Root Proteomics Reveals the Effects of Wood Vinegar on Wheat Growth and Subsequent Tolerance to Drought Stress
Abstract
:1. Introduction
2. Results
2.1. Effects of WV Pretreatment on Phenotype and Growth Parameters of Wheat Seedlings
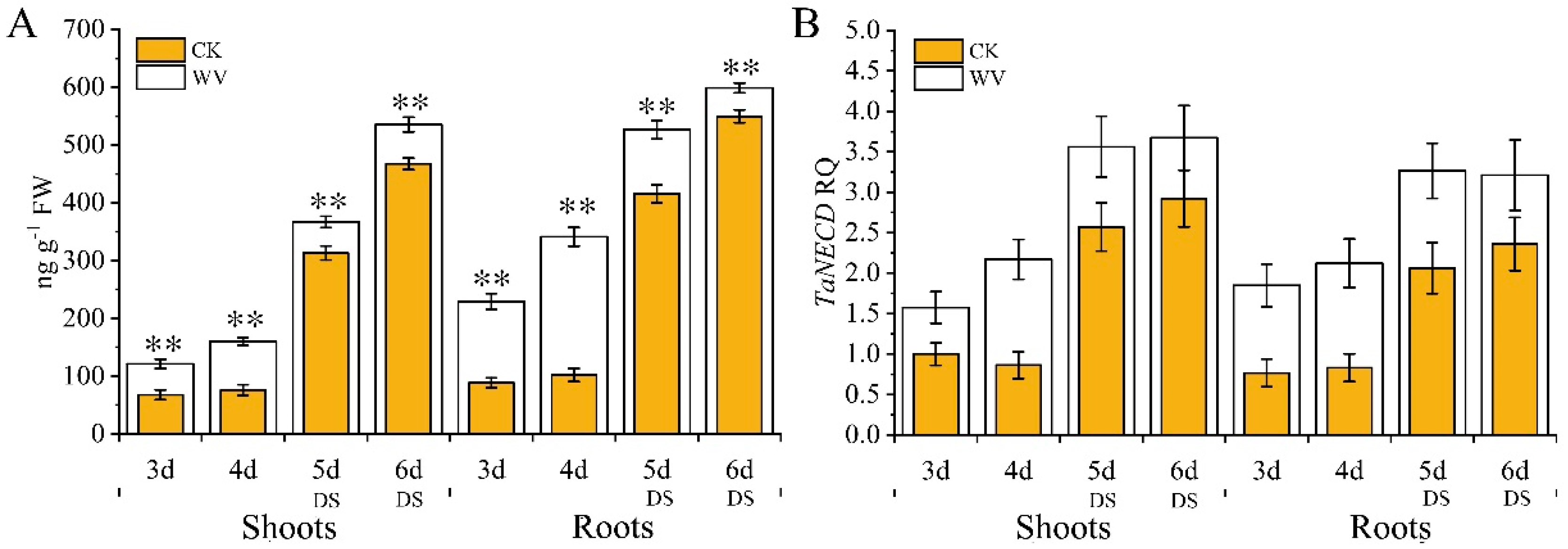
2.2. Physiological Changes in Wheat Seedlings Under Drought Conditions Following WV Pretreatment
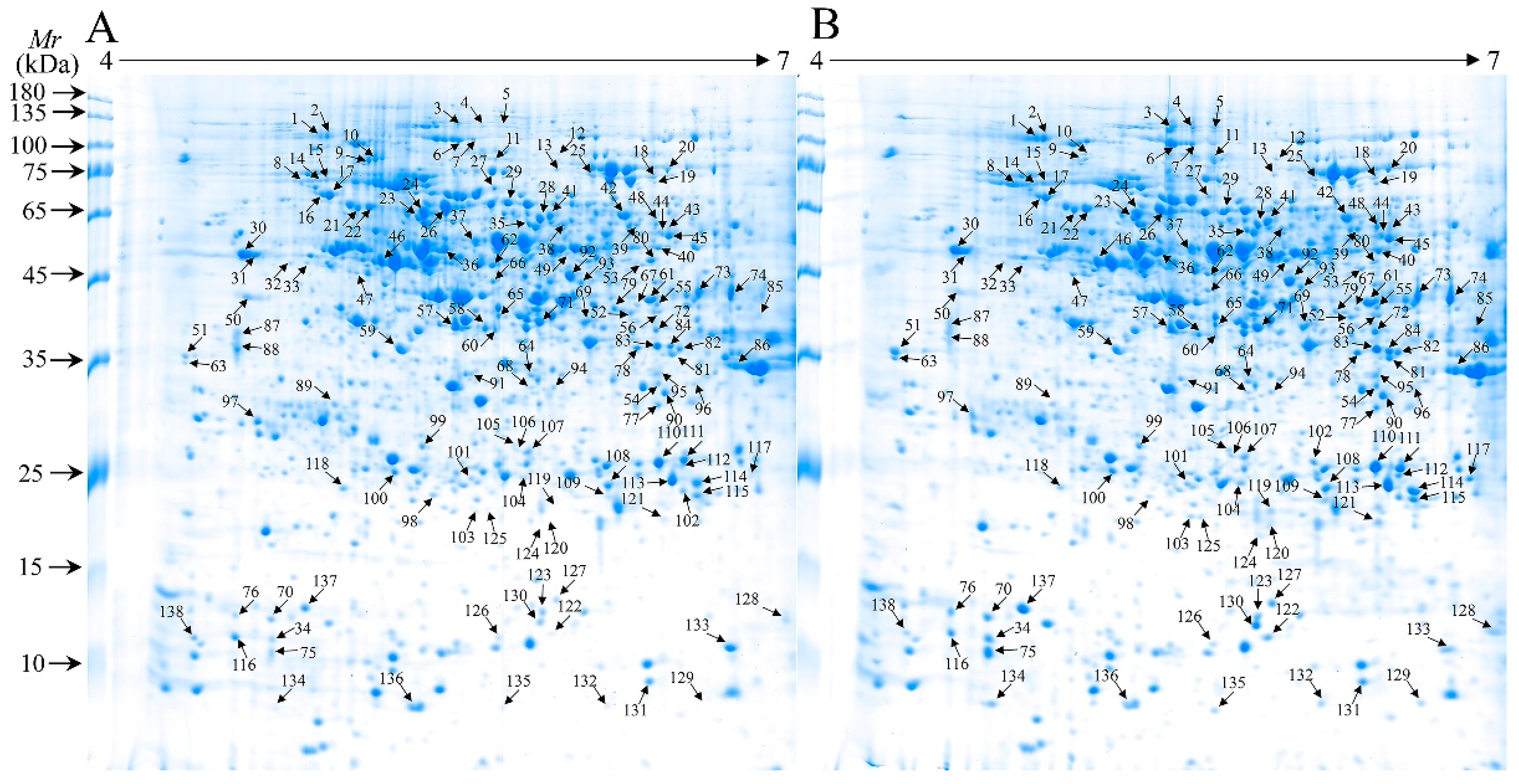
2.3. Analysis of Differentially Accumulated Protein Spots (DAPs) in Control and WV Pretreated Roots Under Drought Tolerance
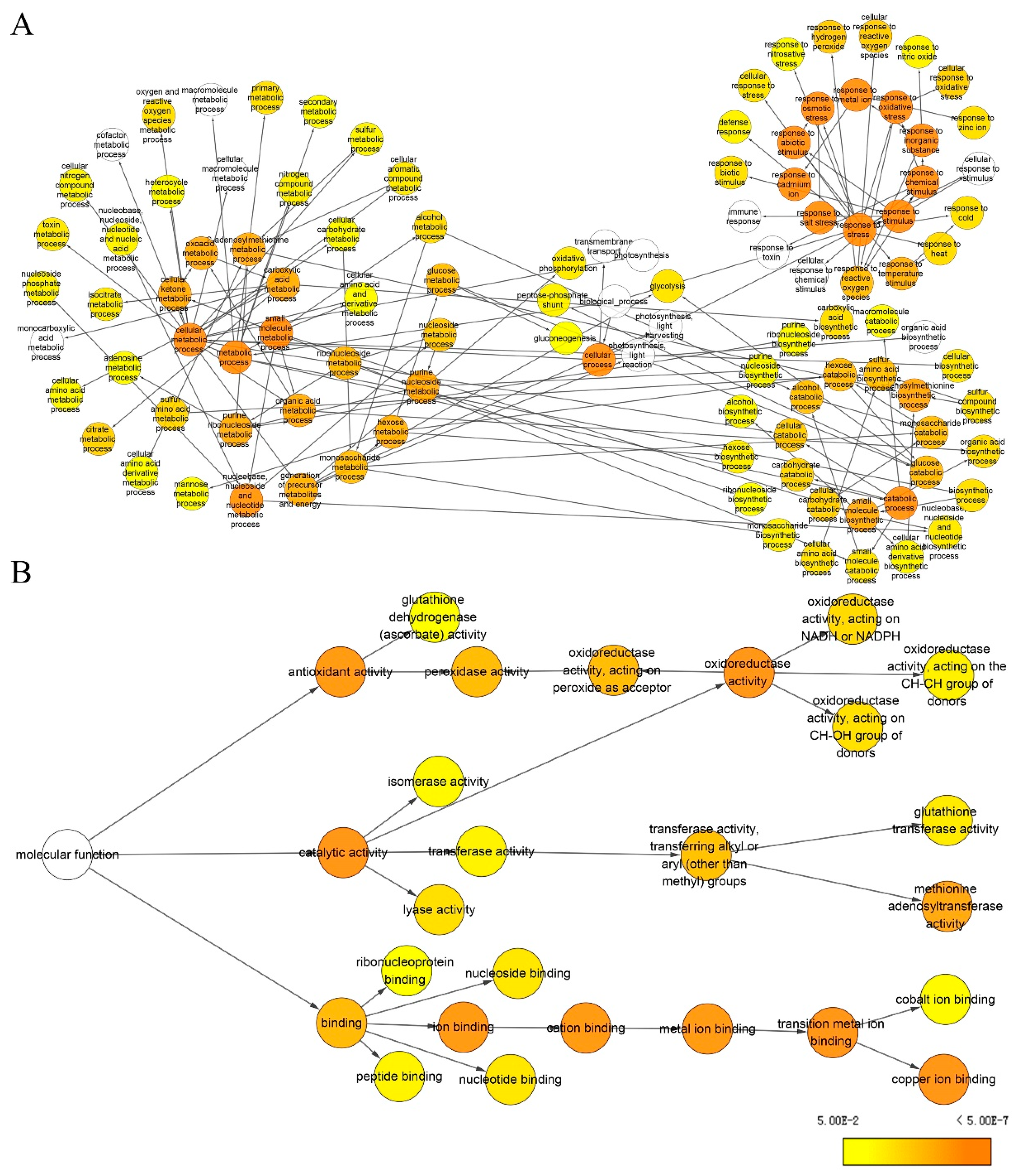
2.4. Identification and Functional Classification of DAPs
2.5. PPI Analysis of Identified Membrane Proteins
3. Discussion
3.1. Morphological and Physiological Response of Wheat Seedlings to Exogenous WV Pretreatment
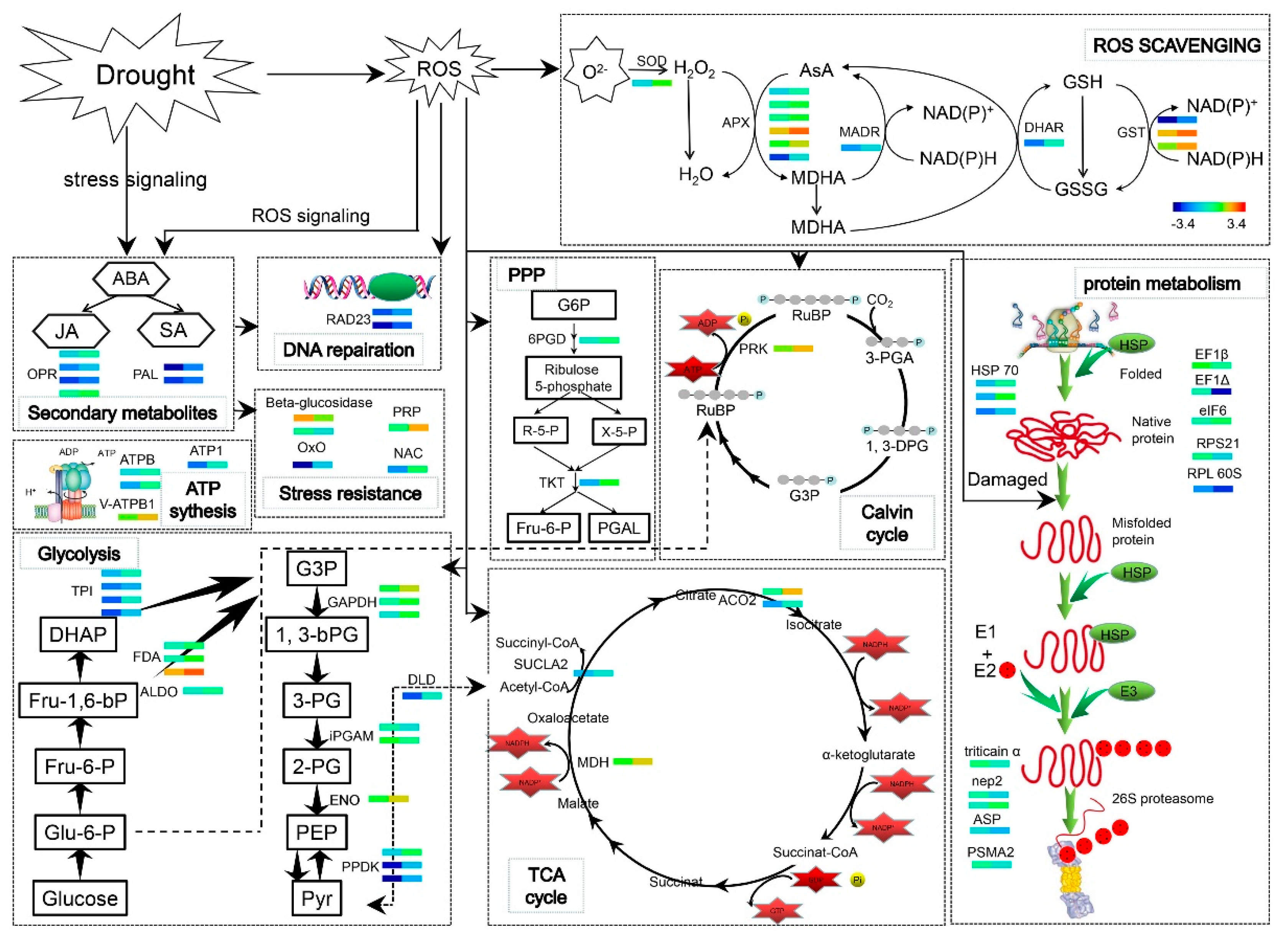
3.2. Protein Species Involved in Carbohydrate Metabolism and Energy Production
3.3. Protein Species Involved in the Stress Response
3.4. Protein Metabolism-Related Proteins
3.5. Proteins Involved in Secondary Metabolism
3.6. WV can Initiate An Early Defense Mechanism to Mitigate Subsequent Drought Stress
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of O2− Formation Rate and H2O2 Content
4.3. Determination of Antioxidant Enzyme Activity
4.4. Determination of MDA Content
4.5. Quantitative Determination of ABA Content
4.6. Protein Extraction
4.7. 2-DE and Gel Image Analysis
4.8. In-gel Digestion and MALDI-TOF/TOF MS Analysis
4.9. Total RNA Isolation and Real-Time PCR
4.10. Bioinformatic Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-DE | Two-dimensional gel electrophoresis |
| 6PGD | 6-phosphogluconate dehydrogenase |
| ABA | Abscisic acid |
| ACO2 | Aconitate hydratase |
| ADH1A | Alcohol dehydrogenase |
| ALDO | Aldolase |
| APX1 | L-ascorbate peroxidase 1 |
| ASP | Aspartic proteinase |
| ATPB | ATP synthase beta subunit |
| CAT | Catalase |
| DEPs | Differential expression proteins |
| DHAR | Dehydroascorbate reductase |
| DLD | Dihydrolipoyl dehydrogenase 1 |
| EF1β | Elongation factor 1-beta |
| EF1Δ | Elongation factor 1-delta |
| eIF6 | Eukaryotic translation initiation factor 6 |
| ENO | Enolase |
| FDA | Fructose-bisphosphate aldolase cytoplasmic isozyme |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GRAVY | Grand average of hydropathicity value |
| GST | Glutathione transferase |
| iPGAM | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase |
| JA | Jasmonic acid |
| MDA | Malonaldehyde |
| MDAR | Monodehydroascorbate reductase |
| MDH | Malate dehydrogenase 1 |
| Mr | Monoisotopic mass |
| NAC | NAC transcription factor |
| NECD | 9-cis-epoxycarotenoid dioxygenase |
| nep2 | Aspartic proteinase nepenthesin-2 |
| OPR | 12-oxophytodienoate reductase |
