Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease
Abstract
:1. Introduction
2. Overview of Cardiovascular Disease
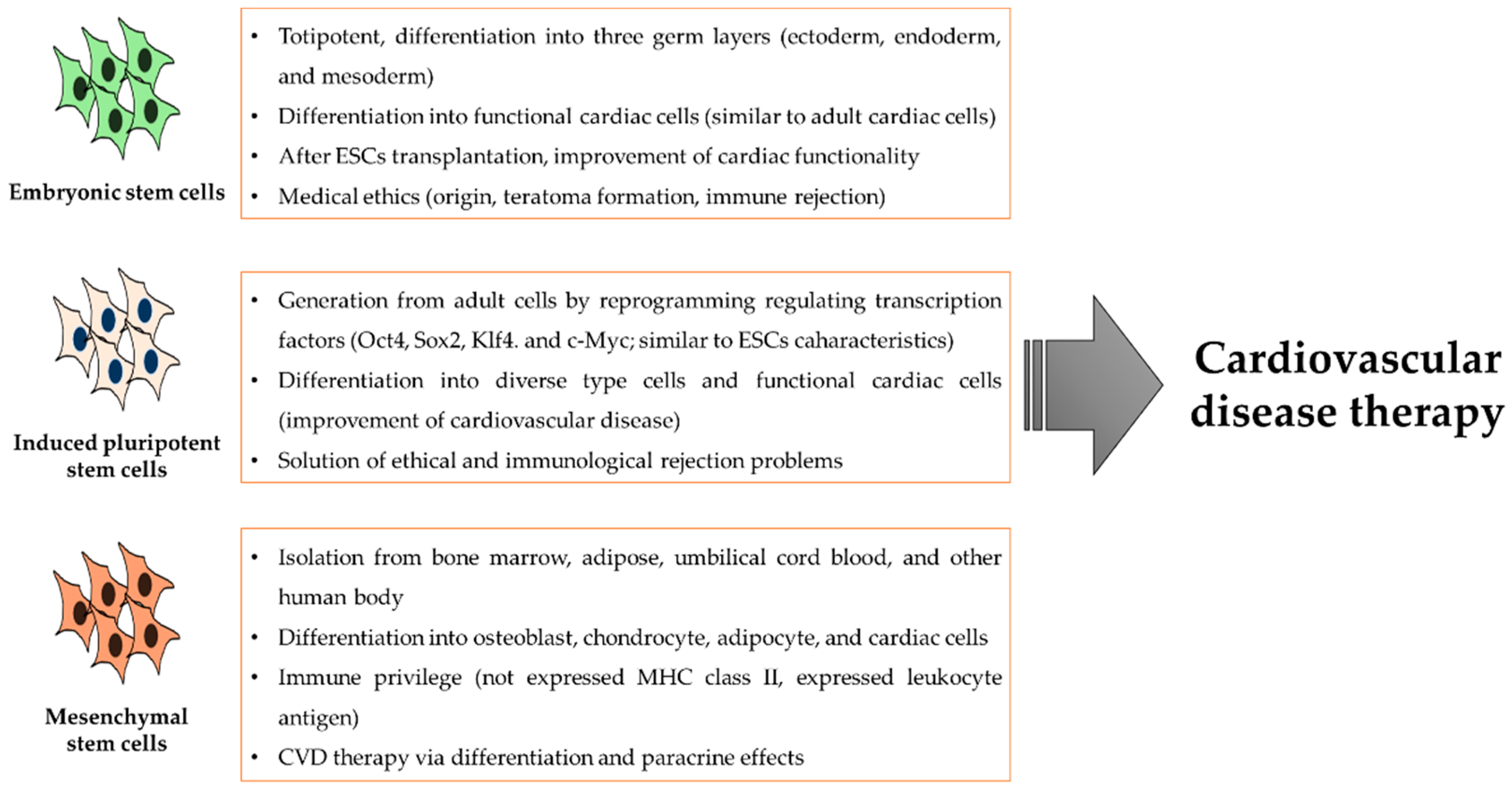
3. Diverse Stem Cell Use for the Treatment of Cardiovascular Disease
4. The Potential Therapeutic Effects of Mesenchymal Stem Cells in Cardiovascular Disease
5. The Treatment of Cardiovascular Disease via Mesenchymal Stem-Cell-Derived Exosomes
6. The Treatment of Cardiovascular Disease via Mesenchymal Stem Cells Enhanced Natural Products
7. Conclusions
Funding
Conflicts of Interest
References
- Writing, C.; Smith, S.C., Jr.; Collins, A.; Ferrari, R.; Holmes, D.R., Jr.; Logstrup, S.; McGhie, D.V.; Ralston, J.; Sacco, R.L.; Stam, H.; et al. Our time: A call to save preventable death from cardiovascular disease (heart disease and stroke). Glob. Heart 2012, 7, 297–305. [Google Scholar] [CrossRef]
- Gupta, A.S. Nanomedicine approaches in vascular disease: A review. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Rentrop, K.P.; Feit, F. Reperfusion therapy for acute myocardial infarction: Concepts and controversies from inception to acceptance. Am. Heart J. 2015, 170, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Spagnoli, V.; Tardif, J.C.; L’Allier, P.L. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis 2015, 240, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki-Ogata, A.; Hashizume, R.; Fu, X.M.; Usui, A.; Narita, Y. Mesenchymal stem cells for treatment of aortic aneurysms. World J. Stem Cells 2014, 6, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Holmes, D.R., Jr. Drug-eluting coronary-artery stents. N. Engl. J. Med. 2013, 368, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y. Effects of mechanical loading on human mesenchymal stem cells for cartilage tissue engineering. J. Cell. Physiol. 2018, 233, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Wan Safwani, W.K.Z.; Choi, J.R.; Yong, K.W.; Ting, I.; Mat Adenan, N.A.; Pingguan-Murphy, B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology 2017, 75, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.; Yong, K.W.; Poon, C.T.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Xu, F.; Wan Safwani, W.K. In situ normoxia enhances survival and proliferation rate of human adipose tissue-derived stromal cells without increasing the risk of tumourigenesis. PLoS ONE 2015, 10, e0115034. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.T.; Lokmic, Z.; Peshavariya, H.; Abberton, K.M.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Hypoxic conditioning enhances the angiogenic paracrine activity of human adipose-derived stem cells. Stem Cells Dev. 2013, 22, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Tang, J.; Nishi, K.; Yan, C.; Dinh, P.U.; Cores, J.; Kudo, T.; Zhang, J.; Li, T.S.; Cheng, K. Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. Circ. Res. 2017, 120, 1768–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsuan, Y.C.; Lin, C.H.; Chang, C.P.; Lin, M.T. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016, 6, e00526. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryu, J.M.; Han, Y.S.; Zia, M.F.; Kwon, H.Y.; Noh, H.; Han, H.J.; Lee, S.H. Fucoidan improves bioactivity and vasculogenic potential of mesenchymal stem cells in murine hind limb ischemia associated with chronic kidney disease. J. Mol. Cell. Cardiol. 2016, 97, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hsieh, A.F.; Dharmarajan, K.; Masoudi, F.A.; Krumholz, H.M. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation 2013, 128, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Hartiala, J.; Schwartzman, W.S.; Gabbay, J.; Ghazalpour, A.; Bennett, B.J.; Allayee, H. The Genetic Architecture of Coronary Artery Disease: Current Knowledge and Future Opportunities. Curr. Atheroscler. Rep. 2017, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Verrier, E.D.; Boyle, E.M., Jr. Endothelial cell injury in cardiovascular surgery. Ann. Thorac. Surg. 1996, 62, 915–922. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front. Physiol. 2015, 6, 365. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- ElAli, A. The implication of neurovascular unit signaling in controlling the subtle balance between injury and repair following ischemic stroke. Neural Regen. Res. 2016, 11, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.A.; Citerio, G.; Smith, M. The intensive care management of acute ischemic stroke: An overview. Intens. Care Med. 2014, 40, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.H. A new penumbra: Transitioning from injury into repair after stroke. Nat. Med. 2008, 14, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.W.; Assimes, T.L.; Li, J.; Quertermous, T.; Cooke, J.P. Genetic susceptibility to peripheral arterial disease: A dark corner in vascular biology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Subherwal, S.; Patel, M.R.; Kober, L.; Peterson, E.D.; Bhatt, D.L.; Gislason, G.H.; Olsen, A.M.; Jones, W.S.; Torp-Pedersen, C.; Fosbol, E.L. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: Results from a nationwide study. Eur. J. Prev. Cardiol. 2015, 22, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Management of peripheral arterial disease (PAD). TransAtlantic Inter-Society Consensus (TASC). Section D: Chronic critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2000, 19, S144–S243. [Google Scholar]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.L.; Chang, D.S.; Sarkar, R.; Wang, R.; Messina, L.M. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J. Vasc. Surg. 2005, 41, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coats, P.; Wadsworth, R. Marriage of resistance and conduit arteries breeds critical limb ischemia. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1044–H1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marston, W.A.; Davies, S.W.; Armstrong, B.; Farber, M.A.; Mendes, R.C.; Fulton, J.J.; Keagy, B.A. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J. Vasc. Surg. 2006, 44, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Bristow, M.R. Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation 2005, 111, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Correction to: 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016, 134, e298. [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Cass Principal Investigators and Their Associates. Coronary artery surgery study (CASS): A randomized trial of coronary artery bypass surgery. Survival data. Circulation 1983, 68, 939–950. [Google Scholar] [CrossRef]
- Piatek, J.; Konstanty-Kalandyk, J.; Kedziora, A.; Hyochan Song, B.; Wierzbicki, K.; Darocha, T.; Milaniak, I.; Kapelak, B. Total arterial myocardial revascularization in patients over 70 years old—A new trend in coronary surgery in elderly. Przeglad Lekarski 2016, 73, 813–815. [Google Scholar] [PubMed]
