.Y.; Feng, L.; Yang, W.F.; Li, J.L.; Feng, Q.J.; Xiao, B.G.; et al. The inhibition of Rho kinase blocks cell migration and accumulation possibly by challenging inflammatory cytokines and chemokines on astrocytes. J. Neurol. Sci. 2014, 343, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Eilam, R.; Segal, M.; Malach, R.; Sela, M.; Arnon, R.; Aharoni, R. Astrocyte disruption of neurovascular communication is linked to cortical damage in an animal model of multiple sclerosis. Glia 2018, 66, 1098–1117. [Google Scholar] [CrossRef]
- Risau, W.; Esser, S.; Engelhardt, B. Differentiation of blood-brain barrier endothelial cells. Pathol. Biol. 1998, 46, 171–175. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, R.; Jiang, Y.; Wang, L.; Gao, F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS ONE 2014, 9, e86407. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ye, Z.; Pan, Y.; Li, X.; Fu, X.; Zhang, B.; Li, Y.; Lin, W.; Li, X.; Gao, Q. Vascular endothelial growth factor aggravates cerebral ischemia and reperfusion-induced blood-brain-barrier disruption through regulating LOC102640519/HOXC13/ZO-1 signaling. Exp. Cell Res. 2018, 369, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Zhang, P.; Gao, Y.; Li, C.L.; Wang, H.J.; Chen, L.C.; Feng, Y.; Li, R.Y.; Li, Y.L.; Jiang, C.L. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol. Med. Rep. 2017, 15, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Reeson, P.; Tennant, K.A.; Gerrow, K.; Wang, J.; Weiser Novak, S.; Thompson, K.; Lockhart, K.L.; Holmes, A.; Nahirney, P.C.; Brown, C.E. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J. Neurosci. 2015, 35, 5128–5143. [Google Scholar] [CrossRef]
- Zhao, F.; Deng, J.; Yu, X.; Li, D.; Shi, H.; Zhao, Y. Protective effects of vascular endothelial growth factor in cultured brain endothelial cells against hypoglycemia. Metab. Brain Dis. 2015, 30, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood–brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef]
- Kim, I.; Moon, S.O.; Park, S.K.; Chae, S.W.; Koh, G.Y. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ. Res. 2001, 89, 477–479. [Google Scholar] [CrossRef]
- Shore, P.M.; Jackson, E.K.; Wisniewski, S.R.; Clark, R.S.; Adelson, P.D.; Kochanek, P.M. Vascular endothelial growth factor is increased in cerebrospinal fluid after traumatic brain injury in infants and children. Neurosurgery 2004, 54, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Matsumoto, N.; Tasaki, O.; Nakamura, H.; Akagaki, F.; Shimazu, T. Delayed progression of edema formation around a hematoma expressing high levels of VEGF and mmp-9 in a patient with traumatic brain injury: Case report. Neurol. Med. Chir. 2013, 53, 609–612. [Google Scholar] [CrossRef]
- Matsuo, R.; Ago, T.; Kamouchi, M.; Kuroda, J.; Kuwashiro, T.; Hata, J.; Sugimori, H.; Fukuda, K.; Gotoh, S.; Makihara, N.; et al. Clinical significance of plasma VEGF value in ischemic stroke-research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Grossetete, M.; Phelps, J.; Arko, L.; Yonas, H.; Rosenberg, G.A. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery 2009, 65, 702–708. [Google Scholar] [CrossRef]
- Liu, C.L.; Chen, C.C.; Lee, H.C.; Cho, D.Y. Matrix metalloproteinase-9 in the ventricular cerebrospinal fluid correlated with the prognosis of traumatic brain injury. Turk. Neurosurg. 2014, 24, 363–368. [Google Scholar]
- Chen, F.; Ohashi, N.; Li, W.; Eckman, C.; Nguyen, J.H. Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 2009, 50, 1914–1923. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Zhang, S.; An, Q.; Wang, T.; Gao, S.; Zhou, G. Autophagy- and MMP-2/9-mediated reduction and redistribution of ZO-1 contribute to hyperglycemia-increased blood-brain barrier permeability during early reperfusion in stroke. Neuroscience 2018, 377, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xu, L.; Wang, X.; Sun, X. MMP-9 expression and activity is concurrent with endothelial cell apoptosis in the basilar artery after subarachnoid hemorrhaging in rats. Neurol. Sci. 2015, 36, 1241–1245. [Google Scholar] [CrossRef]
- Lee, S.R.; Lo, E.H. Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J. Cereb. Blood Flow Metab. 2004, 24, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol. Biol. 2011, 762, 333–345. [Google Scholar]
- Asahi, M.; Wang, X.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; Fini, M.E.; Lo, E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001, 21, 7724–7732. [Google Scholar] [CrossRef] [PubMed]
