Lymphoproliferation Impairment and Oxidative Stress in Blood Cells from Early Parkinson’s Disease Patients
Abstract
:1. Introduction
2. Results
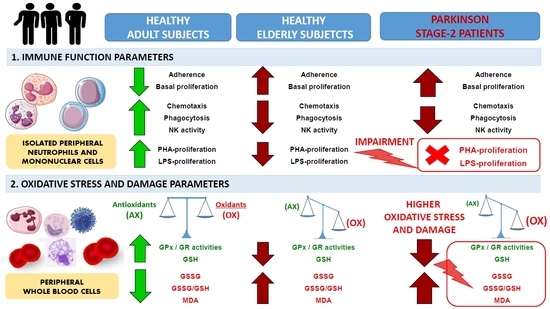
2.1. Impairment of Adaptive Immune Functions Presented in PD Stage 2 Patients
2.2. Oxidative Stress and Lipid Damage in Whole Blood cells from PD Stage 2 Patients
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Collection of Peripheral Whole Blood Cells and Isolation of Blood Neutrophils and Lymphocytes Leukocytes
4.3. Adherence Capacity Assay
4.4. Chemotaxis Assay
4.5. Phagocytosis Assay
4.6. Natural Killer (NK) Cytotoxicity Assay
4.7. Lymphoproliferation Assay
4.8. Glutathione peroxidase (GPx) Activity Assay
4.9. Glutathione Reductase (GR) Activity Assay
4.10. Glutathione Content Assay
4.11. Lipid Peroxidation (MDA) Assay
4.12. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | Bicinchoninic acid |
| BHT | Butylated hydroxytoluene |
| CSF | Cerebrospinal fluid |
| DA | Dopamine |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| L-DOPA | Levodopa |
| LPS | Lipopolysaccharide |
| MDA | Malondialdehyde |
| NK | Natural killer |
| PD | Parkinson’s Disease |
| PBMNs | Peripheral blood mononuclear cells |
| PHA | Phytohemagglutinin |
| SNpc | Substantia nigra pars compacta |
| WB | Whole blood cells |
References
- Massano, J.; Bhatia, K.P. Clinical Approach to Parkinson’s Disease: Features, Diagnosis, and Principles of Management. Cold Spring Harb. Perspect. Med. 2012, 2, a008870. [Google Scholar] [CrossRef]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Forno, L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef]
- Greenamyre, J.T.; Hastings, T.G. Biomedicine: Parkinson’s Divergent Causes, Convergent Mechanisms. Science 2004, 304, 1120–1122. [Google Scholar] [CrossRef]
- Driver, J.A.; Logroscino, G.; Gaziano, J.M.; Kurth, T. Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology 2009, 72, 432–438. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D.L. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Maaroufa, C.L.; Beachb, T.G.; Adlerc, C.H.; Shilld, H.A.; Sabbaghd, M.N.; Wue, T.; Walkerf, D.G.; Kokjohna, T.A.; Rohera, A.E.; Consortium, A.P. Cerebrospinal fluid biomarkers of neuropathologically diagnosed Parkinson’s disease subjects. Neurol. Res. 2012, 34, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr Staging Scale: Status and Recommendations. The Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Dovero, S.; Prunier, C.; Ravenscroft, P.; Chalon, S.; Guilloteau, D.; Crossman, A.R.; Bioulac, B.; Brotchie, J.M.; Gross, C.E. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridinelesioned macaque model of Parkinson’s disease. J. Neurosci. 2001, 21, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M. Crosstalk between the nervous and the immune systems in health and sickness. Curr. Pharm. Des. 2014, 20, 4605–4607. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; Maté, I.; Vida, C.; Cruces, J.; De la Fuente, M. Immune function parameters as markers of biological age and predictors of longevity. Aging (Albany NY). 2016, 8, 3110–3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, D.K.; Reynolds, A.D.; Mosley, R.L.; Gendelman, H.E. Innate and Adaptive Immunity for the Pathobiology of Parkinson’s Disease. Antioxid. Redox Signal. 2009, 11, 2151–2166. [Google Scholar] [CrossRef] [PubMed]
- Kannarkat, G.T.; Boss, J.M.; Tansey, M.G. The role of innate and adaptive immunity in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Singh, S. Updates on immunity and inflammation in Parkinson disease pathology. J. Neurosci. Res. 2018, 96, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.; D’Angelo, C.; Reale, M. The role of immunosenescence in neurodegenerative diseases. Mediat. Inflamm. 2018, 6039171. [Google Scholar] [CrossRef]