- Back, M.; Hansson, G.K. Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.R.; Chen, C.C.; Goto, S.; Lin, P.Y.; Wei, F.C.; Chen, C.L. Mesenchymal stem cells as immunomodulators in a vascularized composite allotransplantation. Clin. Dev. Immunol. 2012, 2012, 854846. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, Y.S.; Lee, S.H. Potentiation of biological effects of mesenchymal stem cells in ischemic conditions by melatonin via upregulation of cellular prion protein expression. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Rolletschek, A.; Blyszczuk, P.; Wobus, A.M. Embryonic stem cell-derived cardiac, neuronal and pancreatic cells as model systems to study toxicological effects. Toxicol. Lett. 2004, 149, 361–369. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, C.W.; Elliott, D.A.; Braam, S.R.; Mummery, C.L.; Davis, R.P. Differentiation of Human Pluripotent Stem Cells to Cardiomyocytes Under Defined Conditions. Methods Mol. Biol. 2016, 1353, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Boheler, K.R.; Czyz, J.; Tweedie, D.; Yang, H.T.; Anisimov, S.V.; Wobus, A.M. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ. Res. 2002, 91, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Fijnvandraat, A.C.; van Ginneken, A.C.; Schumacher, C.A.; Boheler, K.R.; Lekanne Deprez, R.H.; Christoffels, V.M.; Moorman, A.F. Cardiomyocytes purified from differentiated embryonic stem cells exhibit characteristics of early chamber myocardium. J. Mol. Cell. Cardiol. 2003, 35, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Kenyagin-Karsenti, D.; Snir, M.; Segev, H.; Amit, M.; Gepstein, A.; Livne, E.; Binah, O.; Itskovitz-Eldor, J.; Gepstein, L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Investig. 2001, 108, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chase, M.W. Multiple disseminated granulomata in sensitized guinea pigs. Ryumachi 1975, 15, 389–390. [Google Scholar] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, K.P.; Uppal, H. Direct reprogramming of human fibroblasts to hepatocyte-like cells by synthetic modified mRNAs. PLoS ONE 2014, 9, e100134. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Miki, K.; Ito, E.; Sougawa, N.; Kawamura, T.; Daimon, T.; Shimizu, T.; et al. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation 2013, 128, S87–S94. [Google Scholar] [CrossRef] [PubMed]
- Ptaszek, L.M.; Mansour, M.; Ruskin, J.N.; Chien, K.R. Towards regenerative therapy for cardiac disease. Lancet 2012, 379, 933–942. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Wernig, M.; Meissner, A.; Foreman, R.; Brambrink, T.; Ku, M.; Hochedlinger, K.; Bernstein, B.E.; Jaenisch, R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007, 448, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoo, J.L.; Chang, W. Arthroscopic Harvest of Adipose-Derived Mesenchymal Stem Cells from the Infrapatellar Fat Pad. Am. J. Sports Med. 2017, 45, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, M.C.; Malpasso, G.; Musaro, P.; Turco, V.; Gnecchi, M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. Methods Mol. Biol. 2016, 1416, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Shake, J.G.; Gruber, P.J.; Baumgartner, W.A.; Senechal, G.; Meyers, J.; Redmond, J.M.; Pittenger, M.F.; Martin, B.J. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann. Thorac. Surg. 2002, 73, 1919–1925. [Google Scholar] [CrossRef]
