Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine
Abstract
:1. Physiological Oxygen Levels In Vivo
2. Stem Cell Niches in Adult Tissues
- In lungs, two main stem cell populations have been described. Basal stem cells (BSCs) have the capacity to self-renew and to form secretory and ciliated cells [74,75,76]. Distal alveolar stem cells (DASCs), which are present in the distal airways after H1N1 influenza virus infection and have the capacity to replace injured alveolar cells [77,78].
- In the skin, epithelial stem cells are found in the bulge area of the hair follicles [79], while the exact components of skin niche have not been fully identified yet, although critical regulatory cues derive from the dermal papilla. These stem cells are important in regeneration of hair follicles while scattered stem cells attached to the basal membrane that separates epidermis from dermis (basal keratinocytes) are involved in replacement of interfollicular epidermis [80]. Sebaceous glands are maintained by cells at the base of each gland [81], but their niche is still largely unknown.
- While our knowledge of brown, white and beige adipose tissue is rapidly increasing, little is still known about marrow adipose tissue and its progenitors, despite recent studies demonstrating possible roles for marrow adipose tissue in regulating the hematopoietic space [82]. Inconclusive results have been published about the in situ location or “niche” of adipocyte progenitors (APs). Regardless of the high vascularity of white adipose tissue (WAT), it has also been reported that only a fraction of cells with markers of APs are found in close proximity to blood vessels [83]. Therefore, the ontogeny of WAT and the AP niche are still a matter of some debate.
- The vasculature needs to have capacity for cell turnover, growth, and repair to maintain normal homeostasis. It has emerged during the past decade that there exists an array of ancestral progenitor cells resident within the mural layers of macro- and micro-vessels [84,85]. These consist of lineage-committed endothelial progenitor cells (EPCs) [86] and smooth muscle progenitor cells (SPCs) [87], multipotent vascular stem cells (MVSCs) [88], mesenchymal stem/stromal cells (MSCs) [89], adventitial macrophage progenitor cells (AMPCs), and circulation-derived hematopoietic stem cells (HSCs) [90]. The inner adventitia, adjacent to the external elastic lamina, has emerged as the prime candidate for the vascular stem cell niche.
- In the heart, the myocardium lacks the basal-apical orientation typical of epithelial organs, making it difficult to delineate the precise localization of cardiac stem cell (CSC) niches. The epicardial lining has been employed to define anatomically several classes of niches in the adult heart [91,92,93,94,95,96]. However, cardiac niches are not limited to the subepicardium and are dispersed throughout the myocardium. CSC niches are more numerous in the atria and apex, which represent protected anatomical areas characterized by low hemodynamic stress [97,98]. Recently, these CSC have been put into controversy: a study provided in vivo genetic evidence for nonmyocyte to myocyte conversion in embryonic but not adult hearts, arguing again the myogenic potential of putative stem cell populations for cardiac regeneration in the adult stage [99].
- Regarding the central nervous system, several researchers have identified the lateral subventricular zone (SVZ) and in the subgranular zone (SGZ) of the dentate gyrus within the hippocampus [100,101,102]. Astrocytes in SVZ and SGZ are able to give rise to neuroblasts and subsequently mature neurons. However, the presence of a stem cell niche in the adult human brain is under debate [103,104]. Considering the hypoxic nature of human brain, it is conceivable that neural stem cells (NSCs) in the brain would be located in a relatively hypoxic environment. When it comes to embryonic development and early stages of life, there is evidence that cell fate decision in neural stem cells (NSCs), which can generate both neurons and glia, is affected by oxygen tension [105].
- The liver has a high regenerative capacity that involves stem/progenitor cells when the proliferation of hepatocytes is impaired. Liver stem/progenitor cells, termed hepatic progenitor cells (HPCs) [106], emerge when hepatocyte proliferation is overwhelmed by persistent or severe liver injury. There is evidence that hepatic progenitor cells can originate from niches in the canals of Hering; in addition, the space of Disse may also serve as a stem cell niche during foetal haematopoiesis and constitute a niche for stellate cells in adults [107].