| Oxo | Oxalate oxidase |
| PA | Pyroligneous acid |
| PAL | Phenylalanine ammonia-lyase |
| pI | Isoelectric point |
| POD | Guaiacol peroxidase |
| POX1 | Peroxidase 1 |
| PPDK | Phosphate dikinase 1 |
| PPI | Protein–protein interaction |
| PRK | Phosphoribulokinase |
| PRP | Pathogenesis-related protein |
| PSMA2 | Proteasome subunit alpha type-2 |
| q-PCR | Real-time PCR |
| RAD23 | DNA repair protein RAD23 |
| ROS | Reactive oxygen species |
| RPL 60S | 60s acidic ribosomal protein-like protein |
| RPS21 | 40S ribosomal protein S21 |
| RQ | Relative quantification |
| SA | Salicylic acid |
| SOD | Superoxide dismutase |
| SUCLA2 | Succinyl-CoA ligase [ADP-forming] subunit beta |
| TKT | Transketolase |
| TMDs | Transmembrane domains |
| TPI | Triosephosphate isomerase |
| V-ATPB1 | Vacuolar ATPase subunit B1 |
| WV | Wood vinegar |
References
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrolysis 2010, 87, 24–28. [Google Scholar] [CrossRef]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Pimenta, A.S.; Fasciotti, M.; Monteiro, T.V.C.; Lima, K.M.G. Chemical composition of pyroligneous acid obtained from eucalyptus gg100 clone. Molecules 2018, 23, 426. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Cai, K.; Jiang, S.; Ren, C.; He, Y. Significant damage-rescuing effects of wood vinegar extract in living Caenorhabditis elegans under oxidative stress. Sci Food 2012, 92, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dissatian, A.; Sanitchon, J.; Pongdontri, P.; Jongrungklang, N.; Jothityangkoon, D. Potential of wood vinegar for enhancing seed germination of three upland rice varieties by suppressing malondialdehyde production. J. Agric. Sci. 2018, 40, 371–380. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.; Steele, P. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Jung, K. Growth inhibition effect of pyroligneous acid on pathogenic fungus, Alternaria mali, the agent of Alternaria blotch of apple. Biotechnol. Bioprocess Eng. 2007, 12, 318–322. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Sparg, S.G.; Light, M.E.; van Staden, J. Stimulation of rice (Oryza sativa L.) seedling vigour by smoke-water and butenolide. J. Agron. Crop Sci. 2006, 192, 395–398. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Loo, A.Y.; Jain, K.; Darah, I. Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant, Rhizophora apiculata. Food Chem. 2007, 104, 300–307. [Google Scholar] [CrossRef]