- Brilha, S.; Ong, C.W.M.; Weksler, B.; Romero, N.; Couraud, P.O.; Friedland, J.S. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci. Rep. 2017, 7, 16031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.S.; Jin, S.M.; Kim, H.J.; Lee, Y.C. Matrix metalloproteinase inhibitor regulates inflammatory cell migration by reducing ICAM-1 and VCAM-1 expression in a murine model of toluene diisocyanate-induced asthma. J. Allergy Clin. Immunol. 2003, 111, 1278–1284. [Google Scholar] [CrossRef]
- Jiang, L.; Pan, C.L.; Wang, C.Y.; Liu, B.Q.; Han, Y.; Hu, L.; Liu, L.; Yang, Y.; Qu, J.W.; Liu, W.T. Selective suppression of the JNK-MMP2/9 signal pathway by tetramethylpyrazine attenuates neuropathic pain in rats. J. Neuroinflamm. 2017, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.J.; Cirrito, J.R.; Yan, P.; Hu, X.; Xiao, Q.; Pan, X.; Bateman, R.; Song, H.; Hsu, F.F.; Turk, J.; et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 2006, 26, 10939–10948. [Google Scholar] [CrossRef]
- Min, H.; Hong, J.; Cho, I.H.; Jang, Y.H.; Lee, H.; Kim, D.; Yu, S.W.; Lee, S.; Lee, S.J. TLR2-induced astrocyte MMP9 activation compromises the blood brain barrier and exacerbates intracerebral hemorrhage in animal models. Mol. Brain 2015, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.F.; Puebla, M.; Figueroa, X.F. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front. Cell Neurosci. 2015, 9, 59. [Google Scholar] [CrossRef]
- Liu, B.; Neufeld, A.H. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia 2000, 30, 178–186. [Google Scholar] [CrossRef]
- Buskila, Y.; Abu-Ghanem, Y.; Levi, Y.; Moran, A.; Grauer, E.; Amitai, Y. Enhanced astrocytic nitric oxide production and neuronal modifications in the neocortex of a NOS2 mutant mouse. PLoS ONE 2007, 2, e843. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.N.; Pahan, K. Signals for the induction of nitric oxide synthase in astrocytes. Neurochem. Int. 2006, 49, 154–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Zheng, G.; Xu, M.; Li, Y.; Chen, X.; Zhu, W.; Tong, Y.; Chung, S.K.; Liu, K.J.; Shen, J. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J. Neurochem. 2012, 120, 147–156. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, C.; Arrick, D.M.; Yang, S.; Baluna, A.E.; Sun, H. Role of nitric oxide synthases in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS ONE 2014, 9, e93134. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Wang, X.L.; Wilcken, D.E. Nitric oxide induces and inhibits apoptosis through different pathways. FEBS Lett. 1998, 433, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, Y.; Deng, X.; Jiang, W.; Li, B.; Fu, Z.; Du, M.; Ding, R. Hemoglobin-induced nitric oxide synthase overexpression and nitric oxide production contribute to blood-brain barrier disruption in the rat. J. Mol. Neurosci. 2013, 51, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.D.; Hines, I.; Houghton, J.; Warren, A.; Jackson, T.H.; Jawahar, A.; Nanda, A.; Elrod, J.W.; Long, A.; Chi, A.; et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Deli, M.A.; Veszelka, S.; Hayashi, K.; Hennig, B.; Toborek, M. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J. Cereb. Blood Flow Metab. 2007, 27, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hogan-Cann, A.D.; Globa, A.K.; Lu, P.; Nagy, J.I.; Bamji, S.X.; Anderson, C.M. Astrocytes drive cortical vasodilatory signaling by activating endothelial NMDA receptors. J. Cereb. Blood Flow Metab. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Vazana, U.; Veksler, R.; Pell, G.S.; Prager, O.; Fassler, M.; Chassidim, Y.; Roth, Y.; Shahar, H.; Zangen, A.; Raccah, R.; et al. Glutamate-mediated blood-brain barrier opening: Implications for neuroprotection and drug delivery. J. Neurosci. 2016, 36, 7727–7739. [Google Scholar] [CrossRef]
- Liu, X.; Hunter, C.; Weiss, H.R.; Chi, O.Z. Effects of blockade of ionotropic glutamate receptors on blood-brain barrier disruption in focal cerebral ischemia. Neurol. Sci. 2010, 31, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Salonia, R.; Empey, P.E.; Poloyac, S.M.; Wisniewski, S.R.; Klamerus, M.; Ozawa, H.; Wagner, A.K.; Ruppel, R.; Bell, M.J.; Feldman, K.; et al. Endothelin-1 is increased in cerebrospinal fluid and associated with unfavorable outcomes in children after severe traumatic brain injury. J. Neurotrauma 2010, 27, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Li, W.J.; Dou, X.J.; Jia, R.; Yang, H.; Liu, X.G.; Xu, C.B.; Liu, J.; Cao, Y.X.; Luo, G.G. Role of endothelin-1 and its receptors in cerebral vasospasm following subarachnoid hemorrhage. Mol. Med. Rep. 2018, 27, 5229–5236. [Google Scholar] [CrossRef] [PubMed]