- The existence, phenotype, and anatomical location of stem/progenitors in the adult pancreas are actively debated [108]. Although some reports claim the existence of multipotent stem cells within the pancreas [109], most suggest that these cells are rare in the postnatal pancreas [110]. Ongoing studies suggest that postnatal pancreatic stem cells (PSCs) reside within the biliary tree, primarily the hepato-pancreatic common duct, and are rare in the pancreas proper [111].
- In adult kidneys, it has been proved that, after an injury, tubules can recover completely, but this is not the case for nephrons, which are not able to regenerate. Several cellular types with stem cell properties have been isolated from human adult kidneys [112,113]. These cells have been identified as a subset of parietal epithelial cells (PEC) in the Bowman’s capsule, which exhibits coexpression of the stem cell markers CD24 and CD133. However, their ability to differentiate and form new tissue in vivo is less studied and still controversial.
- Turnover of the epithelial cell lineages within the gastrointestinal tract is a constant process under normal homeostasis and increases after damage. This process is regulated by multipotent stem cells, which give rise to all gastrointestinal epithelial cell lineages and can regenerate whole intestinal crypts and gastric glands. The stem cells of the gastrointestinal tract are yet undefined, although it is generally agreed that they are located within a ‘niche’ in the intestinal crypts and gastric glands [114]:
- ○
- Two niches seem to co-exist in the gastric unit: one in the isthmus region and the other at the base of the gland, although the precise features of the cell populations and the two niches are currently under debate [115]. The current evidence suggests that gastric stem cells in every gastric gland give rise to four functionally distinct cell lineages: parietal, surface mucous (pit), zymogenic, and enteroendocrine.
- ○
- Nearly 90% of the intestinal epithelium is replaced every 3–4 days by cells newly generated from the crypt epithelium; however, long-lived intestinal stem cells (ISCs) are harboured in the crypt bottom interdigitated between Paneth cells, where cells are physically shielded from the content of the lumen [116]. To replenish the large amount of disposable functional epithelium, ISCs produce rapidly cycling progenitor cells, referred to as transit-amplifying (TA) cells. As they proliferate, TA cells migrate up the crypt-villus axis and differentiate into mature epithelial cells that are eventually shed off into the lumen [117].
- Human endometrium is the mucosal lining of the uterus and is a highly regenerative tissue, undergoing more than 400 cycles of proliferation, differentiation, and shedding during a woman’s reproductive life. During the last 10 years, an MSC subpopulation has been identified and characterized in human endometrium and in menstrual blood. Endometrial mesenchymal stem/stromal cells (eMSCs) are easily isolated from endometrial biopsy tissue [118].
- In bone marrow, hematopoietic stem cells (HSCs) reside along the endosteal surface close to osteoblastic cells [121,122] and in proximity to the blood vessels [123,124]. According to Keeley and Mann, both MSCs and HSCs originate from the bone marrow, but their sites of action extend throughout the organism. Indeed, it has been postulated that changes in partial oxygen pressure as cells exit the marrow into the systemic bloodstream serve as a key trigger for terminal differentiation into one cell type or another. For example, osteogenic and adipogenic differentiation of MSCs is hampered under low oxygen pressure, whereas chondrogenesis may be enhanced [125].
- Oral tissues, including tooth, periodontal ligament, and gingiva are also an important source of MSCs. Oral MSCs involve dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSCs), dental follicle stem cells (DFCs), stem cells from apical papilla (SCAP) and gingival stem cells (GMSCs) [126].