- Baba, N.; Rubio, M.; Sarfati, M. Interplay between CD45RA+ regulatory T cells and TNF- in the regulation of human Th17 differentiation. Int. Immunol. 2010, 22, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, C.; Seksenyan, A.; Koronyo, Y.; Rentsendorj, A.; Sarayba, D.; Wu, H.; Gragg, A.; Siegel, E.; Thomas, D.; Espinosa, A.; et al. T-Lymphocyte Deficiency Exacerbates Behavioral Deficits in the 6-OHDA Unilateral Lesion Rat Model for Parkinson’s Disease. J. Neurol. Neurophysiol. 2014, 5. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral cytokines profile in Parkinson’s disease. Brain. Behav. Immun. 2009, 23, 55–63. [Google Scholar] [CrossRef]
- Scalzo, P.; de Miranda, A.S.; Guerra Amaral, D.C.; de Carvalho Vilela, M.; Cardoso, F.; Teixeira, A.L. Serum Levels of Chemokines in Parkinson’s Disease. Neuroimmunomodulation 2011, 18, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Olanow, C.W. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 1996, 47, S161–S170. [Google Scholar] [CrossRef] [PubMed]
- Younes-Mhenni, S.; Frih-Ayed, M.; Kerkeni, A.; Bost, M.; Chazot, G. Peripheral blood markers of oxidative stress in Parkinson’s disease. Eur. Neurol. 2007, 58, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, S.; Maiani, E.; Bellusci, G.; Diederich, M.; Gonfloni, S. Parkinson’s Disease: A Complex Interplay of Mitochondrial DNA Alterations and Oxidative Stress. Int. J. Mol. Sci. 2013, 14, 2388–2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Mythri, R.B.; Venkateshappa, C.; Harish, G.; Mahadevan, A.; Muthane, U.B.; Yasha, T.C.; Srinivas Bharath, M.M.; Shankar, S.K. Evaluation of Markers of Oxidative Stress, Antioxidant Function and Astrocytic Proliferation in the Striatum and Frontal Cortex of Parkinson’s Disease Brains. Neurochem. Res. 2011, 36, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Venkateshappa, C.; Harish, G.; Mythri, R.B.; Mahadevan, A.; Bharath, M.M.S.; Shankar, S.K. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: Implications for Parkinson’s disease. Neurochem. Res. 2012, 37, 358–369. [Google Scholar] [CrossRef]
- Jenner, P.; Olanow, C.W. Understanding cell death in Parkinson’s disease. Ann. Neurol. 1998, 44, S72–S84. [Google Scholar] [CrossRef]
- Dexter, D.T.; Holley, A.E.; Flitter, W.D.; Slater, T.F.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: An HPLC and ESR study. Mov. Disord. 1994, 9, 92–97. [Google Scholar] [CrossRef]
- Good, P.F.; Hsu, A.; Werner, P.; Perl, D.P.; Olanow, C.W. Protein nitration in Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1998, 57, 338–342. [Google Scholar] [CrossRef]
- Schapira, A.H.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial complex I deficiency in Parkinson’s disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Ilić, T.; Jovanović, M.; Jovicić, A.; Tomović, M. Oxidative stress and Parkinson’s disease. Vojnosanit. Pregl. 1998, 55, 463–468. [Google Scholar] [PubMed]
- Agil, A.; Durán, R.; Barrero, F.; Morales, B.; Araúzo, M.; Alba, F.; Miranda, M.T.; Prieto, I.; Ramírez, M.; Vives, F. Plasma lipid peroxidation in sporadic Parkinson’s disease. Role of the L-dopa. J. Neurol. Sci. 2006, 240, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Prigione, A.; Begni, B.; Galbussera, A.; Beretta, S.; Brighina, L.; Garofalo, R.; Andreoni, S.; Piolti, R.; Ferrarese, C. Oxidative stress in peripheral blood mononuclear cells from patients with Parkinson’s disease: Negative correlation with levodopa dosage. Neurobiol. Dis. 2006, 23, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Vinish, M.; Anand, A.; Prabhakar, S. Altered oxidative stress levels in Indian Parkinson’s disease patients with PARK2 mutations. Acta Biochim. Pol. 2011, 58, 165–169. [Google Scholar]