- Petrov, T.; Steiner, J.; Braun, B.; Rafols, J.A. Sources of endothelin-1 in hippocampus and cortex following traumatic brain injury. Neuroscience 2002, 115, 275–283. [Google Scholar] [CrossRef]
- Hammond, T.R.; Gadea, A.; Dupree, J.; Kerninon, C.; Nait-Oumesmar, B.; Aguirre, A.; Gallo, V. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron 2014, 81, 588–602. [Google Scholar] [CrossRef]
- Ranno, E.; D’Antoni, S.; Spatuzza, M.; Berretta, A.; Laureanti, F.; Bonaccorso, C.M.; Pellitteri, R.; Longone, P.; Spalloni, A.; Iyer, A.M.; et al. Endothelin-1 is over-expressed in amyotrophic lateral sclerosis and induces motor neuron cell death. Neurobiol. Dis. 2014, 65, 160–171. [Google Scholar] [CrossRef]
- Yeung, P.K.; Shen, J.; Chung, S.S.; Chung, S.K. Targeted over-expression of endothelin-1 in astrocytes leads to more severe brain damage and vasospasm after subarachnoid hemorrhage. BMC Neurosci. 2013, 14, 131. [Google Scholar] [CrossRef]
- Lo, A.C.; Chen, A.Y.; Hung, V.K.; Yaw, L.P.; Fung, M.K.; Ho, M.C.; Tsang, M.C.; Chung, S.S.; Chung, S.K. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J. Cereb. Blood Flow Metab. 2005, 25, 998–1011. [Google Scholar] [CrossRef]
- Hung, V.K.; Yeung, P.K.; Lai, A.K.; Ho, M.C.; Lo, A.C.; Chan, K.C.; Wu, E.X.; Chung, S.S.; Cheung, C.W.; Chung, S.K. Selective astrocytic endothelin-1 overexpression contributes to dementia associated with ischemic stroke by exaggerating astrocyte-derived amyloid secretion. J. Cereb. Blood Flow Metab. 2015, 35, 1687–1696. [Google Scholar] [CrossRef]
- Narushima, I.; Kita, T.; Kubo, K.; Yonetani, Y.; Momochi, C.; Yoshikawa, I.; Ohno, N.; Nakashima, T. Highly enhanced permeability of blood-brain barrier induced by repeated administration of endothelin-1 in dogs and rats. Pharmacol. Toxicol. 2003, 92, 21–26. [Google Scholar] [CrossRef]
- Reijerkerk, A.; Lakeman, K.A.; Drexhage, J.A.; van Het Hof, B.; van Wijck, Y.; van der Pol, S.M.; Kooij, G.; Geerts, D.; de Vries, H.E. Brain endothelial barrier passage by monocytes is controlled by the endothelin system. J. Neurochem. 2012, 121, 730–737. [Google Scholar] [CrossRef] [PubMed]
- McCarron, R.M.; Wang, L.; Stanimirovic, D.B.; Spatz, M. Endothelin induction of adhesion molecule expression on human brain microvascular endothelial cells. Neurosci. Lett. 1993, 156, 31–34. [Google Scholar] [CrossRef]
- Matsuo, Y.; Mihara, S.; Ninomiya, M.; Fujimoto, M. Protective effect of endothelin type A receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke 2001, 32, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Ryu, H.J.; Kang, T.C. Status epilepticus induces vasogenic edema via tumor necrosis factor-α/endothelin-1-mediated two different pathways. PLoS ONE 2013, 8, e74458. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ko, A.R.; Hyun, H.W.; Kang, T.C. ETB receptor-mediated MMP-9 activation induces vasogenic edema via ZO-1 protein degradation following status epilepticus. Neuroscience 2015, 304, 355–367. [Google Scholar] [CrossRef]
- Sabirzhanov, B.; Faden, A.I.; Aubrecht, T.; Henry, R.; Glaser, E.; Stoica, B.A. MicroRNA-711-induced downregulation of angiopoietin-1 mediates neuronal cell death. J. Neurotrauma 2018, 35, 2462–2481. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, W.J.; Choi, Y.K.; Song, H.S.; Son, M.J.; Gelman, I.H.; Kim, Y.J.; Kim, K.W. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat. Med. 2003, 9, 900–906. [Google Scholar] [CrossRef]
- Zacharek, A.; Chen, J.; Cui, X.; Li, A.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1684–1691. [Google Scholar] [CrossRef]
- Koyama, Y.; Maebara, Y.; Hayashi, M.; Nagae, R.; Tokuyama, S.; Michinaga, S. Endothelins reciprocally regulate VEGF-A and angiopoietin-1 production in cultured rat astrocytes: Implications on astrocytic proliferation. Glia 2012, 60, 1954–1963. [Google Scholar] [CrossRef]
- Xia, Y.P.; He, Q.W.; Li, Y.N.; Chen, S.C.; Huang, M.; Wang, Y.; Gao, Y.; Huang, Y.; Wang, M.D.; Mao, L.; Hu, B. Recombinant human sonic hedgehog protein regulates the expression of ZO-1 and occludin by activating angiopoietin-1 in stroke damage. PLoS ONE 2013, 8, e68891. [Google Scholar] [CrossRef]
- Kawamura, K.; Takahashi, T.; Kanazawa, M.; Igarashi, H.; Nakada, T.; Nishizawa, M.; Shimohata, T. Effects of angiopoietin-1 on hemorrhagic transformation and cerebral edema after tissue plasminogen activator treatment for ischemic stroke in rats. PLoS ONE 2014, 9, e98639. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Li, M.; He, Q.; Jiang, S.; Zhang, X.; Xiao, J.; Bai, Y. Ectopic expression of human angiopoietin-1 promotes functional recovery and neurogenesis after focal cerebral ischemia. Neuroscience 2014, 267, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, L.; Croll, S.D.; Chopp, M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience 2002, 113, 683–687. [Google Scholar] [CrossRef]