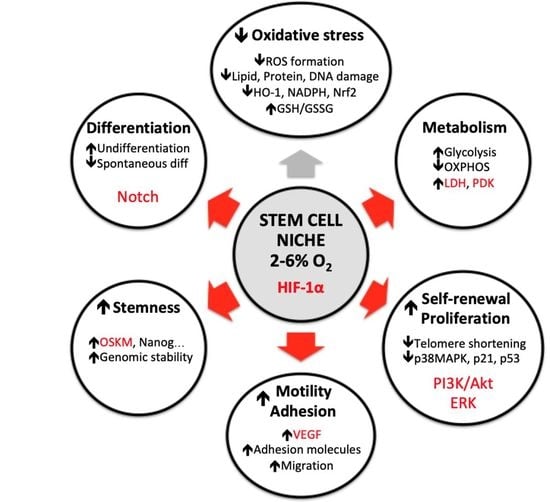
3. Oxygen Alterations In Vitro Affects Many Stem Cell Parameters
3.1. Reactive Oxygen Species (ROS) Formation and Antioxidant Defense
3.2. Metabolism
3.3. Self-Renewal and Proliferation Rate
3.4. Motility and Adhesion
3.5. Differentiation Fate
3.6. Stemness Maintenance
3.7. Reprogramming Efficiency
4. Stem Cells Defense Pathways Activated by Oxygen
4.1. Autophagy
4.2. Apoptosis
4.3. Senescence
5. Perspectives Regarding Stem Cell Culture Oxygen Condition for Stem Cell Therapy
Funding
Conflicts of Interest
References
- Carrel, A. On the Permanent Life of Tissues Outside of the Organism. J. Exp. Med. 1912, 15, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Shooter, R.A.; Gey, G.O. Studies of the mineral requirements of mammalian cells. Br. J. Exp. Pathol. 1952, 33, 98–103. [Google Scholar] [PubMed]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, M.C.; Pouyssegur, J. Oxygen, a source of life and stress. FEBS Lett. 2007, 581, 3582–3591. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef]
- Ward, J.P. Oxygen sensors in context. Biochim. Biophys. Acta 2008, 1777, 1–14. [Google Scholar] [CrossRef]
- Deninger, A.J.; Eberle, B.; Ebert, M.; Grossmann, T.; Heil, W.; Kauczor, H.; Lauer, L.; Markstaller, K.; Otten, E.; Schmiedeskamp, J.; et al. Quantification of regional intrapulmonary oxygen partial pressure evolution during apnea by (3)He MRI. J. Magn. Reson. 1999, 141, 207–216. [Google Scholar] [CrossRef]
- Wild, J.M.; Fichele, S.; Woodhouse, N.; Paley, M.N.; Kasuboski, L.; van Beek, E.J. 3D volume-localized pO2 measurement in the human lung with 3He MRI. Magn. Reson. Med. 2005, 53, 1055–1064. [Google Scholar] [CrossRef]
- Miller, G.W.; Mugler, J.P., 3rd; Altes, T.A.; Cai, J.; Mata, J.F.; de Lange, E.E.; Tobias, W.A.; Cates, G.D.; Brookeman, J.R. A short-breath-hold technique for lung pO2 mapping with 3He MRI. Magn. Reson. Med. 2010, 63, 127–136. [Google Scholar] [CrossRef]
- Hamedani, H.; Kadlecek, S.J.; Ishii, M.; Emami, K.; Kuzma, N.N.; Xin, Y.; Rossman, M.; Rizi, R.R. A variability study of regional alveolar oxygen tension measurement in humans using hyperpolarized (3) He MRI. Magn. Reson. Med. 2013, 70, 1557–1566. [Google Scholar] [CrossRef]
- Hamedani, H.; Shaghaghi, H.; Kadlecek, S.J.; Xin, Y.; Han, B.; Siddiqui, S.; Rajaei, J.; Ishii, M.; Rossman, M.; Rizi, R.R. Vertical gradients in regional alveolar oxygen tension in supine human lung imaged by hyperpolarized 3He MRI. NMR Biomed. 2014, 27, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Morosin, M.; Vignati, C.; Novi, A.; Salvioni, E.; Veglia, F.; Alimento, M.; Merli, G.; Sciomer, S.; Sinagra, G.; Agostoni, P. The alveolar to arterial oxygen partial pressure difference is associated with pulmonary diffusing capacity in heart failure patients. Respir. Physiol. Neurobiol. 2016, 233, 1–6. [Google Scholar] [CrossRef] [PubMed]
- White, R.A.; Nolan, L.; Harley, D.; Long, J.; Klein, S.; Tremper, K.; Nelson, R.; Tabrisky, J.; Shoemaker, W. Noninvasive evaluation of peripheral vascular disease using transcutaneous oxygen tension. Am. J. Surg. 1982, 144, 68–75. [Google Scholar] [CrossRef]
- Jaszczak, P. Skin oxygen tension, skin oxygen consumption, and skin blood flow measured by a tc-pO2 electrode. Acta Physiol. Scand. Suppl. 1991, 603, 53–57. [Google Scholar] [PubMed]
- Falstie-Jensen, N.; Spaun, E.; Brochner-Mortensen, J.; Falstie-Jensen, S. The influence of epidermal thickness on transcutaneous oxygen pressure measurements in normal persons. Scand. J. Clin. Lab. Investig. 1988, 48, 519–523. [Google Scholar] [CrossRef]