- Curtis, T.; Halford, N.G. Food security: the challenge of increasing wheat yield and the importance of not compromising food safety. Ann. Appl. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.; Li, G.; Xu, W.; Peng, X.; Han, Q.; Zhu, Y.; Guo, T. Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. J. Proteome Res. 2012, 11, 6066–6079. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.M.; Sakuma, Y.; Inouhe, M. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J. Plant Res. 2012, 125, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Chakraborty, S.; Datta, A.; Chakraborty, N. Proteomics approach to identify dehydration responsive nuclear proteins from chickpea (Cicer arietinum L.). Mol. Cell. Proteomics 2008, 7, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Choudhury, S.R.; Pandey, S. Comparative quantitative proteomics analysis of the ABA response of roots of drought-sensitive and drought-tolerant wheat varieties identifies proteomic signatures of drought adaptability. J. Proteome Res. 2014, 13, 1688–1701. [Google Scholar] [CrossRef] [PubMed]
- Faghani, E.; Gharechahi, J.; Komatsu, S.; Mirzaei, M.; Khavarinejad, R.A.; Najafi, F.; Farsad, L.K.; Salekdeh, G.H. Comparative physiology and proteomic analysis of two wheat genotypes contrasting in drought tolerance. J. Proteom. 2015, 114, 1–15. [Google Scholar] [CrossRef]
- Liu, H.; Sultan, M.A.R.F.; Liu, X.L.; Zhang, J.; Yu, F.; Zhao, H.X. Physiological and comparative proteomic analysis reveals different drought responses in roots and leaves of drought-tolerant wild wheat (Triticum boeoticum). PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Wang, M.C.; Li, F.; Lv, H.J.; Li, C.L.; Xia, G.M. A Proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Mol. Cell. Proteomics 2009, 8, 2676–2686. [Google Scholar] [CrossRef]
- Agrawal, L.; Gupta, S.; Mishra, S.K.; Pandey, G.; Kumar, S.; Chauhan, P.S.; Chakrabarty, D.; Nautiyal, C.S. Elucidation of complex nature of peg induced drought-stress response in rice root using comparative proteomics approach. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Yang, A.J.; James, A.T. Comparative proteomic analysis of drought response in roots of two soybean genotypes. Crop Pasture Sci. 2017, 68, 609–619. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.P.; Moieni, A.; Komatsu, S. Comparative proteome analysis of drought-sensitive and drought-tolerant rapeseed roots and their hybrid F1 line under drought stress. Amino Acids 2012, 43, 2137–2152. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Negri, A.S.; Failla, O.; Scienza, A.; Espen, L. Root proteomic and metabolic analyses reveal specific responses to drought stress in differently tolerant grapevine rootstocks. BMC Plant Biol. 2018, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, H.; Apweiler, R.; Holzhütter, H.G.; Jungblut, P.R. Finding one’s way in proteomics: A protein species nomenclature. Chem. Cent. J. 2009, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jia, J.; Zhang, C.; Li, H.; Liu, T.; Jiang, X.; Polle, A.; Peng, C.; Luo, Z.B. Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol. Plant. 2014, 151, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.B.; Janz, D.; Jiang, X.; Gobel, C.; Wildhagen, H.; Tan, Y.; Rennenberg, H.; Feussner, I.; Polle, A. Upgrading root physiology for stress tolerance by ectomycorrhizas: Insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiol. 2009, 151, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Pan, K. Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ. Sci. Pollut. Res. 2013, 20, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular mechanism for the regulation of ABA homeostasis during plant development and stress responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.M.; Carvalho, R.F.; Richardson, D.N.; Duque, P. Abscisic acid (ABA) regulation of Arabidopsis SR protein gene expression. Int. J. Mol. Sci. 2014, 15, 17541–17564. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Kumar, V. Responses of wild type and abscisic acid mutants of Arabidopsis thaliana to cadmium. J. Plant Physiol. 2002, 159, 1323–1327. [Google Scholar] [CrossRef]
- Disante, K.B.; Cortina, J.; Vilagrosa, A.; Fuentes, D.; Hernandez, E.I.; Ljung, K. Alleviation of Zn toxicity by low water availability. Physiol. Plant. 2014, 150, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Cruz, D.S.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 2012, 25, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Stroiński, A.; Chadzinikolau, T.; Giżewska, K.; Zielezińska, M. ABA or cadmium induced phytochelatin synthesis in potato tubers. Bio. Plant. 2010, 54, 117–120. [Google Scholar] [CrossRef]
- Trinh, N.N.; Huang, T.L.; Chi, W.C.; Fu, S.F.; Chen, C.C.; Huang, H.J. Chromium stress response effect on signal transduction and expression of signaling genes in rice. Physiol. Plant. 2014, 150, 205–224. [Google Scholar] [CrossRef] [PubMed]
- López-Castillo, L.M.; Jiménez-Sandoval, P.; Baruch-Torres, N.; Trasviňa-Arenas, C.H.; Diaz-Quezada, C.; Lara-González, S.; Winkler, R.; Brieba, L.G. Structural basis for redox regulation of cytoplasmic and chloroplastic triosephosphate isomerases from Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1817. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Knuesting, J.; Birkholz, O.; Heinisch, J.J.; Scheibe, R. Cytosolic GAPDH as a redox-dependent regulator of energy metabolism. BMC Plant Biol. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, M.; Zaffagnini, M.; Festa, M.; Trost, P.; Lo Schiavo, F.; Costa, A. Nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in cadmium-stressed Arabidopsis roots. Plant Physiol. 2013, 162, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.P.; Skipsey, M.; Grundy, N.M.; Edwards, R. Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol. 2005, 138, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Žd’arska, M.; Zatloukalová, P.; Benítez, M.; Šedo, O.; Potěšil, D.; Novák, O.; Svačinova, J.; Pešek, B.; Malbeck, J.; Vašíčkova, J.; et al. Proteome analysis in Arabidopsis reveals shoot- and root-specific targets of cytokinin action and differential regulation of hormonal homeostasis. Plant Physiol. 2013, 161, 918–930. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.P.; Romero-Rodriguez, M.C.; Sanchez-Lucas, R.; Navarro-Cerrillo, R.M.; Medina-Aunon, J.A.; Jorrin-Novo, J.V. 2-DE proteomics analysis of drought treated seedlings of Quercus ilex supports a root active strategy for metabolic adaptation in response to water shortage. Front. Plant. Sci. 2015, 6, 627. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Ratcliffe, R.G.; Gupta, K.J. Plant mitochondria: Source and target for nitric oxide. Mitochondrion 2014, 19, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, M.; Xia, Y.J.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef]
- Kosova, K.; Vitamvas, P.; Prasil, I.T. Proteomics of stress responses in wheat and barley-search for potential protein markers of stress tolerance. Front. Plant. Sci. 2014, 5, 711. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: the new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Herrero, J.; Esteban-Carrasco, A.; Zapata, J.M. Looking for Arabidopsis thaliana peroxidases involved in lignin biosynthesis. Plant Physiol. Biochem. 2013, 67, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Komatsu, S. Proteomic analysis of soybean root including hypocotyl during recovery from drought stress. J. Proteom. 2016, 144, 39–50. [Google Scholar] [CrossRef]
- Navrot, N.; Finnie, C.; Svensson, B.; Hägglund, P. Plant redox proteomics. J. Proteom. 2011, 74, 1450–1462. [Google Scholar] [CrossRef]
- Bhatt, I.; Tripathi, B.N. Plant peroxiredoxins: catalytic mechanisms, functional significance and future perspectives. Biotechnol. Adv. 2011, 29, 850–859. [Google Scholar] [CrossRef]
- Dixon, D.P.; Skipsey, M.; Edwards, R. Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 2010, 71, 338–350. [Google Scholar] [CrossRef]
- Bazargani, M.M.; Sarhadi, E.; Bushehri, A.A.; Matros, A.; Mock, H.P.; Naghavi, M.R.; Hajihoseini, V.; Mardi, M.; Hajirezaei, M.R.; Moradi, F.; et al. A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat. J. Proteom. 2011, 74, 1959–1973. [Google Scholar] [CrossRef]
- Gong, B.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Overexpression of S-adenosylmethionine synthetase 1 enhances tomato callus tolerance to alkali stress through polyamine and hydrogen peroxide cross-linked networks. Plant Cell Tissue Organ. Cult. 2015, 124, 377–391. [Google Scholar] [CrossRef]
