Region-Specific Neuroprotective Features of Astrocytes against Oxidative Stress Induced by 6-Hydroxydopamine
Abstract
:1. Introduction
2. Results
2.1. Regional Difference in Astroglial Neuroprotective Effects
2.2. Regional Difference in Glia Conditioned Medium (GCM)
2.3. Altering Expression of Genes Induced by 6-OHDA in Astrocytes
2.4. GSH Levels in 6-OHDA-Treated Astrocytes
2.5. Expression of Nrf2 and Its Regulating Phase-II Detoxifying Molecules in 6-OHDA-Treated Astrocytes
2.6. Induction of Oxidative Stress in 6-OHDA-Treated Astrocytes
2.7. Quinone Formation in 6-OHDA-Treated Astrocytes or Neuron-Astrocyte Co-Cultures
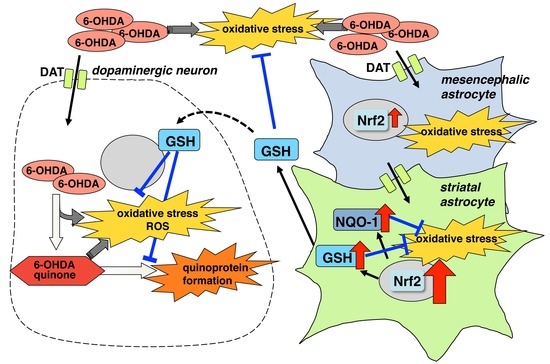
3. Discussion
4. Materials and Methods
4.1. Reagents and Animals
4.2. Cell Cultures
4.3. Preparation of Conditioned Medium
4.4. Cell Treatments
4.5. Immunohistochemistry
4.6. cDNA Microarray
4.7. Measurement of Total Glutathione (GSH)
4.8. Western Blot Analysis
4.9. Measurement of Protein-Bound Quinone (Quinoprotein)
4.10. Measurement of Intracellular Superoxide Anions
4.11. Protein Determination
4.12. Satistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allaman, I.; Belanger, M.; Magistretti, P.J. Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011, 34, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Schilling, K.; Steinhauser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M. Therapeutic strategy of targeting astrocytes for neuroprotection in Parkinson′s disease. Curr. Pharm. Des. 2017, 23, 4936–4947. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef]
- Shih, A.Y.; Erb, H.; Sun, X.; Toda, S.; Kalivas, P.W.; Murphy, T.H. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 2006, 26, 10514–10523. [Google Scholar] [CrossRef]
- Wang, X.F.; Cynader, M.S. Astrocytes provide cysteine to neurons by releasing glutathione. J. Neurochem. 2000, 74, 1434–1442. [Google Scholar] [CrossRef]
- Aschner, M. Astrocyte metallothioneins (MTs) and their neuroprotective role. Ann. N. Y. Acad. Sci. 1997, 825, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Penkowa, M. Metallothioneins are multipurpose neuroprotectants during brain pathology. FEBS J. 2006, 273, 1857–1870. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M.; Kikkawa, Y.; Takeshima, M.; Murakami, S.; Miyoshi, K.; Sogawa, N.; Kita, T. Astrocyte-derived metallothionein protects dopaminergic neurons from dopamine quinone toxicity. Glia 2011, 59, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T.H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003, 23, 3394–3406. [Google Scholar] [CrossRef]
- Asanuma, M.; Miyazaki, I.; Ogawa, N. Dopamine- or L-DOPA-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of Parkinson′s disease. Neurotox. Res. 2003, 5, 165–176. [Google Scholar] [CrossRef]
- Haque, M.E.; Asanuma, M.; Higashi, Y.; Miyazaki, I.; Tanaka, K.; Ogawa, N. Apoptosis-inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim. Biophys. Acta 2003, 1619, 39–52. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M.; Hozumi, H.; Miyoshi, K.; Sogawa, N. Protective effects of metallothionein against dopamine quinone-induced dopaminergic neurotoxicity. FEBS Lett. 2007, 581, 5003–5008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asanuma, M.; Miyazaki, I.; Diaz-Corrales, F.J.; Kimoto, N.; Kikkawa, Y.; Takeshima, M.; Miyoshi, K.; Murata, M. Neuroprotective effects of zonisamide target astrocyte. Ann. Neurol. 2010, 67, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, I.; Murakami, S.; Torigoe, N.; Kitamura, Y.; Asanuma, M. Neuroprotective effects of levetiracetam target xCT in astrocytes in parkinsonian mice. J. Neurochem. 2016, 136, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M.; Murakami, S.; Takeshima, M.; Torigoe, N.; Kitamura, Y.; Miyoshi, K. Targeting 5-HT1A receptors in astrocytes to protect dopaminergic neurons in Parkinsonian models. Neurobiol. Dis. 2013, 59, 244–256. [Google Scholar] [CrossRef]