- Evans, N.T.; Naylor, P.F. The dynamics of changes in dermal oxygen tension. Respir. Physiol. 1966, 2, 61–72. [Google Scholar] [CrossRef]
- Spence, V.A.; Walker, W.F. Measurement of oxygen tension in human skin. Med. Biol. Eng. 1976, 14, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kabon, B.; Nagele, A.; Reddy, D.; Eagon, C.; Fleshman, J.W.; Sessler, D.I.; Kurz, A. Obesity decreases perioperative tissue oxygenation. Anesthesiology 2004, 100, 274–280. [Google Scholar] [CrossRef]
- Goossens, G.H.; Bizzarri, A.; Venteclef, N.; Essers, Y.; Cleutjens, J.P.; Konings, E.; Jocken, J.W.; Cajlakovic, M.; Ribitsch, V.; Clement, K.; et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 2011, 124, 67–76. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Bizzarri, A.; Koehler, H.; Cajlakovic, M.; Pasic, A.; Schaupp, L.; Klimant, I.; Ribitsch, V. Continuous oxygen monitoring in subcutaneous adipose tissue using microdialysis. Anal. Chim. Acta 2006, 573–574, 48–56. [Google Scholar] [CrossRef]
- Vorp, D.A.; Wang, D.H.; Webster, M.W.; Federspiel, W.J. Effect of intraluminal thrombus thickness and bulge diameter on the oxygen diffusion in abdominal aortic aneurysm. J. Biomech. Eng. 1998, 120, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Vorp, D.A.; Lee, P.C.; Wang, D.H.; Makaroun, M.S.; Nemoto, E.M.; Ogawa, S.; Webster, M.W. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 2001, 34, 291–299. [Google Scholar] [CrossRef]
- Pittman, R.N. Oxygen gradients in the microcirculation. Acta Physiol. 2011, 202, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.G.; Johnson, P.C.; Intaglietta, M. Oxygen gradients in the microcirculation. Physiol. Rev. 2003, 83, 933–963. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, D.J.; Toth, A.; Tsai, A.G.; Intaglietta, M.; Johnson, P.C. Oxygen tension distribution in postcapillary venules in resting skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1980–H1985. [Google Scholar] [CrossRef] [PubMed]
- Angell, C.S.; Lakatta, E.G.; Weisfeldt, M.L.; Shock, N.W. Relationship of intramyocardial oxygen tension and epicardial ST segment changes following acute coronary artery ligation: Effects of coronary perfusion pressure. Cardiovasc. Res. 1975, 9, 12–18. [Google Scholar] [CrossRef]
- Bjerrum, J.T.; Perko, M.J.; Beck, B. Myocardial oxygen tension during surgical revascularization. A clinical comparison between blood cardioplegia and crystalloid cardioplegia. Eur. J. Cardio-Thorac. Surg. 2006, 29, 181–185. [Google Scholar] [CrossRef]
- Feola, M.; Azar, D.; Wiener, L. Improved oxygenation of ischemic myocardium by hemodilution with stroma-free hemoglobin solution. Chest 1979, 75, 369–375. [Google Scholar] [CrossRef]
- Rivera, B.K.; Naidu, S.K.; Subramanian, K.; Joseph, M.; Hou, H.; Khan, N.; Swartz, H.M.; Kuppusamy, P. Real-time, in vivo determination of dynamic changes in lung and heart tissue oxygenation using EPR oximetry. Adv. Exp. Med. Biol. 2014, 812, 81–86. [Google Scholar] [CrossRef]
- Wiener, L.; Santamore, W.P.; Venkataswamy, A.; Plzak, L.; Templeton, J. Postoperative monitoring of myocardial oxygen tension: Experience in 51 coronary artery bypass patients. Clin. Cardiol. 1982, 5, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Winbury, M.M.; Howe, B.B.; Weiss, J.R. Effect of nitroglycerin and dipyridamole on epicardial and endocardial oxygen tension--further evidence for redistribution of myocardial blood flow. J. Pharmacol. Exp. Ther. 1971, 176, 184–199. [Google Scholar] [PubMed]