- Venkat, P.; Yan, T.; Chopp, M.; Zacharek, A.; Ning, R.; Van Slyke, P.; Dumont, D.; Landschoot-Ward, J.; Liang, L.; Chen, J. Angiopoietin-1 mimetic peptide promotes neuroprotection after stroke in type 1 diabetic rats. Cell Transplant. 2018, 20, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Fei, Z.H.; Wang, Y.Q.; Yang, J.G.; Zhao, C.H.; Cai, Y.; Zhong, X.M. Angiopoietin-1 and angiopoietin-2 expression imbalance influence in early period after subarachnoid hemorrhage. Int. Neurourol. J. 2016, 20, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Brickler, T.R.; Hazy, A.; Guilhaume Correa, F.; Dai, R.; Kowalski, E.J.A.; Dickerson, R.; Chen, J.; Wang, X.; Morton, P.D.; Whittington, A.; et al. Angiopoietin/Tie2 axis regulates the age-at-injury cerebrovascular response to traumatic brain injury. J. Neurosci. 2018, 38, 9618–9634. [Google Scholar] [CrossRef]
- Golledge, J.; Clancy, P.; Maguire, J.; Lincz, L.; Koblar, S.; McEvoy, M.; Attia, J.; Levi, C.; Sturm, J.; Almeida, O.P.; et al. Plasma angiopoietin-1 is lower after ischemic stroke and associated with major disability but not stroke incidence. Stroke 2014, 45, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, T.; Arias, S.; Rodríguez-González, R.; Brea, D.; Silva, Y.; de la Ossa, N.P.; Agulla, J.; Blanco, M.; Pumar, J.M.; Serena, J.; et al. High serum levels of growth factors are associated with good outcome in intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2009, 29, 1968–1974. [Google Scholar] [CrossRef]
- Nag, S.; Manias, J.L.; Kapadia, A.; Stewart, D.J. Molecular changes associated with the protective effects of angiopoietin-1 during blood-brain barrier breakdown post-injury. Mol. Neurobiol. 2017, 54, 4232–4242. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Liu, X.; Xu, Y.; Qi, S. Angiopoietin-1 protects the endothelial cells against advanced glycation end product injury by strengthening cell junctions and inhibiting cell apoptosis. J. Cell. Physiol. 2015, 230, 1895–1905. [Google Scholar] [CrossRef]
- Yu, H.; Wang, P.; An, P.; Xue, Y. Recombinant human angiopoietin-1 ameliorates the expressions of ZO-1, occludin, VE-cadherin, and PKCα signaling after focal cerebral ischemia/reperfusion in rats. J. Mol. Neurosci. 2012, 46, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, Z.; Rikani, A.A.; Choudhry, A.M.; Tariq, S.; Zakaria, F.; Asghar, M.W.; Sarfraz, M.K.; Haider, K.; Shafiq, A.A.; Mobassarah, N.J. Sonic hedgehog signalling pathway: A complex network. Ann. Neurosci. 2014, 21, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Seifert, T.; Bauer, J.; Weissert, R.; Fazekas, F.; Storch, M.K. Differential expression of sonic hedgehog immunoreactivity during lesion evolution in autoimmune encephalomyelitis. J. Neuropathol. Exp. Neurol. 2005, 64, 404–411. [Google Scholar] [CrossRef]
- Amankulor, N.M.; Hambardzumyan, D.; Pyonteck, S.M.; Becher, O.J.; Joyce, J.A.; Holland, E.C. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J. Neurosci. 2009, 29, 10299–10308. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef]
- He, Q.W.; Xia, Y.P.; Chen, S.C.; Wang, Y.; Huang, M.; Huang, Y.; Li, J.Y.; Li, Y.N.; Gao, Y.; Mao, L.; et al. Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol. Neurobiol. 2013, 47, 976–987. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Wang, Y.; Mao, L.; Gao, Y.; He, Q.; Huang, M.; Chen, S.; Hu, B. Sonic hedgehog (Shh) regulates the expression of angiogenic growth factors in oxygen-glucose-deprived astrocytes by mediating the nuclear receptor NR2F2. Mol. Neurobiol. 2013, 47, 967–975. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Sonobe, Y.; Cheng, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS ONE 2014, 9, e110024. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, G.; Li, W.; Zhang, J.; Che, Y.; Song, M.; Gao, S.; Zeng, B.; Wang, Y. MiR-155 targets PTCH1 to mediate endothelial progenitor cell dysfunction caused by high glucose. Exp. Cell Res. 2018, 366, 55–62. [Google Scholar] [CrossRef]