- Gong, B.; Li, X.; VandenLangenberg, K.M.; Wen, D.; Sun, S.; Wei, M.; Li, Y.; Yang, F.; Shi, Q.; Wang, X. Overexpression of S-adenosyl-L-methionine synthetase increased tomato tolerance to alkali stress through polyamine metabolism. Plant Biotechnol. J. 2014, 12, 694–708. [Google Scholar] [CrossRef]
- Saikawa, N.; Akiyama, Y.; Ito, K. FtsH exists as an exceptionally large complex containing HflKC in the plasma membrane of Escherichia coli. J. Struct. Biol. 2004, 146, 123–129. [Google Scholar] [CrossRef]
- Nagaraj, S.; Senthil-Kumar, M.; Ramu, V.S.; Wang, K.; Mysore, K.S. Plant Ribosomal Proteins, RPL12 and RPL19, Play a Role in Nonhost Disease Resistance against Bacterial Pathogens. Front. Plant. Sci. 2015, 6, 1192. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H. Plant heat-shock proteins: A mini review. J. King Saud Univ. Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Huang, Y.H.; Huang, G.J.; Huang, S.S.; Chow, T.J.; Lin, Y.H. Sweet potato SPAP1 is a typical aspartic protease and participates in ethephon-mediated leaf senescence. J. Plant Physiol. 2015, 180, 1–17. [Google Scholar] [CrossRef]
- Fendrych, M.; van Hautegem, T.; van Durme, M.; Olvera-Carrillo, Y.; Huysmans, M.; Karimi, M.; Lippens, S.; Guérin, C.J.; Krebs, M.; Schumacher, K.; et al. Programmed cell death controlled by ANAC033/SOMBRERO determines root cap organ size in Arabidopsis. Curr. Biol. 2014, 24, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Liang, W.; Yang, X.; Jin, W.; Wilson, Z.A.; Hu, J.; Zhang, D. EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat. Commun. 2013, 4, 1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, H.A.; Iacuone, S.; Li, S.F.; Parish, R.W. The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell 2011, 23, 2209–2224. [Google Scholar] [CrossRef] [PubMed]
- Van Durme, M.; Nowack, M.K. Mechanisms of developmentally controlled cell death in plants. Curr. Opin. Plant Biol. 2016, 29, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L. The role of ubiquitin and the 26S proteasome in plant abiotic stress signaling. Front. Plant. Sci. 2014, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Volko, S.M.; Ledford, H.; Ausubel, F.M.; Dong, X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 2000, 12, 2175–2190. [Google Scholar] [CrossRef]
- Kamal, A.H.M.; Komatsu, S. Jasmonic acid induced protein response to biophoton emissions and flooding stress in soybean. J. Proteom. 2016, 133, 33–47. [Google Scholar] [CrossRef]
- Rao, M.V.; Lee, H.; Creelman, R.A.; Mullet, J.E.; Davis, K.R. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 2000, 12, 1633–1646. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J. Proteom. 2017, 163, 28–51. [Google Scholar] [CrossRef]
- Gutiérrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565. [Google Scholar] [CrossRef]