- Vida, C.; Martinez de Toda, I.; Garrido, A.; Carro, E.; Molina, J.A.; De la Fuente, M. Impairment of Several Immune Functions and Redox State in Blood Cells of Alzheimer’s Disease Patients. Relevant Role of Neutrophils in Oxidative Stress. Front. Immunol. 2018, 8, 1974. [Google Scholar] [CrossRef] [PubMed]
- Maté, I.; Cruces, J.; Giménez-Llort, L.; De La Fuente, M. Function and redox state of peritoneal leukocytes as preclinical and prodromic markers in a longitudinal study of triple-transgenic mice for Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 43, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Akazawa-Ogawa, Y.; Matsumura, A.; Saigoh, K.; Itoh, S.; Sutou, K.; Kobayashi, M.; Mita, Y.; Shichiri, M.; Hisahara, S.; et al. Oxidation and interaction of DJ-1 with 20S proteasome in the erythrocytes of early stage Parkinson’s disease patients. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Mackie, P.; Lebowitz, J.; Saadatpour, L.; Nickoloff, E.; Gaskill, P.; Khoshbouei, H. The dopamine transporter: An unrecognized nexus for dysfunctional peripheral immunity and signaling in Parkinson’s Disease. Brain Behav. Immun. 2018, 70, 21–35. [Google Scholar] [CrossRef]
- Bessler, H.; Djaldetti, R.; Salman, H.; Bergman, M.; Djaldetti, M. IL-1β, IL-2, IL-6 and TNF-α production by peripheral blood mononuclear cells from patients with Parkinson’s disease. Biomed. Pharmacother. 1999, 53, 141–145. [Google Scholar] [CrossRef]
- Grozdanov, V.; Bliederhaeuser, C.; Ruf, W.P.; Roth, V.; Fundel-Clemens, K.; Zondler, L.; Brenner, D.; Martin-Villalba, A.; Hengerer, B.; Kassubek, J.; et al. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 2014, 128, 651–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cruzzocre, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Rad. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef]
- De Toda, I.M.; Vida, C.; De La Fuente, M. An appropriate modulation of lymphoproliferative response and cytokine release as possible contributors to longevity. Int. J. Mol. Sci. 2017, 18, 1598. [Google Scholar] [CrossRef]
- Wikby, A.; Ferguson, F.; Forsey, R.; Thompson, J.; Strindhall, J.; Lofgren, S.; Nilsson, B.O.; Ernerudh, J.; Pawelec, G.; Johansson, B. An immune risk phenotype, cognitive impairment, and survival in very late life: Impact of allostatic load in Swedish octogenarian and nonagenarian humans. J. Gerontol. Biol. Sci. Med. Sci. 2005, 60, 556–565. [Google Scholar] [CrossRef]
- De la Rosa, O.; Pawelec, G.; Peralbo, E.; Wikby, A.; Mariani, E.; Mocchegiani, E.; Tarazona, R.; Solana, R. Immunological biomarkers of ageing in man: Changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology 2006, 7, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.H.; Rowe, D.; Morel-Kopp, M.C.; Orr, C.; Russell, T.; Ranola, M.; Ward, C.; Halliday, G.M. Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol. 2012, 252, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Fiszer, U.; Mix, E.; Fredrikson, S.; Kostulas, V.; Link, H. Parkinson’s disease and immunological abnormalities: Increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol. Scand. 1994, 90, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Kluter, H.; Vieregge, P.; Stolze, H.; Kirchner, H. Defective production of Interleukin-2 in patients with idiopathic Parkinson’s disease. J. Neurol. Sci. 1995, 133, 134–139. [Google Scholar] [CrossRef]
- Marttila, R.J.; Eskola, J.; Soppi, E.; Rinne, U.K. Immune functions in Parkinson’s disease, lymphocyte subsets, concanavabn A-induced suppressor cell activity and in vitro immunoglobulin production. J. Neurol. Sci. 1985, 69, 121–131. [Google Scholar] [CrossRef]
