Ginseng Berry Extract Rich in Phenolic Compounds Attenuates Oxidative Stress but not Cardiac Remodeling post Myocardial Infarction
Abstract
:1. Introduction
2. Results
2.1. Phenolic Content and Antioxidant Capacity of GBE
2.2. Body Weight and Heart Weight Characteristics after MI
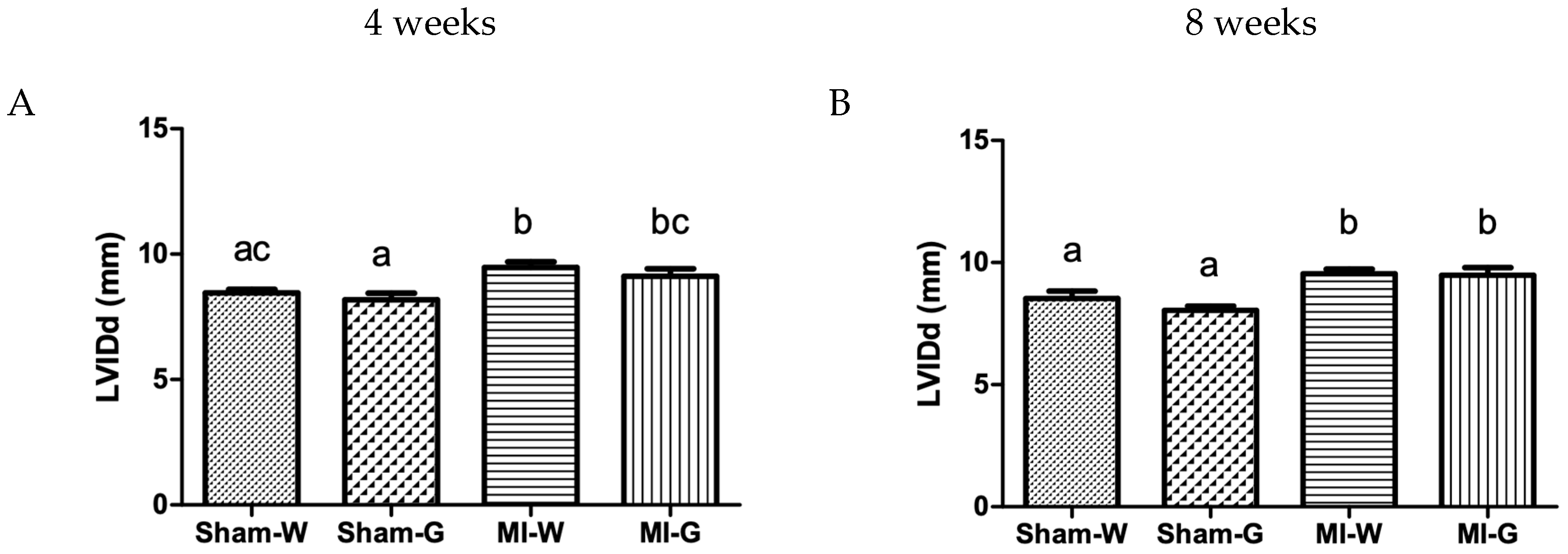
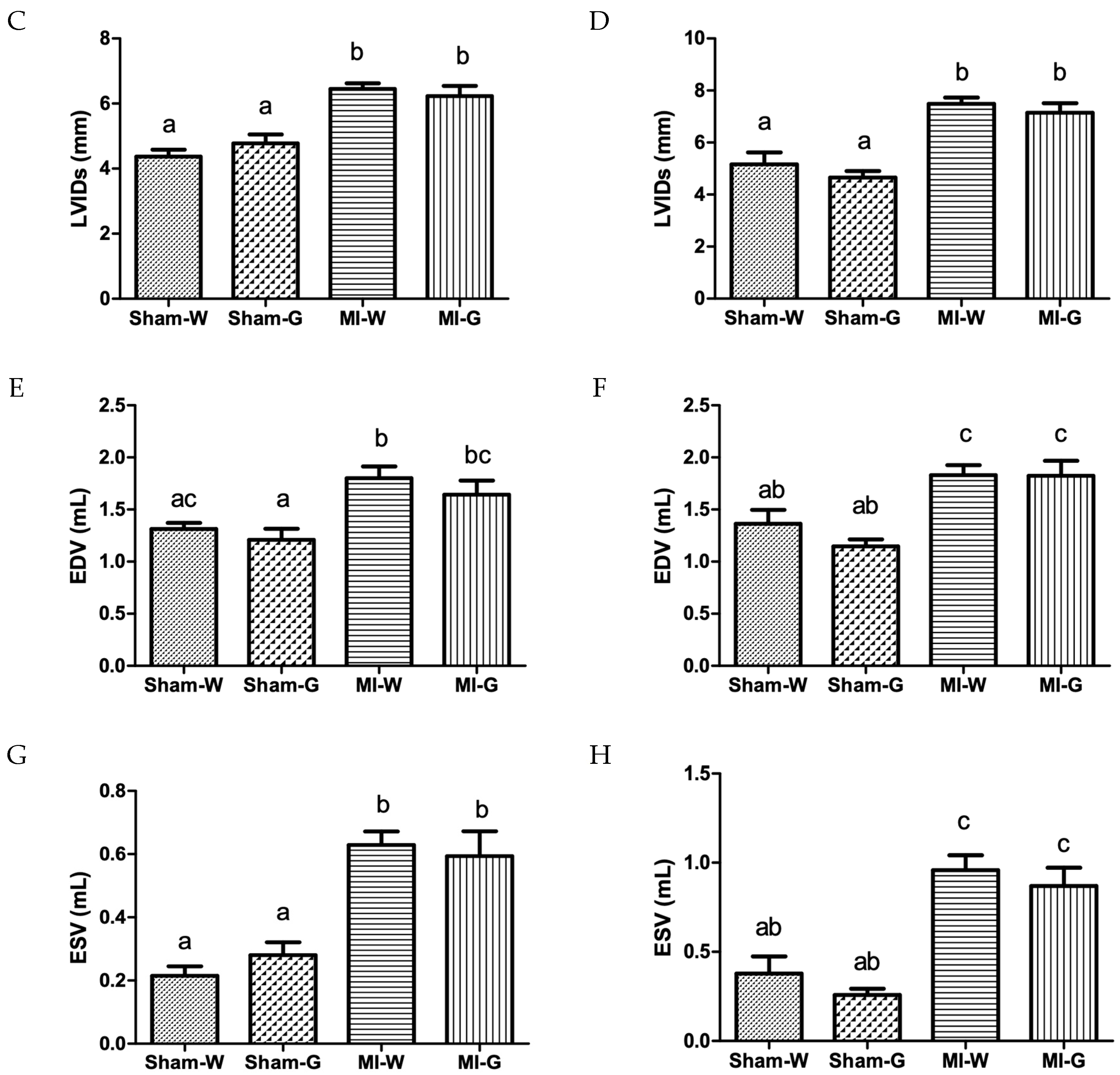
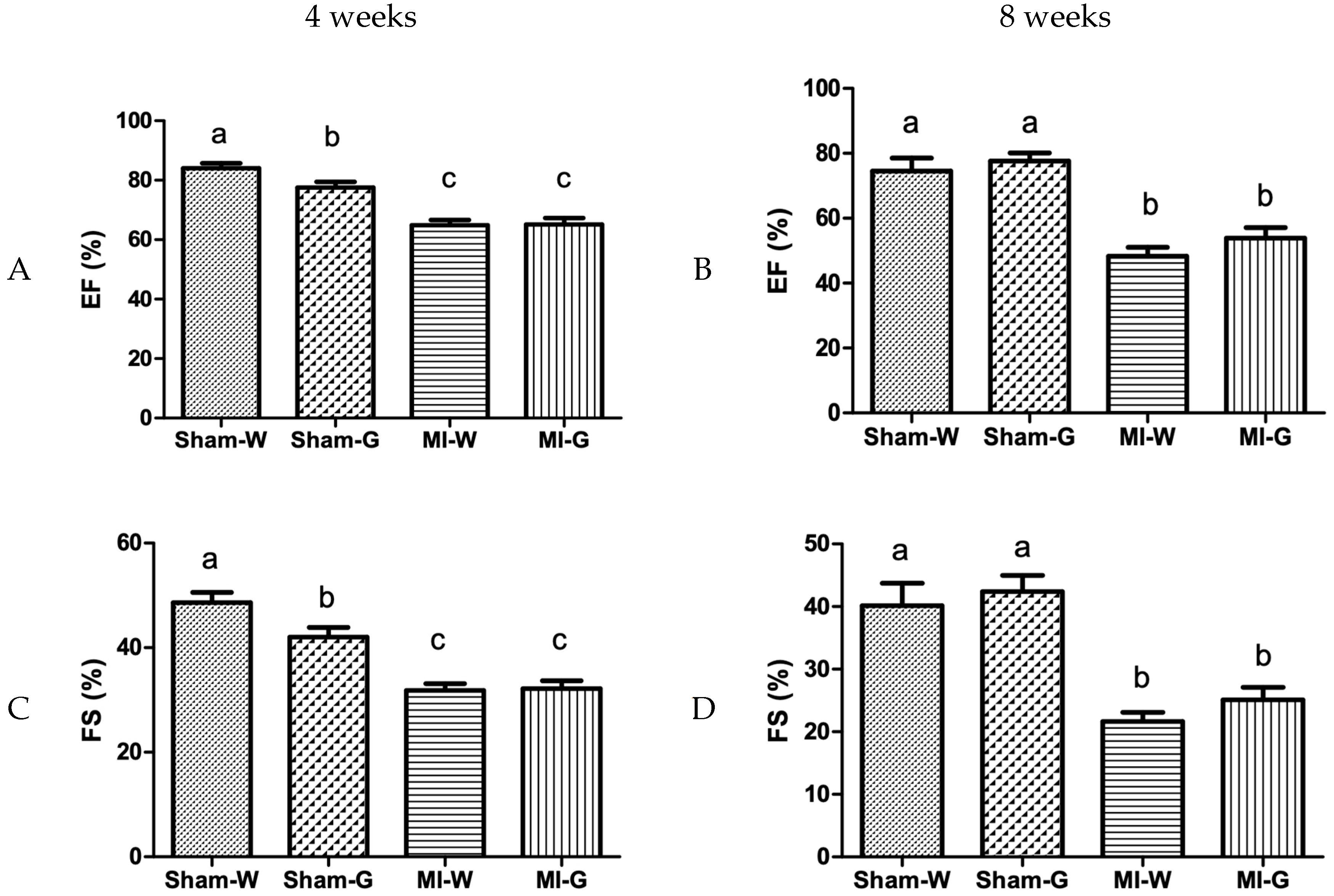
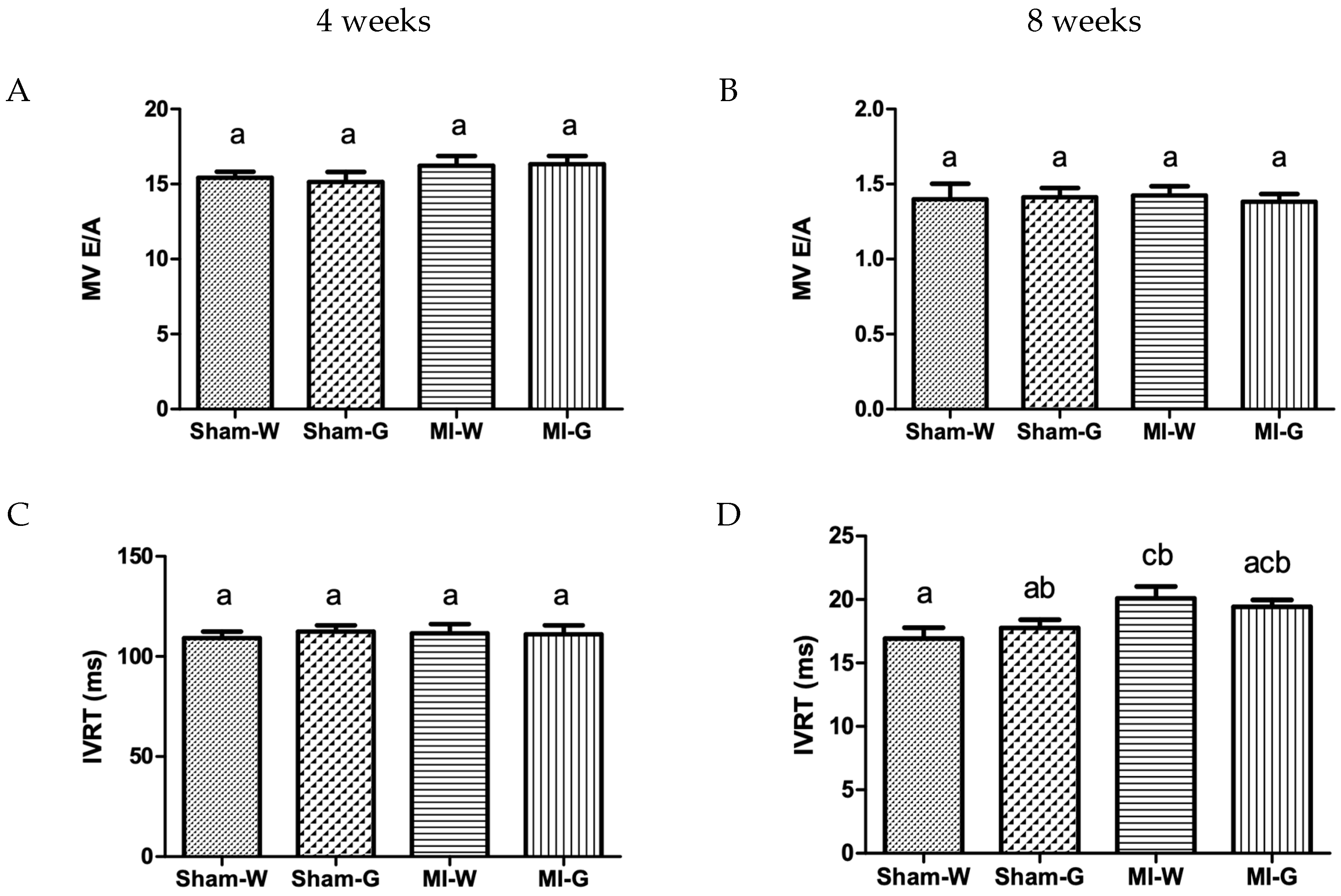
2.3. Lack of Improvement in Cardiac Structure and Function with GBE Treatment
2.4. Reduction in Oxidative Stress and Inflammation with Ginseng Berry Extract Treatment
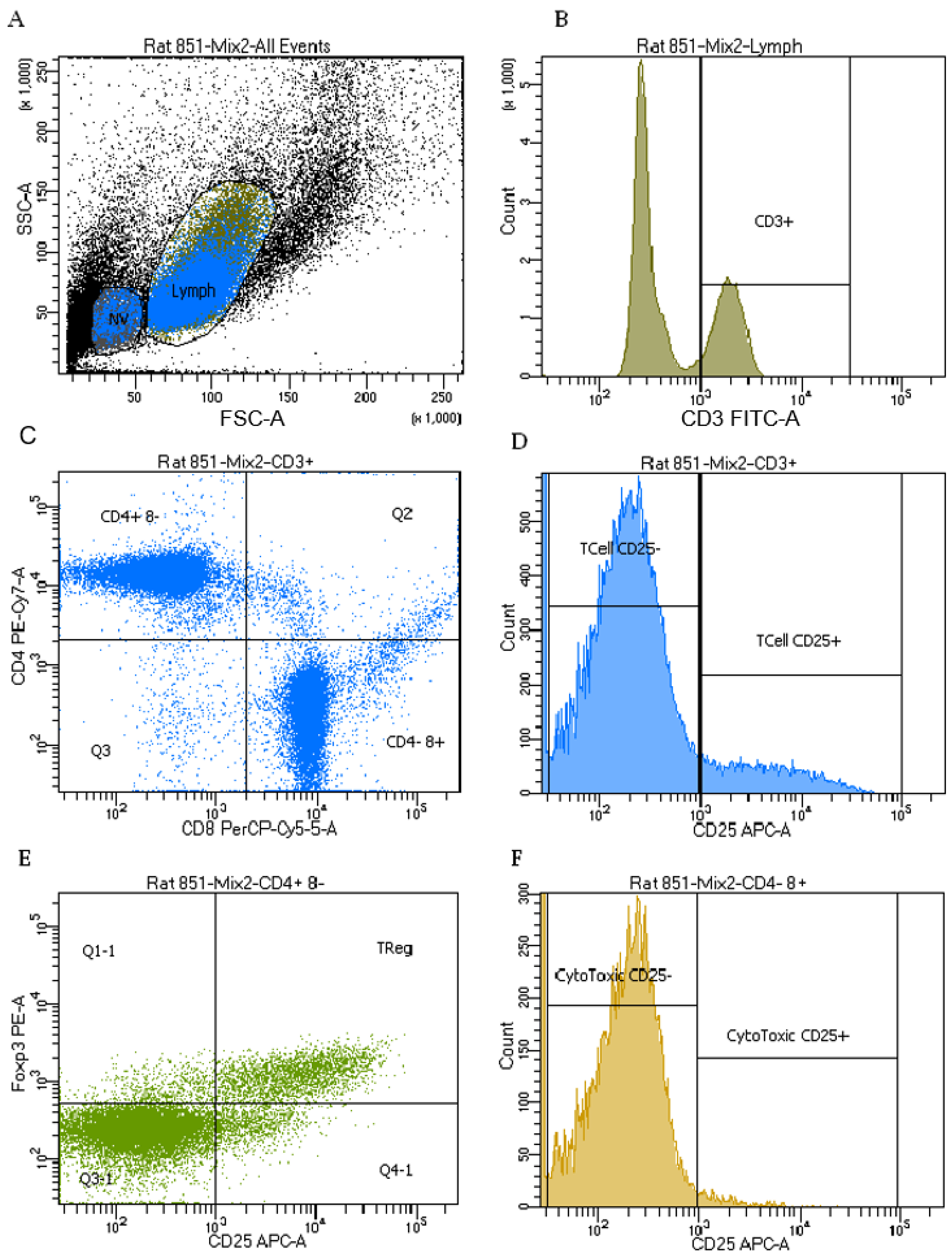
2.5. Immune Cell Phenotypes
2.6. Cytokines Production
3. Discussion
Limitations and Future Opportunities
4. Materials and Methods
4.1. Ginseng Berry Pulp Extract
4.2. Quantification of Phenolic Content and Antioxidant Activity
4.3. Proximate Analysis
4.4. Animal Study
4.4.1. Transthoracic Echocardiography
4.4.2. Biological Sample Collection and Analysis
4.5. T-cell Phenotypes and Function
4.5.1. Isolation of T-cells
4.5.2. Cytokine Determination
4.5.3. Phenotyping
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vuksan, V.; Xu, Z.Z.; Jovanovski, E.; Jenkins, A.L.; Beljan-Zdravkovic, U.; Sievenpiper, J.L.; Mark Stavro, P.; Zurbau, A.; Duvnjak, L.; Li, M.Z.C. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double-blind, randomized, cross-over clinical trial. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Chung, I.M.; Lim, J.J.; Ahn, M.S.; Jeong, H.N.; An, T.J.; Kim, S.H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016, 40, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Mehendale, S.R.; Wang, C.Z.; Shao, Z.H.; Li, C.Q.; Xie, J.T.; Aung, H.H.; Yuan, C.S. Chronic pretreatment with American ginseng berry and its polyphenolic constituents attenuate oxidant stress in cardiomyocytes. Eur. J. Pharmacol. 