- Amado, L.C.; Saliaris, A.P.; Schuleri, K.H.; St John, M.; Xie, J.S.; Cattaneo, S.; Durand, D.J.; Fitton, T.; Kuang, J.Q.; Stewart, G.; et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA 2005, 102, 11474–11479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szaraz, P.; Gratch, Y.S.; Iqbal, F.; Librach, C.L. In Vitro Differentiation of Human Mesenchymal Stem Cells into Functional Cardiomyocyte-like Cells. J. Vis. Exp. JoVE 2017. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, H.F.; Xue, T.; Lau, C.P.; Siu, C.W.; Wang, K.; Zhang, Q.Y.; Tomaselli, G.F.; Akar, F.G.; Li, R.A. Bioartificial sinus node constructed via in vivo gene transfer of an engineered pacemaker HCN Channel reduces the dependence on electronic pacemaker in a sick-sinus syndrome model. Circulation 2006, 114, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J.; Gnecchi, M.; Pachori, A.S. Enhancing stem cell therapy through genetic modification. J. Am. Coll. Cardiol. 2005, 46, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, F.; Garry, D.J. Dystrophin-Deficient Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Atoui, R.; Chiu, R.C. Concise review: Immunomodulatory properties of mesenchymal stem cells in cellular transplantation: Update, controversies, and unknowns. Stem Cells Transl. Med. 2012, 1, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Abomaray, F.M.; Alshabibi, M.A.; AlAskar, A.S.; Kalionis, B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 2017, 59, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Blanc, K.; Ringden, O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med. 2007, 262, 509–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuhara, S.; Tomita, S.; Yamashiro, S.; Morisaki, T.; Yutani, C.; Kitamura, S.; Nakatani, T. Direct cell-cell interaction of cardiomyocytes is key for bone marrow stromal cells to go into cardiac lineage in vitro. J. Thorac. Cardiovasc. Surg. 2003, 125, 1470–1480. [Google Scholar] [CrossRef]
- Karantalis, V.; Hare, J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ. Res. 2015, 116, 1413–1430. [Google Scholar] [CrossRef] [PubMed]
- White, I.A.; Sanina, C.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cells in Cardiology. Methods Mol. Biol. 2016, 1416, 55–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Zhang, C. Effects of Hyperlipidemia and Cardiovascular Diseases on Proliferation, Differentiation and Homing of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2017, 12, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.Y.; Wang, L.; Zemskova, M.; Deppen, J.; Xu, K.; Strobel, F.; Garcia, A.J.; Tirouvanziam, R.; Levit, R.D. Adenosine Production by Biomaterial-Supported Mesenchymal Stromal Cells Reduces the Innate Inflammatory Response in Myocardial Ischemia/Reperfusion Injury. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Chou, C.C.; Frantzen, M.; Miyamoto, T.; Kar, S.; Lee, S.; Shah, P.K.; Martin, B.J.; Lill, M.; Forrester, J.S.; et al. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int. J. Cardiol. 2006, 111, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Saito, A.; Komoda, H.; Kitagawa-Sakakida, S.; Miyagawa, S.; Kondoh, H.; Ichikawa, H.; Sawa, Y. Allogenic mesenchymal stem cell transplantation has a therapeutic effect in acute myocardial infarction in rats. J. Mol. Cell. Cardiol. 2008, 44, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Han, Y.S.; Lee, J.H.; Lee, S.H. Combination of MSC spheroids wrapped within autologous composite sheet dually protects against immune rejection and enhances stem cell transplantation efficacy. Tissue Cell 2018, 53, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.L.; Goethe, J.W. The effect of being reared with an alcoholic half-sibling: A classic study reanalyzed. Fam. Process 1990, 29, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Chen, A.; Zhao, Q. Mesenchymal stem cells promote cardiac muscle repair via enhanced neovascularization. Cell. Physiol. Biochem. 