- Zhu, S.L.; Luo, M.Q.; Peng, W.X.; Li, Q.X.; Feng, Z.Y.; Li, Z.X.; Wang, M.X.; Feng, X.X.; Liu, F.; Huang, J.L. Sonic hedgehog signalling pathway regulates apoptosis through Smo protein in human umbilical vein endothelial cells. Rheumatology 2015, 54, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Drannik, A.; Martin, J.; Peterson, R.; Ma, X.; Jiang, F.; Turnbull, J. Cerebrospinal fluid from patients with amyotrophic lateral sclerosis inhibits sonic hedgehog function. PLoS ONE 2017, 12, e0171668. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ba, H.; Lu, C.; Dai, J.; Sun, J. Glial cell line-derived neurotrophic factor (GDNF) promotes angiogenesis through the demethylation of the fibromodulin (FMOD) promoter in glioblastoma. Med. Sci. Monit. 2018, 24, 6137–6143. [Google Scholar] [CrossRef]
- Utsumi, H.; Chiba, H.; Kamimura, Y.; Osanai, M.; Igarashi, Y.; Tobioka, H.; Mori, M.; Sawada, N. Expression of GFRalpha-1, receptor for GDNF, in rat brain capillary during postnatal development of the BBB. Am. J. Physiol. Cell Physiol. 2000, 279, C361–C368. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Chiba, H.; Utsumi, H.; Miyajima, H.; Ishizaki, T.; Gotoh, T.; Kuwahara, K.; Tobioka, H.; Satoh, M.; Mori, M.; et al. Expression of receptors for glial cell line-derived neurotrophic factor (GDNF) and neurturin in the inner blood-retinal barrier of rats. Cell. Struct. Funct. 2000, 25, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Sano, Y.; Saito, K.; Abe, M.A.; Maeda, T.; Haruki, H.; Kanda, T. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem. Res. 2012, 37, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, W.; Chen, W.; Sun, L.; Li, X.; Zhang, C.; Yang, H. GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation. Mol. Neurobiol. 2014, 50, 274–289. [Google Scholar] [CrossRef]
- Mizee, M.R.; Nijland, P.G.; van der Pol, S.M.; Drexhage, J.A.; van Het Hof, B.; Mebius, R.; van der Valk, P.; van Horssen, J.; Reijerkerk, A.; de Vries, H.E. Astrocyte-derived retinoic acid: A novel regulator of blood-brain barrier function in multiple sclerosis. Acta Neuropathol. 2014, 128, 691–703. [Google Scholar] [CrossRef]
- Mizee, M.R.; Wooldrik, D.; Lakeman, K.A.; van het Hof, B.; Drexhage, J.A.; Geerts, D.; Bugiani, M.; Aronica, E.; Mebius, R.E.; Prat, A.; et al. Retinoic acid induces blood-brain barrier development. J. Neurosci. 2013, 33, 1660–1671. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Y.; Wang, X.J.; Wang, X.T.; Zhao, Y.; Wang, L.M.; Chen, Z.Y. Retinoic acid ameliorates blood-brain barrier disruption following ischemic stroke in rats. Pharmacol. Res. 2015, 99, 125–136. [Google Scholar] [CrossRef]
- Gille, J.; Paxton, L.L.; Lawley, T.J.; Caughman, S.W.; Swerlick, R.A. Retinoic acid inhibits the regulated expression of vascular cell adhesion molecule-1 by cultured dermal microvascular endothelial cells. J. Clin. Investig. 1997, 99, 492–500. [Google Scholar] [CrossRef]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liang, H.; Liu, H.; Li, D.; Chen, X.; Li, L.; Zhang, C.Y.; Zen, K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J. Immunol. 2014, 192, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, A.I.; Borrajo, A.; Diaz-Ruiz, C.; Garrido-Gil, P.; Labandeira-Garcia, J.L. Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: Role in neuroinflammation and aging. Oncotarget 2016, 7, 30049–30067. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.; Wilcox, K.C.; Tortelli, V.; Diniz, L.P.; Oliveira, M.S.; Dobbins, C.; Yu, X.W.; Nandamuri, S.; Gomes, F.C.A.; DiNunno, N.; et al. Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers. Mol. Biol. Cell. 2017, 28, 2623–2636. [Google Scholar] [CrossRef] [Green Version]
- Madathil, S.K.; Carlson, S.W.; Brelsfoard, J.M.; Ye, P.; D’Ercole, A.J.; Saatman, K.E. Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS ONE 2013, 8, e67204. [Google Scholar] [CrossRef] [PubMed]
- Okoreeh, A.K.; Bake, S.; Sohrabji, F. Astrocyte-specific insulin-like growth factor-1 gene transfer in aging female rats improves stroke outcomes. Glia 2017, 65, 1043–1058. [Google Scholar] [CrossRef]
- Bake, S.; Okoreeh, A.K.; Alaniz, R.C.; Sohrabji, F. Insulin-like growth factor (IGF)-I modulates endothelial blood-brain barrier function in ischemic middle-aged female rats. Endocrinology 2016, 157, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Bake, S.; Okoreeh, A.; Khosravian, H.; Sohrabji, F. Insulin-like Growth Factor (IGF)-1 treatment stabilizes the microvascular cytoskeleton under ischemic conditions. Exp. Neurol. 2019, 311, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Morikawa, M.; Fryer, J.D.; Sullivan, P.M.; Christopher, E.A.; Wahrle, S.E.; DeMattos, R.B.; O’Dell, M.A.; Fagan, A.M.; Lashuel, H.A.; Walz, T.; et al. Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-beta. Neurobiol. Dis. 2005, 19, 66–76. [Google Scholar] [CrossRef]