2006, 553, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Cho, C.W.; Lee, Y.; Kim, S.S.; Lee, S.H.; Kim, K.T. Comparison of Ginsenoside and Phenolic Ingredient Contents in Hydroponically-cultivated Ginseng Leaves, Fruits, and Roots. J. Ginseng Res. 2012, 36, 425–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, T.K.; Jang, K.I. Are berries useless by-products of ginseng? Recent research on the potential health benefits of ginseng berry. Excli J. 2017, 16, 780–784. [Google Scholar] [PubMed]
- Dey, L.; Xie, J.T.; Wang, A.; Wu, J.; Maleckar, S.A.; Yuan, C.S. Anti-hyperglycemic effects of ginseng: Comparison between root and berry. Phytomedicine 2003, 10, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, J.; Jung, S.; Kim, J.W.; Shin, J.H.; Lee, H.J. The involvement of ginseng berry extract in blood flow via regulation of blood coagulation in rats fed a high-fat diet. J. Ginseng Res. 2017, 41, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Kim, S.; Lee, S.J.; Oh, B.C.; Jun, H.S. Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients 2015, 7, 3038–3053. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, S.; Kim, M.J.; Kim, M.S.; Kim, J.; Park, C.W.; Seo, D.; Shin, S.S.; Oh, S.W. Efficacy and safety of Panax ginseng berry extract on glycemic control: A 12-wk randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng Res. 2018, 42, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ichikawa, T.; Jin, Y.; Hofseth, L.J.; Nagarkatti, P.; Nagarkatti, M.; Windust, A.; Cui, T. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J. Ethnopharmacol. 