- Bieganowska, K.; Członkowska, A.; Bidziński, A.; Mierzewska, H.; Korlak, J. Immunological changes in the MPTP-induced Parkinson’s disease mouse model. J. Neuroimmunol. 1993, 42, 33–37. [Google Scholar] [CrossRef]
- Arranz, L.; Caamano, J.H.; Lord, J.M.; De la Fuente, M. Preserved Immune Functions and Controlled Leukocyte Oxidative Stress in Naturally Long-lived Mice: Possible Role of Nuclear Factor Kappa B. J. Gerontol. Ser. Biol. Sci. Med. Sci. 2010, 65A, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Alquézar, C.; Bartolomé, F.; de la Encarnación, A.; Bermejo-Pareja, F.; Molina, J.A.; Martín-Requero, Á. G1/S Cell Cycle Checkpoint Dysfunction in Lymphoblasts from Sporadic Parkinson’s Disease Patients. Mol. Neurobiol. 2015, 52, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Niwa, F.; Kuriyama, N.; Nakagawa, M.; Imanishi, J. Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson’s disease. Geriatr. Gerontol. Int. 2012, 12, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Nakashima, M.; Kuroiwa, A.; Akitake, Y.; Ono, K.; Hosokawa, M.; Yamada, T.; Takahashi, M. Natural killer cells of Parkinson’s disease patients are set up for activation: A possible role for innate immunity in the pathogenesis of this disease. Parkinsonism Relat. Disord. 2008, 14, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gao, H.; Luo, Q.; Wang, P.; Yang, X. The correlation of lymphocyte subsets, natural killer cell, and Parkinson’s disease: A meta-analysis. Neurol. Sci. 2017, 38, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Ilic, T.; Jovanovic, M.; Jovicic, A.; Tomovic, M. Indices of Oxidative Stress in the Peripheral Blood of De Novo Patients with Parkinson’s Disease. In Progress in Alzheimer’s and Parkinson’s Disease; Fisher, A., Hanin, I., Yoshida, M., Eds.; Plenum Press: New York, NY, USA, 1999; pp. 779–787. ISBN 0306459035. [Google Scholar]
- Migliore, L.; Petrozzi, L.; Lucetti, C.; Gambaccini, G.; Bernardini, S.; Scarpato, R.; Trippi, F.; Barale, R.; Frenzilli, G.; Rodilla, V.; et al. Oxidative damage and cytogenetic analysis in leukocytes of Parkinson’s disease patients. Neurology 2002, 58, 1809–1815. [Google Scholar] [CrossRef]
- Chen, C.M.; Liu, J.L.; Wu, Y.R.; Chen, Y.C.; Cheng, H.S.; Cheng, M.L.; Chiu, D.T. yee Increased oxidative damage in peripheral blood correlates with severity of Parkinson’s disease. Neurobiol. Dis. 2009, 33, 429–435. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Uitti, R.J.; Low, P.A.; Tyce, G.M.; Nickander, K.K.; Petersen, R.C.; Kokmen, E. No evidence for systemic oxidant stress in Parkinson’s or Alzheimer’s disease. Mov. Disord. 1995, 10, 566–573. [Google Scholar] [CrossRef]
- Mischley, L.K.; Standish, L.J.; Weiss, N.S.; Padowski, J.M.; Kavanagh, T.J.; White, C.C.; Rosenfeld, M.E. Glutathione as a Biomarker in Parkinson’s Disease: Associations with Aging and Disease Severity. Oxid. Med. Cell. Longev. 2016, 2016, 9409363. [Google Scholar] [CrossRef]
- Serra, J.A.; Domínguez, R.O.; de Lustig, E.S.; Guareschi, E.M.; Famulari, A.L.; Bartolomé, E.L.; Marschoff, E.R. Parkinson’s disease is associated with oxidative stress: Comparison of peripheral antioxidant profiles in living Parkinson’s, Alzheimer’s and vascular dementia patients. J. Neural Transm. 2001, 108, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Soundararajan, C.C.; Vivekanandhan, S.; Behari, M. Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J. Med. Res. 2005, 121, 111–115. [Google Scholar] [PubMed]