- Koo, A.J.; Gao, X.; Jones, A.D.; Howe, G.A. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J. 2009, 59, 974–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, K.C.; Mizuuchi, K.; Koshio, A.; Kaneko, K.; Mitsui, T.; Takeno, K. Stress enhances the gene expression and enzyme activity of phenylalanine ammonia-lyase and the endogenous content of salicylic acid to induce flowering in pharbitis. J. Plant Physiol. 2014, 171, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, S.; Zhang, G.; Li, Y.; Li, Z.; Guo, J.; Niu, N.; Wang, J.; Ma, S. Comparative proteomic analysis of a membrane-enriched fraction from flag leaves reveals responses to chemical hybridization agent SQ-1 in wheat. Front. Plant. Sci. 2015, 6, 669. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.G.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus × canescens exposed to excess zinc. Plant Cell Environ. 2015, 38, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Valledor, L.; Castillejo, M.A.; Lenz, C.; Rodriguez, R.; Cañal, M.J.; Jorrín, J. Proteomic analysis of Pinus radiata needles: 2-DE map and protein identification by LC/MS/MS and substitution-tolerant database searching. J. Proteome Res. 2008, 7, 2616–2631. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, T.; He, J.; Chang, W.; Zhang, R.; Liu, M.; Yu, M.; Fan, Y.; Ma, J.; Sun, W.; et al. qPrimerDB: A thermodynamics-based gene-specific qPCR primer database for 147 organisms. Nucleic Acids Res. 2018, 46, D1229–D1236. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef]
- Scholtz, J.J.; Visser, B. Reference gene selection for qPCR gene expression analysis of rust-infected wheat. Physiol. Mol. Plant Pathol. 2013, 81, 22–25. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Valledor, L.; Jorrín, J. Back to the basics: Maximizing the information obtained by quantitative two dimensional gel electrophoresis analyses by an appropriate experimental design and statistical analyses. J. Proteom. 2011, 74, 1–18. [Google Scholar] [CrossRef]
- Valledor, L.; Jorrín, J.V.; Rodriguez, J.L.; Lenz, C.; Meijon, M.; Rodriguez, R.; Canal, M.J. Combined proteomic and transcriptomic analysis identifies differentially expressed pathways associated to Pinus radiata needle maturation. J. Proteome Res. 2010, 9, 3954–3979. [Google Scholar] [CrossRef] [PubMed]





| Treatments | CK | 1:300 | 1:600 | 1:900 | 1:1200 | 1:1500 | |
|---|---|---|---|---|---|---|---|
| 2d | FW(mg)1 | 71.1 ± 1.75b | 60.8 ± 1.87a | 76.9 ± 1.5d | 78.2 ± 1.6d | 74.1 ± 1.43c | 73.1 ± 1.25bc |
| DW(mg)1 | 7.7 ± 0.35b | 6.9 ± 0.11a | 10.9 ± 0.49d | 12.9 ± 0.61e | 9.9 ± 0.51c | 9.6 ± 0.25c | |
| 3d | FW(mg)1 | 116.6 ± 6.26b | 90 ± 2.2a | 127.6 ± 4.05de | 134.7 ± 5.29e | 126.5 ± 3.65cd | 119.7 ± 1.89bc |