2010, 130, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moey, M.; Gan, X.T.; Huang, C.X.; Rajapurohitam, V.; Martinez-Abundis, E.; Lui, E.M.; Karmazyn, M. Ginseng reverses established cardiomyocyte hypertrophy and postmyocardial infarction-induced hypertrophy and heart failure. Circ. Heart Fail. 2012, 5, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E. Polyphenols from red wine are potent modulators of innate and adaptive immune responsiveness. Proc. Nutr. Soc. 2010, 69, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.-K.; Cho, D.H.; Lee, K.-S.; Lee, D.-K.; Park, C.-W.; Kim, W.G.; Lee, S.J.; Ha, K.-S.; Goo Taeg, O.; Kwon, Y.-G.; Kim, Y.-M. Ginseng Berry Extract Prevents Atherogenesis via Anti-Inflammatory Action by Upregulating Phase II Gene Expression. Evid. -Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Zhou, Y.P.; Xie, J.T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kim, H.J.; Park, M.S.; Ji, G.E. Effects of fermented ginseng root and ginseng berry on obesity and lipid metabolism in mice fed a high-fat diet. J. Ginseng Res. 2018, 42, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-H.; Xie, J.-T.; Vanden Hoek, T.L.; Mehendale, S.; Aung, H.; Li, C.-Q.; Qin, Y.; Schumacker, P.T.; Becker, L.B.; Yuan, C.-S. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim. Biophys. Acta Gen. Subj. 2004, 1670, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Eaton, L.W.; Weiss, J.L.; Bulkley, B.H.; Garrison, J.B.; Weisfeldt, M.L. Regional Cardiac Dilatation after Acute Myocardial Infarction. N. Engl. J. Med. 1979, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J.A.; Weiss, J.L.; Eaton, L.W.; Kallman, C.; Weisfeldt, M.L.; Bulkley, B.H. Late effects of acute infarct dilation on heart size: A two dimensional echocardiographic study. Am. J. Card. 1982, 49, 1120–1126. [Google Scholar] [CrossRef]