- Murakami, S.; Miyazaki, I.; Asanuma, M. Neuroprotective effect of fermented papaya preparation by activation of Nrf2 pathway in astrocytes. Nutr. Neurosci. 2018, 21, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, M.; Miyazaki, I.; Murakami, S.; Kita, T.; Asanuma, M. L-Theanine protects against excess dopamine-induced neurotoxicity in the presence of astrocytes. J. Clin. Biochem. Nutr. 2016, 59, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson′s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, M.; Miyazaki, I.; Murakami, S.; Diaz-Corrales, F.J.; Ogawa, N. Striatal astrocytes act as a reservoir for L-DOPA. PLoS ONE 2014, 9, e106362. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Ichikawa, E.; Kondo, Y.; Miyazaki, I.; Asanuma, M.; Ogawa, N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J. Neurochem. 1999, 72, 2334–2344. [Google Scholar] [CrossRef]
- Engele, J.; Bohn, M.C. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalic glia. J. Neurosci. 1991, 11, 3070–3078. [Google Scholar] [CrossRef] [PubMed]
- Engele, J.; Schubert, D.; Bohn, M.C. Conditioned media derived from glial cell lines promote survival and differentiation of dopaminergic neurons in vitro: Role of mesencephalic glia. J. Neurosci. Res. 1991, 30, 359–371. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, E.K.; Sieber, B.A.; Morrison, R.S.; Black, I.B.; Dreyfus, C.F. Nigral type I astrocytes release a soluble factor that increases dopaminergic neuron survival through mechanisms distinct from basic fibroblast growth factor. Brain Res. 1994, 647, 83–90. [Google Scholar] [CrossRef]
- Yoshida, M.; Saito, H.; Katsuki, H. Neurotrophic effects of conditioned media of astrocytes isolated from different brain regions on hippocampal and cortical neurons. Experientia 1995, 51, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Sagara, J.; Makino, N.; Bannai, S. Glutathione efflux from cultured astrocytes. J. Neurochem. 1996, 66, 1876–1881. [Google Scholar] [CrossRef]
- Langeveld, C.H.; Jongenelen, C.A.; Schepens, E.; Stoof, J.C.; Bast, A.; Drukarch, B. Cultured rat striatal and cortical astrocytes protect mesencephalic dopaminergic neurons against hydrogen peroxide toxicity independent of their effect on neuronal development. Neurosci. Lett. 1995, 192, 13–16. [Google Scholar] [CrossRef]
- Maher, J.M.; Dieter, M.Z.; Aleksunes, L.M.; Slitt, A.L.; Guo, G.; Tanaka, Y.; Scheffer, G.L.; Chan, J.Y.; Manautou, J.E.; Chen, Y.; et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology 2007, 46, 1597–1610. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sato, H.; Kuriyama-Matsumura, K.; Sato, K.; Maebara, K.; Wang, H.; Tamba, M.; Itoh, K.; Yamamoto, M.; Bannai, S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 2002, 277, 44765–44771. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Weerachayaphorn, J.; Cai, S.Y.; Soroka, C.J.; Boyer, J.L. Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G126–G135. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Lai, L.; Yeo, H.C.; Goh, B.C.; Tan, T.M. Multidrug resistance protein 4 (MRP4/ABCC4) mediates efflux of bimane-glutathione. Int. J. Biochem. Cell Biol. 2004, 36, 247–257. [Google Scholar] [CrossRef]
- Leier, I.; Jedlitschky, G.; Buchholz, U.; Cole, S.P.; Deeley, R.G.; Keppler, D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994, 269, 27807–27810. [Google Scholar] [PubMed]
- Huang, Y.; Sadee, W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006, 239, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Heikkila, R.E. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J. Biol. Chem. 1974, 249, 2447–2452. [Google Scholar] [PubMed]