- Ito, J.; Nagayasu, Y.; Miura, Y.; Yokoyama, S.; Michikawa, M. Astrocyte׳s endogenous apoE generates HDL-like lipoproteins using previously synthesized cholesterol through interaction with ABCA1. Brain Res. 2014, 1570, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Lin, S.; Wu, X.; Esterman, M.; Koger, D.; Hanson, J.; Higgs, R.; Liu, F.; Malkani, S.; Bales, K.R.; et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 2004, 10, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Jiang, Y.; Wu, Y.; Zhong, J.; Liu, J.; Qin, X.; Chen, L.; Vitek, M.P.; Li, F.; Xu, L.; et al. Apolipoprotein E-Mimetic COG1410 Reduces Acute Vasogenic Edema following Traumatic Brain Injury. J. Neurotrauma 2016, 33, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Z.; Guo, Z.; Zhong, J.; Cheng, C.; Huang, Z.; Wu, Y.; Tang, S.; Luo, C.; Peng, X.; Wu, H.; et al. ApoE Influences the Blood-Brain Barrier Through the NF-κB/MMP-9 Pathway After Traumatic Brain Injury. Sci. Rep. 2017, 7, 6649. [Google Scholar] [CrossRef]
- Zheng, M.; Wei, J.; Tang, Y.; Yang, C.; Wei, Y.; Yin, X.; Liu, Q. ApoE-deficient promotes blood-brain barrier disruption in experimental autoimmune encephalomyelitis via alteration of MMP-9. J. Mol. Neurosci. 2014, 54, 282–290. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Therapeutic strategy of targeting astrocytes for neuroprotection in Parkinson’s Disease. Curr. Pharm. Des. 2017, 23, 4936–4947. [Google Scholar] [CrossRef]
- Assefa, B.T.; Gebre, A.K.; Altaye, B.M. Reactive astrocytes as drug target in Alzheimer’s Disease. Biomed. Res. Int. 2018, 2018, 4160247. [Google Scholar] [CrossRef]
- Ponath, G.; Park, C.; Pitt, D. The role of astrocytes in multiple sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Dubner, R. Neuron-glia crosstalk gets serious: Role in pain hypersensitivity. Curr. Opin. Anaesthesiol. 2008, 21, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Magistretti, P.J. A new outlook on mental illnesses: Glial involvement beyond the glue. Front. Cell. Neurosci. 2015, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016, 3, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte activation and reactive gliosis-a new target in stroke? Neurosci. Lett. 2018, 18, 30490–30497. [Google Scholar] [CrossRef]
- Arevalo, M.A.; Santos-Galindo, M.; Acaz-Fonseca, E.; Azcoitia, I.; Garcia-Segura, L.M. Gonadal hormones and the control of reactive gliosis. Horm. Behav. 2013, 63, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Acaz-Fonseca, E.; Sanchez-Gonzalez, R.; Azcoitia, I.; Arevalo, M.A.; Garcia-Segura, L.M. Role of astrocytes in the neuroprotective actions of 17β-estradiol and selective estrogen receptor modulators. Mol. Cell. Endocrinol. 2014, 389, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Wen, Y.; Perez, E.; Wang, X.; Day, A.L.; Simpkins, J.W.; Yang, S.H. 17beta-estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005, 1060, 55–61. [Google Scholar] [CrossRef]
- Xiao, H.; Deng, M.; Yang, B.; Hu, Z.; Tang, J. Pretreatment with 17β-estradiol attenuates cerebral ischemia-induced blood-brain barrier disruption in aged rats: Involvement of antioxidant signaling. Neuroendocrinology 2018, 106, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Asl, S.Z.; Khaksari, M.; Khachki, A.S.; Shahrokhi, N.; Nourizade, S. Contribution of estrogen receptors alpha and beta in the brain response to traumatic brain injury. J. Neurosurg. 2013, 119, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaksari, M.; Hajializadeh, Z.; Shahrokhi, N.; Esmaeili-Mahani, S. Changes in the gene expression of estrogen receptors involved in the protective effect of estrogen in rat’s traumatic brain injury. Brain Res. 2015, 1618, 1–8. [Google Scholar] [CrossRef]
- Na, W.; Lee, J.Y.; Kim, W.S.; Yune, T.Y.; Ju, B.G. 17β-estradiol ameliorates tight junction disruption via repression of MMP transcription. Mol. Endocrinol. 2015, 29, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Ardelt, A.A.; McCullough, L.D.; Korach, K.S.; Wang, M.M.; Munzenmaier, D.H.; Hurn, P.D. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke 2005, 36, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Pei, F. Estradiol Inhibits Cytokine-Induced Expression of VCAM-1 and ICAM-1 in Cultured Human Endothelial Cells Via AMPK/PPARα Activation. Cell Biochem. Biophys. 2015, 72, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.D.; Wisdom, A.J.; Cao, Y.; Hill, H.M.; Mongerson, C.R.; Stapornkul, B.; Itoh, N.; Sofroniew, M.V.; Voskuhl, R.R. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERα signaling on astrocytes but not through ERβ signaling on astrocytes or neurons. J. Neurosci. 2013, 33, 10924–10933. [Google Scholar] [CrossRef]