- Gaudron, P.; Eilles, C.; Kugler, I.; Ertl, G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation 1993, 87, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Rigo, P.; Murray, M.; Strauss, H.W.; Taylor, D.; Kelly, D.; Weisfeldt, M.; Pitt, B. Left Ventricular Function in Acute Myocardial Infarction Evaluated by Gated Scintiphotography. Circulation 1974, 50, 678–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, J.E.; Hillis, G.S.; Oh, J.K.; Reeder, G.S.; Gersh, B.J.; Pellikka, P.A. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am. Heart J. 2006, 151, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Multicenter Postinfarction Research Group. Risk Stratification and Survival after Myocardial Infarction. N. Engl. J. Med. 1983, 309, 331–336. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Norris, R.M.; Brown, M.A.; Brandt, P.W.; Whitlock, R.M.; Wild, C.J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987, 76, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Janahmadi, Z.; Nekooeian, A.A.; Moaref, A.R.; Emamghoreishi, M. Oleuropein Offers Cardioprotection in Rats with Acute Myocardial Infarction. Cardiovasc. Toxicol. 2015, 15, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, J.; Hong, G. Minocycline improves cardiac function after myocardial infarction in rats by inhibiting activation of PARP-1. Biomed. Pharmacother. 2018, 97, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Narasimhan, M.; Sakthivel, R.; Kumar, R.R.; Davidson, C.; Palaniappan, S.; Claycomb, W.W.; Hoidal, J.R.; Darley-Usmar, V.M.; Rajasekaran, N.S. A biphasic effect of TNF-alpha in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox Biol. 2016, 9, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kurrelmeyer, K.M.; Michael, L.H.; Baumgarten, G.; Taffet, G.E.; Peschon, J.J.; Sivasubramanian, N.; Entman, M.L.; Mann, D.L. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc. Natl. Acad. Sci. USA 2000, 97, 5456–5461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ani, D.; Philipchik, I.; Stav, H.; Levi, M.; Zerbib, J.; Shainberg, A. Tumor necrosis factor alpha protects heart cultures against hypoxic damage via activation of PKA and phospholamban to prevent calcium overload. Can. J. Physiol. Pharmacol. 2014, 92, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Utheim, T.P. Chapter 7: Why Test BCG in Sjögren’s Syndrome? In The Value of BCG and TNF in Autoimmunity; Faustman, D.L., Ed.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 105–125. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; McCallum, J.L.; Kirby, C.; Grewal, G.; Yu, L.; Wigle, J.T.; Netticadan, T. Effects of cyanidin 3-0-glucoside on cardiac structure and function in an animal model of myocardial infarction. Food Funct. 2017, 8, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Zhao, S.; Jassal, D.S.; Dixon, I.M. Effect of AT1 receptor blockade on cardiac collagen remodeling after myocardial infarction. Cardiovasc. Res. 1997, 35, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Blewett, H.J.; Mohankumar, S.K.; Rech, L.; Rector, E.S.; Taylor, C.G. The reduced proportion of New splenic T-cells in the zinc-deficient growing rat is not due to increased susceptibility to apoptosis. Immunobiology 2014, 219, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Blewett, H.J.; Gerdung, C.A.; Ruth, M.R.; Proctor, S.D.; Field, C.J. Vaccenic acid favourably alters immune function in obese JCR:LA-cp rats. Br. J. Nutr. 2009, 102, 526–536. [Google Scholar] [CrossRef] [PubMed]


| Constituent | Value (%) |
|---|---|
| Moisture | 8.19 |
| Dry matter | 91.81 |
| Crude protein | 8.61 |
| Crude fibre | 1.54 |
| Fat | 0.90 |
| Ash | 12.50 |
| Non-fibre carbohydrates | 68.26 |
| Total digestible nutrients | 69.77 |