2015, 35, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Price, M.J.; Lill, M.; Frantzen, M.; Takizawa, K.; Kleisli, T.; Zheng, J.; Kar, S.; McClelan, R.; Miyamota, T.; et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, W.; Sun, S.; Ristagno, G.; Xu, T.; Weil, M.H. Improved outcomes of cardiopulmonary resuscitation in rats with myocardial infarction treated with allogenic bone marrow mesenchymal stem cells. Crit. Care Med. 2009, 37, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Tano, N.; Kaneko, M.; Ichihara, Y.; Ikebe, C.; Coppen, S.R.; Shiraishi, M.; Shintani, Y.; Yashiro, K.; Warrens, A.; Suzuki, K. Allogeneic Mesenchymal Stromal Cells Transplanted onto the Heart Surface Achieve Therapeutic Myocardial Repair Despite Immunologic Responses in Rats. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Ishikane, S.; Hosoda, H.; Yamahara, K.; Akitake, Y.; Kyoungsook, J.; Mishima, K.; Iwasaki, K.; Fujiwara, M.; Miyazato, M.; Kangawa, K.; et al. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation 2013, 96, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, H.C.; Hatzistergos, K.E.; Oskouei, B.N.; Feigenbaum, G.S.; Rodriguez, J.E.; Valdes, D.; Pattany, P.M.; Zambrano, J.P.; Hu, Q.; McNiece, I.; et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 14022–14027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, S.M.; Ghods, S.; Kolodgie, F.D.; Parcham-Azad, K.; Keane, M.; Hamamdzic, D.; Young, R.; Rippy, M.K.; Virmani, R.; Litt, H.; et al. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur. Heart J. 2008, 29, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, E.G.; Koch, J.M.; Hacker, T.A.; Hatt, C.R.; Tomkowiak, M.T.; Vigen, K.K.; Hendren, N.; Leitzke, C.; Zhao, Y.Q.; Li, Z.; et al. Intravenous Followed by X-ray Fused with MRI-Guided Transendocardial Mesenchymal Stem Cell Injection Improves Contractility Reserve in a Swine Model of Myocardial Infarction. J. Cardiovasc. Transl. Res. 2015, 8, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen. Med. 2011, 6, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Jenjaroenpun, P.; Kremenska, Y.; Nair, V.M.; Kremenskoy, M.; Joseph, B.; Kurochkin, I.V. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ 2013, 1, e201. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.; Lim, S.K. Mesenchymal stem cell exosomes. Sem. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, C.; Song, B.; Xiao, Y.; Fang, M.; Feng, J.; Wang, P. CD4+CD25+ regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model. Cell. Immunol. 2013, 285, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Buschow, S.I.; van Balkom, B.W.; Aalberts, M.; Heck, A.J.; Wauben, M.; Stoorvogel, W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 2010, 88, 851–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Wang, Y.; Lin, L.; Wang, J.; Xiao, H.; Li, J.; Peng, X.; Dai, H.; Li, L. Mast Cell-Derived Exosomes Promote Th2 Cell Differentiation via OX40L-OX40 Ligation. J. Immunol. Res. 2016, 2016, 3623898. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Guo, S.C.; Zhang, C.Q. Platelet-derived Extracellular Vesicles: An Emerging Therapeutic Approach. Int. J. Biol. Sci. 2017, 13, 828–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taverna, S.; Amodeo, V.; Saieva, L.; Russo, A.; Giallombardo, M.; De Leo, G.; Alessandro, R. Exosomal shuttling of miR-126 in endothelial cells modulates adhesive and migratory abilities of chronic myelogenous leukemia cells. Mol. Cancer 2014, 13, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eirin, A.; Riester, S.M.; Zhu, X.Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014, 551, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosca, A.M.; Rayia, D.M.; Tutuianu, R. Emerging Role of Stem Cells-Derived Exosomes as Valuable Tools for Cardiovascular Therapy. Curr. Stem Cell Res. Ther. 2017, 12, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteomics 2012, 2012, 971907. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jin, X.; Hu, C.F.; Li, R.; Zhou, Z.; Shen, C.X. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell. Physiol. Biochem. 2017, 43, 52–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Gong, M.; Wang, Y.; Millard, R.W.; Pasha, Z.; Yang, Y.; Ashraf, M.; Xu, M. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS ONE 2013, 8, e73304. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell. Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zheng, S.; Zhou, C.; Yuan, W.; Wang, J.; Wang, T. Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: microRNAs as novel regulators. J. Cell. Mol. Med. 2012, 16, 657–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopatina, T.; Bruno, S.; Tetta, C.; Kalinina, N.; Porta, M.; Camussi, G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. CCS 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Wang, Y.J.; Chen, L.J.; Pan, Y.H.; Zhang, L.; Weintraub, N.L. Cardiac-derived stem cell-based therapy for heart failure: Progress and clinical applications. Exp. Biol. Med. 2013, 238, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Kuroda, S.; Ito, M.; Shichinohe, H.; Houkin, K.; Kuge, Y.; Tamaki, N. Timing and cell dose determine therapeutic effects of bone marrow stromal cell transplantation in rat model of cerebral infarct. Neuropathology 2013, 33, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Kuroda, S.; Zhao, S.; Magota, K.; Shichinohe, H.; Houkin, K.; Kuge, Y.; Tamaki, N. Bone marrow stromal cell transplantation enhances recovery of local glucose metabolism after cerebral infarction in rats: A serial 18F-FDG PET study. J. Nucl. Med. 2013, 54, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Magota, K.; Zhao, S.; Kubo, N.; Kuge, Y.; Shichinohe, H.; Houkin, K.; Tamaki, N.; Kuroda, S. 123I-iomazenil single photon emission computed tomography visualizes recovery of neuronal integrity by bone marrow stromal cell therapy in rat infarct brain. Stroke 2013, 44, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Muller-Ehmsen, J.; Tschope, C.; Bonarjee, V.; Larsen, A.I.; May, A.E.; Empen, K.; Chorianopoulos, E.; Tebbe, U.; et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: The BOOST-2 randomised placebo-controlled clinical trial. Eur. Heart J. 2017, 38, 2936–2943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, G.; Hou, Y.; Chen, J.; Wang, J.; Zou, C.; Li, D.; Li, H.; Zhang, Q.; Wang, A.; et al. Transplantation of microencapsulated Schwann cells and mesenchymal stem cells augment angiogenesis and improve heart function. Mol. Cell. Biochem. 2012, 366, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Youn, T.J.; Kwon, J.S.; Kim, Y.H.; Bae, J.W.; Bora, S.; Kim, D.W.; Cho, M.C.; Lee, M.M.; Park, Y.B. Effects of bone marrow derived mesenchymal stem cells transplantation in acutely infarcting myocardium. Eur. J. Heart Fail. 2005, 7, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamihata, H.; Matsubara, H.; Nishiue, T.; Fujiyama, S.; Tsutsumi, Y.; Ozono, R.; Masaki, H.; Mori, Y.; Iba, O.; Tateishi, E.; et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001, 104, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.P.; Browner, W.S. Ginkgo biloba: A living fossil. Am. J. Med. 2000, 108, 341–342. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhou, Y.; Sun, L.; Wen, J.T.; Teng, S.J.; Yang, L.; Du, D.S. Protective effects of Gingko biloba extract 761 on myocardial infarction via improving the viability of implanted mesenchymal stem cells in the rat heart. Mol. Med. Rep. 2014, 9, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Y.; Zhuang, P.; Zhang, J.; Zhou, H.; Zhang, M.; Yang, X.; Wang, J.; Liu, D.; Tong, Y. Protective effect of Suxiao jiuxin pill, a traditional Chinese medicine, against acute myocardial ischemia in dogs. BMC Complement. Altern. Med. 2015, 15, 373. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, D.; Zheng, H.; Lv, J.; Leng, J.; Zhang, L.; Zhang, J.; Fan, H.; Liang, F. Acupoint application in patients with chronic stable angina pectoris: Study protocol of a randomized, double-blind, controlled trial. Evid.-Based Complement. Altern. Med. eCAM 2014, 2014, 619706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhuang, P.; Lu, Z.; Zhang, M.; Zhang, T.; Zhang, Y.; Wang, J.; Liu, D.; Tong, Y. Suxiaojiuxin pill enhances atherosclerotic plaque stability by modulating the MMPs/TIMPs balance in ApoE-deficient mice. J. Cardiovasc. Pharmacol. 2014, 64, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.F.; Ju, C.W.; Shen, Y.; Liu, Y.T.; Kim, I.M.; Yu, H.; Weintraub, N.; Wang, X.L.; Tang, Y. Suxiao Jiuxin pill promotes exosome secretion from mouse cardiac mesenchymal stem cells in vitro. Acta Pharmacol. Sin. 2018, 39, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.F.; Li, Y.J.; Ju, C.W.; Shen, Y.; Lei, W.; Chen, C.; Li, Y.; Yu, H.; Liu, Y.T.; Kim, I.M.; et al. Exosomes from Suxiao Jiuxin pill-treated cardiac mesenchymal stem cells decrease H3K27 demethylase UTX expression in mouse cardiomyocytes in vitro. Acta Pharmacol. Sin. 2018, 39, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Castro-Caldas, M.; Carvalho, A.N.; Rodrigues, E.; Henderson, C.J.; Wolf, C.R.; Rodrigues, C.M.; Gama, M.J. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Mol. Neurobiol. 2012, 46, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.J.; Coulombe, R.A.; Sharma, R.P.; Grover, T.A.; Piette, L.H. Oxidation of NADH by vanadium compounds in the presence of thiols. Arch. Biochem. Biophys. 1989, 271, 40–48. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, D.; Xu, J.; Chi, B. Effect of Tauroursodeoxycholic Acid and 4-Phenylbutyric Acid on Metabolism of Copper and Zinc in Type 1 Diabetic Mice Model. Biol. Trace Elem. Res. 2016, 170, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.M.; Lee, J.H.; Yun, S.P.; Han, Y.S.; Yun, C.W.; Lee, H.J.; Noh, H.; Lee, S.J.; Han, H.J.; Lee, S.H. Tauroursodeoxycholic acid reduces ER stress by regulating of Akt-dependent cellular prion protein. Sci. Rep. 2016, 6, 39838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocrine Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.J.; Lopez-Pingarron, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; Garcia-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramirez, J.M.; Bernal-Perez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Zhang, N.; Zhang, C. Stem cell-based therapies in ischemic heart diseases: A focus on aspects of microcirculation and inflammation. Basic Res. Cardiol. 2011, 106, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Monsel, A.; Zhu, Y.G.; Gennai, S.; Hao, Q.; Liu, J.; Lee, J.W. Cell-based therapy for acute organ injury: Preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 2014, 121, 1099–1121. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn-Brouwer, F.C.C.; Gremmels, H.; Fledderus, J.O.; Verhaar, M.C. Mesenchymal Stromal Cell Characteristics and Regenerative Potential in Cardiovascular Disease: Implications for Cellular Therapy. Cell Transp. 2018, 27, 765–785. [Google Scholar] [CrossRef] [PubMed]