- Ma, Y.L.; Qin, P.; Feng, D.Y.; Li, Y.; Zhang, L.X.; Liu, Z.Y.; Yin, A.Q.; Tang, W.H.; Dong, H.L.; Meng, L.Z.; et al. Estrogen regulates the expression of Ndrg2 in astrocytes. Brain Res. 2014, 1569, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stary, C.M.; Xu, L.; Li, L.; Sun, X.; Ouyang, Y.B.; Xiong, X.; Zhao, J.; Giffard, R.G. Inhibition of miR-181a protects female mice from transient focal cerebral ischemia by targeting astrocyte estrogen receptor-α. Mol. Cell. Neurosci. 2017, 82, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Duckles, S.P.; Weiss, J.H.; Li, X.; Krause, D.N. 17β-estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Radic. Biol. Med. 2012, 52, 2151–2160. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Wade, F.M.; Mahesh, V.B.; Brann, D.W. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: Involvement of nonclassical genomic signaling pathways. Endocrinology 2005, 146, 2749–2759. [Google Scholar] [CrossRef]
- Karki, P.; Smith, K.; Johnson, J., Jr.; Lee, E. Astrocyte-derived growth factors and estrogen neuroprotection: Role of transforming growth factor-α in estrogen-induced upregulation of glutamate transporters in astrocytes. Mol. Cell. Endocrinol. 2014, 389, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.D.; Peters, C.M.; Pomonis, J.D.; Hagiwara, H.; Ghilardi, J.R.; Mantyh, P.W. Endothelin B receptors are expressed by astrocytes and regulate astrocyte hypertrophy in the normal and injured CNS. Glia 2003, 41, 180–190. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Li, L.; Pekna, M.; Berthold, C.H.; Blom, S.; Eliasson, C.; Renner, O.; Bushong, E.; Ellisman, M.; Morgan, T.E.; et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J. Neurosci. 2004, 24, 5016–5021. [Google Scholar] [CrossRef]
- LeComte, M.D.; Shimada, I.S.; Sherwin, C.; Spees, J.L. Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. Proc. Natl. Acad. Sci. USA 2015, 112, 8726–8731. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Tanaka, K. Intracerebroventricular administration of an endothelin ET(B)-receptor agonist increases expression of matrix metalloproteinase-2 and -9 in rat brain. J. Pharmacol. Sci. 2010, 114, 433–443. [Google Scholar] [CrossRef]
- Hind, W.H.; Tufarelli, C.; Neophytou, M.; Anderson, S.I.; England, T.J.; O’Sullivan, S.E. Endocannabinoids modulate human blood-brain barrier permeability in vitro. Br. J. Pharmacol. 2015, 172, 3015–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yun, D.; Zhang, Y.; Tao, Y.; Tan, Q.; Qiao, F.; Luo, B.; Liu, Y.; Fan, R.; Xian, J.; et al. A cannabinoid receptor 2 agonist reduces blood-brain barrier damage via induction of MKP-1 after intracerebral hemorrhage in rats. Brain Res. 2018, 1697, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Shin, W.H.; Baek, J.Y.; Cho, E.J.; Baik, H.H.; Kim, S.R.; Won, S.Y.; Jin, B.K. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2016, 48, e205. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.S.; Sulzer, J.K.; Impastato, R.A.; Teng, S.X.; Rogers, E.K.; Molina, P.E. Endocannabinoid degradation inhibition improves neurobehavioral function, blood-brain barrier integrity, and neuroinflammation following mild traumatic brain injury. J. Neurotrauma 2015, 32, 297–306. [Google Scholar] [CrossRef]
- Meng, X.D.; Wei, D.; Li, J.; Kang, J.J.; Wu, C.; Ma, L.; Yang, F.; Zhu, G.M.; Ou-Yang, T.P.; Liu, Y.Y.; et al. Astrocytic expression of cannabinoid type 1 receptor in rat and human sclerotic hippocampi. Int. J. Clin. Exp. Pathol. 2014, 7, 2825–2837. [Google Scholar]
- Kozela, E.; Juknat, A.; Vogel, Z. Modulation of astrocyte activity by cannabidiol, a nonpsychoactive cannabinoid. Int. J. Mol. Sci. 2017, 18, 1669. [Google Scholar] [CrossRef]
- Hegyi, Z.; Oláh, T.; Kőszeghy, Á.; Piscitelli, F.; Holló, K.; Pál, B.; Csernoch, L.; Di Marzo, V.; Antal, M. CB1 receptor activation induces intracellular Ca2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes. Sci. Rep. 2018, 8, 10562. [Google Scholar] [CrossRef]
- Fernández-Trapero, M.; Espejo-Porras, F.; Rodríguez-Cueto, C.; Coates, J.R.; Pérez-Díaz, C.; de Lago, E.; Fernández-Ruiz, J. Upregulation of CB2 receptors in reactive astrocytes in canine degenerative myelopathy, a disease model of amyotrophic lateral sclerosis. Dis. Models Mech. 2017, 10, 551–558. [Google Scholar] [CrossRef]
- Ma, Q.; Dasgupta, C.; Li, Y.; Huang, L.; Zhang, L. MicroRNA-210 suppresses junction proteins and disrupts blood-brain barrier integrity in neonatal rat hypoxic-ischemic brain injury. Int. J. Mol. Sci. 2017, 18, 1356. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.D.; Xia, Y.P.; Gao, Y.; Zhu, Y.Y.; Chen, S.C.; Mao, L.; He, Q.W.; Yue, Z.Y.; Hu, B. MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J. 2018, 32, 935–944. [Google Scholar] [CrossRef]