| Parameter | Sham-W | Sham-G | MI-W | MI-G |
|---|---|---|---|---|
| Body weight (g) | 573.1 ± 11 | 579.9 ± 21 | 538.4 ± 12 | 549 ± 16 |
| Heart weight (g) | 1.433 ± 0.05 | 1.355 ± 0.05 | 1.458 ± 0.03 | 1.415 ± 0.05 |
| LV weight (g) | 0.70 ± 0.060 | 0.86 ± 0.051 | 0.73 ± 0.03 | 0.82 ± 0.04 |
| % infarct | - | - | 32.37 ± 2.8 | 25.47 ± 2.3 |
| Heart weight/Tibia length (g/cm) | 0.32 ± 0.009 | 0.30 ± 0.01 | 0.33 ± 0.0 | 0.31 ± 0.0 |
| LV weight/Tibia length (g/cm) | 0.15 ± 0.013 | 0.19 ± 0.011 | 0.16 ± 0.01 | 0.18 ± 0.01 |
| Lymphocyte Phenotypes | Model | Treatment | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham n = 16 | MI n = 23 | p-Value | Water n = 20 | GBE n = 19 | p-Value | Sham Water n = 8 | Sham GBE n = 8 | MI Water n = 12 | MI GBE n = 11 | p-Value | |
| Viable Lymphocytes | |||||||||||
| CD3+ % lymphocytes | 30.0 ± 0.9 | 30.0 ± 0.8 | 0.99 | 28.7 ± 0.8 | 31.4 ± 0.9 | 0.03 | 29.2 ± 1.3 ab | 30.9 ± 1.3 ab | 28.2 ± 1.0 b | 31.9 ± 1.1 a | 0.39 |
| 1 CD25+ % of CD3+ | 11.5 ± 0.3 | 10.9 ± 0.3 | 0.14 | 11.5 ± 0.3 | 10.9 ± 0.3 | 0.15 | 11.7 ± 0.5 | 11.3 ± 0.4 | 11.3 ± 0.4 | 10.4 ± 0.4 | 0.65 |
| CD4+CD8− % of CD3+ | 57.1 ± 1.2 | 57.5 ± 1.0 | 0.80 | 59.5 ± 1.1 | 55.1 ± 1.1 | 0.01 | 58.7 ± 1.7 a | 55.5 ± 1.7 ab | 60.4 ± 1.4 a | 54.6 ± 1.5 b | 0.41 |
| 2 CD25+ % of CD3+CD4+CD8− | 16.3 ± 0.4 | 15.0 ± 0.4 | 0.04 | 15.9 ± 0.4 | 15.4 ± 0.4 | 0.40 | 16.7 ± 0.6 a | 15.9 ± 0.6 ab | 15.1 ± 0.6 ab | 14.9 ± 0.6 b | 0.54 |
| 3 Foxp3+CD25+ % of CD3+CD4+CD8− | 12.3 ± 0.4 | 11.1 ± 0.3 | 0.03 | 11.9 ± 0.4 | 11.6 ± 0.4 | 0.60 | 12.8 ± 0.5 a | 11.8 ± 0.5 ab | 10.9 ± 0.5 b | 11.4 ± 0.5 ab | 0.17 |
| CD4−CD8+ % of CD3+ | 39.6 ± 1.2 | 39.2 ± 1.0 | 0.80 | 37.0 ± 1.1 | 41.9 ± 1.1 | 0.004 | 37.8 ± 1.7 ab | 41.5 ± 1.7 a | 36.2 ± 1.4 b | 42.3 ± 1.5 a | 0.46 |