- Lunyak, V.V.; Amaro-Ortiz, A.; Gaur, M. Mesenchymal Stem Cells Secretory Responses: Senescence Messaging Secretome and Immunomodulation Perspective. Front. Genet. 2017, 8, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, K.M.; Hajjar, R.J. Mesenchymal Stem Cells & Endothelial Function. EBioMedicine 2015, 2, 376–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, K.R.; Zhang, Y.; Mihic, A.; Li, M.; Li, S.H.; Xue, P.; Zhang, W.; Basmaji, S.; Tsang, K.; Weisel, R.D.; et al. Role of WNT/beta-catenin signaling in rejuvenating myogenic differentiation of aged mesenchymal stem cells from cardiac patients. Am. J. Pathol. 2012, 181, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.A.; Chen, A.; de-Graft Johnson, E.; Gitter, A.; Kozak, S.; Niquice, C.; Zimmer-Faust, A.G.; Weir, M.H.; Mitchell, J.; Gurian, P. Salmonella risks due to consumption of aquaculture-produced shrimp. Microb. Risk Anal. 2018, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]


| Pathological Condition | Type of Source | Findings | Reference |
|---|---|---|---|
| Acute MI | BM-derived MSC | Increase of adenosine via CD73 activity, reduction of inflammatory responses, mobilization, homing of MSCs, reduction of infarct sites, improvement of cardiac function | [78,79,84,85,86,88,89] |
| Ischemic disease | Ad-derived MSC | Immunomodulation, reduction of T cell proliferation and function, anti-inflammatory effects | [81] |
| Ischemic disease | BM-derived MSC | Neovascularization, recovery of damaged cardiac muscle | [83] |
| MI/R | ES-MSC derived exosome | Recovery of tissue injury, protection of cardiac function, reduction of immune response | [105,113] |
| Stroke | BM-MSC derived exosome | Promotion of angiogenesis, neurite remodeling and neurogenesis | [106] |
| MI/R | BM-MSC derived exosome | Reduction of ROS production, apoptosis, infarct size, enhancement of autophagy, promotion of HUVEC function and angiogenesis, immunomodulation | [107,114,119] |
| MI | miR-22/Mecp2 | Reduction of apoptosis and cardiac fibrosis | [109] |
| Cell ischemic injury | miR-221 | Anti-apoptotic activity, cardioprotective effect | [110] |
| MI | miR-21 | Anti-apoptotic activity, neovascularization | [111] |
| MI | Gingko biloba | Reduction of immune responses, antioxidant activity, anti-apoptotic activity, differentiation of MSCs | [130] |
| In vitro | Suxiao jiuxin pill | Increase of exosome releases, histone remodeling, promotion of cardiomyocyte proliferation | [134,135] |
| Ischemic disease | TUDCA | Reduction of ER stress, antioxidant activity, improvement of ischemic injury site | [139] |
| Ischemic disease | Melatonin | Enhancement of proliferation, antioxidant activity, immunomodulation | [39] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, C.W.; Lee, S.H. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 982. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040982
Yun CW, Lee SH. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. International Journal of Molecular Sciences. 2019; 20(4):982. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040982
Chicago/Turabian StyleYun, Chul Won, and Sang Hun Lee. 2019. "Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease" International Journal of Molecular Sciences 20, no. 4: 982. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040982