- Wan, Y.; Jin, H.J.; Zhu, Y.Y.; Fang, Z.; Mao, L.; He, Q.; Xia, Y.P.; Li, M.; Li, Y.; Chen, X.; et al. MicroRNA-149-5p regulates blood-brain barrier permeability after transient middle cerebral artery occlusion in rats by targeting S1PR2 of pericytes. FASEB J. 2018, 32, 3133–3148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.; Ma, Y.; Tang, G.; Liu, Y.; Chen, X.; Zhang, Z.; Zeng, L.; Wang, Y.; Ouyang, Y.B.; et al. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J. Cereb. Blood Flow Metab. 2015, 35, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.B.; Sun, Z.L.; Feng, D.F. The role of microRNA in traumatic brain injury. Neuroscience 2017, 367, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Reijerkerk, A.; Lopez-Ramirez, M.A.; van Het Hof, B.; Drexhage, J.A.; Kamphuis, W.W.; Kooij, G.; Vos, J.B.; van der Pouw Kraan, T.C.; van Zonneveld, A.J.; Horrevoets, A.J.; et al. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: Implications for multiple sclerosis. J. Neurosci. 2013, 33, 6857–6863. [Google Scholar] [CrossRef]
- Bhalala, O.G.; Pan, L.; Sahni, V.; McGuire, T.L.; Gruner, K.; Tourtellotte, W.G.; Kessler, J.A. microRNA-21 regulates astrocytic response following spinal cord injury. J. Neurosci. 2012, 32, 17935–17947. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.B.; Xu, L.; Lu, Y.; Sun, X.; Yue, S.; Xiong, X.X.; Giffard, R.G. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia 2013, 61, 1784–1794. [Google Scholar] [CrossRef] [Green Version]
- Hong, P.; Jiang, M.; Li, H. Functional requirement of dicer1 and miR-17-5p in reactive astrocyte proliferation after spinal cord injury in the mouse. Glia 2014, 62, 2044–2060. [Google Scholar] [CrossRef]
- Sison, S.L.; Patitucci, T.N.; Seminary, E.R.; Villalon, E.; Lorson, C.L.; Ebert, A.D. Astrocyte-produced miR-146a as a mediator of motor neuron loss in spinal muscular atrophy. Hum. Mol. Genet. 2017, 26, 3409–3420. [Google Scholar]
- Meares, G.P.; Rajbhandari, R.; Gerigk, M.; Tien, C.L.; Chang, C.; Fehling, S.C.; Rowse, A.; Mulhern, K.C.; Nair, S.; Gray, G.K.; et al. MicroRNA-31 is required for astrocyte specification. Glia 2018, 66, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, A.; Broekaart, D.W.M.; van Scheppingen, J.; Anink, J.J.; Baayen, J.C.; Idema, S.; Gorter, J.A.; Aronica, E.; van Vliet, E.A. Increased expression of matrix metalloproteinase 3 can be attenuated by inhibition of microRNA-155 in cultured human astrocytes. J. Neuroinflamm. 2018, 15, 211. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yang, S.H.; Tzeng, S.F. MicroRNA-145 as one negative regulator of astrogliosis. Glia 2015, 63, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Mei, P.J.; Chen, C.; Miao, F.A.; Zhang, H.; Li, Z.L. MiR-29c inhibits glioma cell proliferation, migration, invasion and angiogenesis. J. Neurooncol. 2013, 115, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; He, X.; Wang, Y.; Tang, Y.; Zheng, C.; Cai, H.; Liu, J.; Wang, Y.; Fu, Y.; Yang, G.Y. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 2014, 21, 37–43. [Google Scholar] [CrossRef] [PubMed]
- He, Q.W.; Li, Q.; Jin, H.J.; Zhi, F.; Suraj, B.; Zhu, Y.Y.; Xia, Y.P.; Mao, L.; Chen, X.L.; Hu, B. MiR-150 regulates poststroke cerebral angiogenesis via vascular endothelial growth factor in rats. CNS Neurosci. Ther. 2016, 22, 507–517. [Google Scholar] [CrossRef]
- Deng, X.; Zhong, Y.; Gu, L.; Shen, W.; Guo, J. MiR-21 involve in ERK-mediated upregulation of MMP9 in the rat hippocampus following cerebral ischemia. Brain Res. Bull. 2013, 94, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chopp, M.; Lu, Y.; Buller, B.; Jiang, F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013, 329, 146–154. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michinaga, S.; Koyama, Y. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int. J. Mol. Sci. 2019, 20, 571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030571
Michinaga S, Koyama Y. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. International Journal of Molecular Sciences. 2019; 20(3):571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030571
Chicago/Turabian StyleMichinaga, Shotaro, and Yutaka Koyama. 2019. "Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage" International Journal of Molecular Sciences 20, no. 3: 571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030571