| 4 CD25+ % of CD3+CD4−CD8+ | 2.8 ± 0.2 | 2.7 ± 0.1 | 0.66 | 2.7 ± 0.2 | 2.7 ± 0.1 | 0.97 | 2.7 ± 0.2 | 2.9 ± 0.2 | 2.8 ± 0.2 | 2.6 ± 0.2 | 0.30 |
| Non-Viable lymphocytes | |||||||||||
| CD3+ % non-viable cells | 27.2 ± 1.1 | 25.7 ± 0.9 | 0.31 | 23.1 ± 1.0 | 29.9 ± 1.0 | <0.0001 | 22.9 ± 1.5 b | 31.5 ± 1.5 a | 23.2 ± 1.3 b | 28.2 ± 1.4 a | 0.22 |
| 5 CD25+ % of CD3+ | 4.9 ± 0.2 | 4.3 ± 0.2 | 0.07 | 4.9 ± 0.2 | 4.3 ± 0.2 | 0.06 | 4.9 ± 0.3 a | 4.8 ± 0.3 a | 4.8 ± 0.3 a | 3.9 ± 0.3 b | 0.17 |
| CD4+CD8− % of CD3+ | 75.6 ± 1.3 | 74.4 ± 1.1 | 0.49 | 74.9 ± 1.1 | 75.1 ± 1.2 | 0.94 | 75.7 ± 1.8 | 75.5 ± 1.8 | 74.2 ± 1.5 | 74.7 ± 1.6 | 0.83 |
| 6 CD25+ % of CD3+CD4+CD8− | 4.9 ± 0.2 | 4.5 ± 0.2 | 0.13 | 4.9 ± 0.2 | 4.4 ± 0.2 | 0.05 | 4.9 ± 0.3 a | 4.9 ± 0.3 a | 5.0 ± 0.2 a | 4.0 ± 0.2 b | 0.08 |
| 7 Foxp3+CD25+ % of CD3+CD4+CD8− | 3.1 ± 0.1 | 2.7 ± 0.1 | 0.06 | 3.0 ± 0.1 | 2.8 ± 0.1 | 0.18 | 3.2 ± 0.2 a | 3.0 ± 0.2 ab | 2.9 ± 0.2 ab | 2.6 ± 0.2 b | 0.59 |
| CD4−CD8+ % of CD3+ | 17.5 ± 0.9 | 18.3 ± 0.8 | 0.56 | 17.3 ± 0.9 | 18.5 ± 0.9 | 0.36 | 16.7 ± 1.3 | 18.4 ± 1.3 | 18.0 ± 1.1 | 18.6 ± 1.2 | 0.66 |
| 8 CD25+ % of CD3+CD4−CD8+ | 2.3 ± 0.3 | 1.6 ± 0.2 | 0.05 | 2.2 ± 0.2 | 1.7 ± 0.2 | 0.17 | 2.4 ± 0.4 a | 2.2 ± 0.4 ab | 1.9 ± 0.3 ab | 1.2 ± 0.3 b | 0.54 |
| Cytokines | Model | Treatment | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham n = 16 | MI n = 23 | p-Value | Water n = 20 | GBE n = 19 | p-Value | Sham Water n = 8 | Sham GBE n = 8 | MI Water n = 12 | MI GBE n = 11 | p-Value | |
| IL-2 ConA | 2111 ± 181 | 1890 ± 79 | 0.24 | 1910 ± 133 | 2055 ± 114 | 0.47 | 2067 ± 279 | 2155 ± 248 | 1805 ± 125 | 1983 ± 90 | 0.80 |
| 1 IL-2 UNS | 3.4 ± 0.2 | 4.0 ± 0.4 | 0.35 | 3.9 ± 0.4 | 3.4 ± 0.3 | 0.52 | 3.5 ± 0.6 | 3.3 ± 0.2 | 4.2 ± 0.6 | 3.6 ± 0.5 | 0.72 |
| 2 IFNγ ConA | 383.0 ± 75 | 267.0 ± 35 | 0.13 | 312.9 ± 59 | 312.7 ± 47 | 0.79 | 427.8 ± 131 | 343.7 ± 89 | 245.9 ± 49 | 290.1 ± 51 | 0.40 |