- Heikkila, R.E.; Cohen, G. 6-Hydroxydopamine: Evidence for superoxide radical as an oxidative intermediate. Science 1973, 181, 456–457. [Google Scholar] [CrossRef]
- Solano, R.M.; Casarejos, M.J.; Menendez-Cuervo, J.; Rodriguez-Navarro, J.A.; Garcia de Yebenes, J.; Mena, M.A. Glial dysfunction in parkin null mice: Effects of aging. J. Neurosci. 2008, 28, 598–611. [Google Scholar] [CrossRef]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P. DJ-1, a cancer- and Parkinson′s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef]
- Mullett, S.J.; Hinkle, D.A. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol. Dis. 2009, 33, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullett, S.J.; Hinkle, D.A. DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity. J. Neurochem. 2011, 117, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Van Muiswinkel, F.L.; de Vos, R.A.; Bol, J.G.; Andringa, G.; Jansen Steur, E.N.; Ross, D.; Siegel, D.; Drukarch, B. Expression of NAD(P)H:quinone oxidoreductase in the normal and Parkinsonian substantia nigra. Neurobiol. Aging 2004, 25, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson′s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Vargas, M.R.; Johnson, D.A.; Johnson, J.A. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J. Neurosci. 2012, 32, 17775–17787. [Google Scholar] [CrossRef] [PubMed]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Paz, M.A.; Fluckiger, R.; Boak, A.; Kagan, H.M.; Gallop, P.M. Specific detection of quinoproteins by redox-cycling staining. J. Biol. Chem. 1991, 266, 689–692. [Google Scholar] [PubMed]







| Mesencephalon Log2 Ratio | Control | 6-OHDA | Striatum Log2 Ratio | Control | 6-OHDA | |
|---|---|---|---|---|---|---|
| GST (glutathione S-transferase A3) | 1.87 | 1542.9 | 5646.9 | 5.30 | 315.4 | 12,397.4 |
| GST (glutathione S-transferase Yc2 subunit (Gsta5)) | 1.61 | 718.6 | 2192.1 | 4.94 | 142.0 | 4359.8 |
| Gpx (glutathione peroxidase 2) | 1.31 | 22.0 | 54.5 | 1.10 | 33.2 | 71.1 |
| NQO1 (NAD(P)H dehydrogenase) | 2.48 | 1631.3 | 9083.3 | 3.46 | 1501.1 | 16,562.9 |
| HO-1 (heme oxygenase-1) | 0.78 | 2746.2 | 4718.5 | 3.41 | 2753.0 | 29,313.3 |
| Catalase | 0.64 | 317.3 | 493.7 | 1.13 | 213.3 | 466.7 |
| G6PD (glucose-6-phosphate dehydrogenase) | 0.35 | 11,557.4 | 14,765.4 | 1.83 | 8347.5 | 29,683.0 |
| xCT (Xc-, Slc7a11) | −0.33 | 975.2 | 774.0 | 3.40 | 227.7 | 2398.7 |
| MDR1 (p-glycoprotein, Abcb1) | 0.76 | 7064.8 | 12,045.6 | 1.30 | 3693.6 | 9082.9 |
| MRP4 (multidrug resistance-associated protein 4, Abcc4) | 0.43 | 721.5 | 930.4 | 1.43 | 884.6 | 2335.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asanuma, M.; Okumura-Torigoe, N.; Miyazaki, I.; Murakami, S.; Kitamura, Y.; Sendo, T. Region-Specific Neuroprotective Features of Astrocytes against Oxidative Stress Induced by 6-Hydroxydopamine. Int. J. Mol. Sci. 2019, 20, 598. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030598
Asanuma M, Okumura-Torigoe N, Miyazaki I, Murakami S, Kitamura Y, Sendo T. Region-Specific Neuroprotective Features of Astrocytes against Oxidative Stress Induced by 6-Hydroxydopamine. International Journal of Molecular Sciences. 2019; 20(3):598. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030598
Chicago/Turabian StyleAsanuma, Masato, Nao Okumura-Torigoe, Ikuko Miyazaki, Shinki Murakami, Yoshihisa Kitamura, and Toshiaki Sendo. 2019. "Region-Specific Neuroprotective Features of Astrocytes against Oxidative Stress Induced by 6-Hydroxydopamine" International Journal of Molecular Sciences 20, no. 3: 598. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030598