| DW(mg)1 | 14.1 ± 0.53a | 12.5 ± 0.6a | 19.5 ± 1.35c | 23.5 ± 1.12d | 17.7 ± 0.76b | 16.8 ± 0.64b | |
| 4d | FW(mg)1 | 179 ± 3.63b | 152.9 ± 3.12a | 189.4 ± 3.72c | 203 ± 4.54d | 192.3 ± 2c | 188.7 ± 1.46c |
| DW(mg)1 | 21.8 ± 0.67b | 17.9 ± 0.66a | 26.4 ± 0.38d | 29.2 ± 0.5e | 26.4 ± 0.65d | 23.7 ± 0.23c | |
| 5d | FW(mg)1 | 249.7 ± 1.45b | 211.8 ± 1.71a | 272.1 ± 1.96d | 286.9 ± 2.11e | 270.7 ± 1.45d | 262.7 ± 1.59c |
| DW(mg)1 | 29.9 ± 0.64b | 24.2 ± 0.32a | 37.9 ± 0.75d | 42.2 ± 0.2e | 37.6 ± 0.35d | 32 ± 0.21c | |
| 6d | FW(mg)1 | 312.3 ± 2.07b | 302.9 ± 1.53a | 340.3 ± 1.8d | 363.7 ± 2.1e | 335.3 ± 1.8c | 332.8 ± 2.03c |
| DW(g)1 | 37.7 ± 0.47b | 35.4 ± 0.67a | 48.3 ± 0.72d | 54 ± 0.31e | 45.7 ± 0.47c | 38 ± 0.71b | |
| Treatments | CK | 1:300 | 1:600 | 1:900 | 1:1200 | 1:1500 | |
|---|---|---|---|---|---|---|---|
| 2d | FW(mg)1 | 77.9 ± 1.4b | 72 ± 1.96a | 81.2 ± 1.31c | 90.1 ± 1.42d | 82.3 ± 1.25c | 80.1 ± 1.18bc |
| DW(mg)1 | 5.6 ± 0.35a | 5.2 ± 0.3a | 7.9 ± 0.35c | 10.5 ± 0.2d | 6.7 ± 0.25b | 6.2 ± 0.21b | |
| 3d | FW(mg)1 | 91.5 ± 3.27ab | 89.8 ± 2.39a | 101.1 ± 3.72c | 126.8 ± 3e | 119.8 ± 4.58d | 93.1 ± 2.69b |
| DW(mg)1 | 6.3 ± 0.32a | 6.8 ± 0.36a | 10.1 ± 0.25c | 18.7 ± 0.56d | 10.4 ± 0.53c | 7.7 ± 0.42b | |
| 4d | FW(mg)1 | 169.3 ± 1.65a | 178 ± 1.61b | 184.7 ± 1.55c | 206.6 ± 1.67e | 190.3 ± 1.05d | 171 ± 1.15a |
| DW(mg)1 | 14.3 ± 0.2a | 15.5 ± 0.46b | 19.9 ± 0.45c | 27.4 ± 0.72e | 23.9 ± 0.32d | 15.7 ± 0.2b | |
| 5d | FW(mg)1 | 213.2 ± 2.05a | 218.2 ± 2.11b | 232.5 ± 1.56c | 257.4 ± 1.22d | 217.8 ± 1.76b | 210.9 ± 2.27a |
| DW(mg)1 | 17.8 ± 0.26a | 21.7 ± 1.03b | 26.5 ± 0.6c | 33.7 ± 1.21d | 26.7 ± 0.86c | 18.1 ± 0.99a | |
| 6d | FW(mg)1 | 267.6 ± 2.12a | 286.3 ± 2.03b | 295.1 ± 4.41c | 310.2 ± 1.65d | 282.3 ± 2.04b | 263.2 ± 1.78a |
| DW(mg)1 | 21.4 ± 0.7a | 27.9 ± 0.38b | 35.2 ± 0.36d | 41.5 ± 0.84e | 33.7 ± 0.85c | 21.6 ± 0.45a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root Proteomics Reveals the Effects of Wood Vinegar on Wheat Growth and Subsequent Tolerance to Drought Stress. Int. J. Mol. Sci. 2019, 20, 943. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040943
Wang Y, Qiu L, Song Q, Wang S, Wang Y, Ge Y. Root Proteomics Reveals the Effects of Wood Vinegar on Wheat Growth and Subsequent Tolerance to Drought Stress. International Journal of Molecular Sciences. 2019; 20(4):943. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040943
Chicago/Turabian StyleWang, Yuying, Ling Qiu, Qilu Song, Shuping Wang, Yajun Wang, and Yihong Ge. 2019. "Root Proteomics Reveals the Effects of Wood Vinegar on Wheat Growth and Subsequent Tolerance to Drought Stress" International Journal of Molecular Sciences 20, no. 4: 943. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040943