| IFNγ UNS | ND | ND | / | ND | ND | / | ND | ND | ND | ND | / |
| 3 IL-10 ConA | 49.2 ± 3.3 | 47.3 ± 3.1 | 0.65 | 48.4 ± 4.1 | 47.9 ± 2.3 | 0.81 | 51.9 ± 5.4 | 46.9 ± 4.2 | 45.9 ± 5.9 | 48.7 ± 2.7 | 0.41 |
| 4 IL-10 UNS | 33.1 ± 4.2 | 29.1 ± 1.8 | 0.49 | 28.3 ± 2.4 | 33.1 ± 3.1 | 0.30 | 29.2 ± 6.8 | 35.4 ± 5.6 | 27.9 ± 2.4 | 30.8 ± 2.8 | 0.70 |
| TNFα ConA | 773.6 ± 57 | 727.4 ± 31 | 0.43 | 779.1 ± 42 | 711.8 ± 41 | 0.30 | 792.9 ± 87 | 754.3 ± 79 | 770.0 ± 42 | 680.9 ± 43 | 0.68 |
| TNFα UNS | ND | ND | / | ND | ND | / | ND | ND | ND | ND | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parikh, M.; Raj, P.; Yu, L.; Stebbing, J.-A.; Prashar, S.; Petkau, J.C.; Tappia, P.S.; Pierce, G.N.; Siow, Y.L.; Brown, D.; et al. Ginseng Berry Extract Rich in Phenolic Compounds Attenuates Oxidative Stress but not Cardiac Remodeling post Myocardial Infarction. Int. J. Mol. Sci. 2019, 20, 983. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040983
Parikh M, Raj P, Yu L, Stebbing J-A, Prashar S, Petkau JC, Tappia PS, Pierce GN, Siow YL, Brown D, et al. Ginseng Berry Extract Rich in Phenolic Compounds Attenuates Oxidative Stress but not Cardiac Remodeling post Myocardial Infarction. International Journal of Molecular Sciences. 2019; 20(4):983. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040983
Chicago/Turabian StyleParikh, Mihir, Pema Raj, Liping Yu, Jo-Ann Stebbing, Suvira Prashar, Jay C. Petkau, Paramjit S. Tappia, Grant N. Pierce, Yaw L. Siow, Dan Brown, and et al. 2019. "Ginseng Berry Extract Rich in Phenolic Compounds Attenuates Oxidative Stress but not Cardiac Remodeling post Myocardial Infarction" International Journal of Molecular Sciences 20, no. 4: 983. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040983