- Sharma, A.; Kaur, P.; Kumar, B.; Prabhakar, S.; Gill, K.D. Plasma lipid peroxidation and antioxidant status of Parkinson’s disease patients in the Indian population. Parkinsonism Relat. Disord. 2008, 14, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Sudha, K.; Rao, A.V.; Rao, S.; Rao, A. Free radical toxicity and antioxidants in Parkinson’s disease. Neurol. India 2003, 51, 60–62. [Google Scholar] [PubMed]
- Sanyal, J.; Bandyopadhyay, S.K.; Banerjee, T.K.; Mukherjee, S.C.; Chakraborty, D.P.; Ray, B.C.; Rao, V.R. Plasma levels of lipid peroxides in patients with Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 129–132. [Google Scholar] [PubMed]
- Kalra, J.; Rajput, A.H.; Mantha, S.V.; Prasad, K. Serum antioxidant enzyme activity in Parkinson’s disease. Mol. Cell. Biochem. 1992, 110, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Cristalli, D.O.; Arnal, N.; Marra, F.A.; de Alaniz, M.J.T.; Marra, C.A. Peripheral markers in neurodegenerative patients and their first-degree relatives. J. Neurol. Sci. 2012, 314, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.; Yahr, M. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- De la Fuente, M.; Hernanz, A.; Guayerbas, N.; Victor, V.M.; Arnalich, F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic. Res. 2008, 42, 272–280. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Massey, V.; Williams, C.H. On the reaction mechanism of yeast glutathione reductase. J. Biol. Chem. 1965, 240, 4470–4480. [Google Scholar] [PubMed]
- Hissin, P.J.; Hilf, R. Effects of estrogen to alter amino acid transport in R3230AC mammary carcinomas and its relationship to insulin action. Cancer Res. 1979, 39, 3381–3387. [Google Scholar] [PubMed]



| PD Stage 2 Patients | Non-Medicated | Medicated |
|---|---|---|
| Immune Functions Parameters | [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] | [3,4,5] |
| N adherence (%) | 38± 10 | 35 ± 9 |
| L adherence (%) | 36 ± 14 | 34 ± 16 |
| N chemotaxis (C.I.) | 201 ± 10 | 218 ± 30 |
| L chemotaxis (C.I.) | 223 ± 15 | 213 ± 34 |
| Phagocytosis index (P.I.) | 177 ± 11 | 189 ± 24 |
| Phagocytosis efficiency (%) | 61 ± 4 | 56 ± 10 |
| NK cytotoxic activity (% lysis) | 47 ± 15 | 58 ± 19 |
| Basal lymphoproliferation (cpm) | 3827 ± 306 | 3931 ± 354 |
| PHA-lymphoproliferation (cpm) | 7920 ± 481 | 8090 ± 398 |
| LPS-lymphoproliferation (cpm) | 6306 ± 569 | 6629 ± 639 |
| PHA-stimulated (%) | 259 ± 21 | 284 ± 32 |
| LPS-stimulated (%) | 192 ± 18 | 203 ± 22 |
| Oxidative Stress Parameters | [18,19,20,21] | [5] |
| GPx (U/mg protein) | 5.02 ± 0.52 | 6.28 ± 1.23 |
| GR (U/mg protein) | 33.50 ± 9.70 | 31.24 ± 8.24 |
| GSH (nmol/mg protein) | 10.16 ± 2.10 | 12.24 ± 3.24 |
| GSSG (nmol/mg protein) | 1.85 ± 0.42 | 2.03 ± 0.54 |
| GSSG/GSH | 0.21 ± 0.09 | 0.23 ± 0.10 |
| MDA (nmol/mg protein) | 1.79 ± 0.52 | 1.68 ± 0.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vida, C.; Kobayashi, H.; Garrido, A.; Martínez de Toda, I.; Carro, E.; Molina, J.A.; De la Fuente, M. Lymphoproliferation Impairment and Oxidative Stress in Blood Cells from Early Parkinson’s Disease Patients. Int. J. Mol. Sci. 2019, 20, 771. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030771
Vida C, Kobayashi H, Garrido A, Martínez de Toda I, Carro E, Molina JA, De la Fuente M. Lymphoproliferation Impairment and Oxidative Stress in Blood Cells from Early Parkinson’s Disease Patients. International Journal of Molecular Sciences. 2019; 20(3):771. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030771
Chicago/Turabian StyleVida, Carmen, Hikaru Kobayashi, Antonio Garrido, Irene Martínez de Toda, Eva Carro, José Antonio Molina, and Mónica De la Fuente. 2019. "Lymphoproliferation Impairment and Oxidative Stress in Blood Cells from Early Parkinson’s Disease Patients" International Journal of Molecular Sciences 20, no. 3: 771. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030771