- Roy, S.; Khanna, S.; Wallace, W.A.; Lappalainen, J.; Rink, C.; Cardounel, A.J.; Zweier, J.L.; Sen, C.K. Characterization of perceived hyperoxia in isolated primary cardiac fibroblasts and in the reoxygenated heart. J. Biol. Chem. 2003, 278, 47129–47135. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., 3rd; Smith, W.S.; Sonne, D.C.; Morabito, D.; Manley, G.T. Relationship between brain tissue oxygen tension and CT perfusion: Feasibility and initial results. Am. J. Neuroradiol. 2005, 26, 1095–1100. [Google Scholar] [PubMed]
- Dings, J.; Meixensberger, J.; Jager, A.; Roosen, K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 1998, 43, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.E.; Charbel, F.T.; Edelman, G.; Ausman, J.I. Brain tissue oxygenation in patients with cerebral occlusive disease and arteriovenous malformations. Br. J. Anaesth. 1997, 78, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.E.; Charbel, F.T.; Edelman, G. Brain tissue oxygen, carbon dioxide, and pH in neurosurgical patients at risk for ischemia. Anesth. Analg. 1996, 82, 582–586. [Google Scholar]
- Sakadzic, S.; Roussakis, E.; Yaseen, M.A.; Mandeville, E.T.; Srinivasan, V.J.; Arai, K.; Ruvinskaya, S.; Wu, W.; Devor, A.; Lo, E.H.; et al. Cerebral blood oxygenation measurement based on oxygen-dependent quenching of phosphorescence. J. Vis. Exp. JoVE 2011. [Google Scholar] [CrossRef]
- Sakadzic, S.; Roussakis, E.; Yaseen, M.A.; Mandeville, E.T.; Srinivasan, V.J.; Arai, K.; Ruvinskaya, S.; Devor, A.; Lo, E.H.; Vinogradov, S.A.; et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat. Methods 2010, 7, 755–759. [Google Scholar] [CrossRef]
- Seylaz, J.; Pinard, E. Continuous measurement of gas partial pressures in intracerebral tissue. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 528–533. [Google Scholar] [CrossRef]
- Sezai, S.; Sakurabayashi, S.; Yamamoto, Y.; Morita, T.; Hirano, M.; Oka, H. Hepatic arterial and portal venous oxygen content and extraction in liver cirrhosis. Liver 1993, 13, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tygstrup, N.; Winkler, K.; Mellemgaard, K.; Andreassen, M. Determination of the hepatic arterial blood flow and oxygen supply in man by clamping the hepatic artery during surgery. J. Clin. Investig. 1962, 41, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.J.; Eastwood, J.; Beckingham, I.J.; Girling, K.J. Liver tissue partial pressure of oxygen and carbon dioxide during partial hepatectomy. Br. J. Anaesth. 2004, 92, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Leary, T.S.; Klinck, J.R.; Hayman, G.; Friend, P.; Jamieson, N.V.; Gupta, A.K. Measurement of liver tissue oxygenation after orthotopic liver transplantation using a multiparameter sensor. A pilot study. Anaesthesia 2002, 57, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Wolfle, D.; Jungermann, K. Long-term effects of physiological oxygen concentrations on glycolysis and gluconeogenesis in hepatocyte cultures. Eur. J. Biochem. 1985, 151, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Jungermann, K.; Kietzmann, T. Role of oxygen in the zonation of carbohydrate metabolism and gene expression in liver. Kidney Int. 1997, 51, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Samuelson, P.; Angell, C.; Minken, S.L. Polarographic evaluation of transmural oxygen availabitlity in intact muscular arteries. J. Atheroscler. Res. 1968, 8, 803–810. [Google Scholar] [CrossRef]
- Zhang, J.L.; Morrell, G.; Rusinek, H.; Warner, L.; Vivier, P.H.; Cheung, A.K.; Lerman, L.O.; Lee, V.S. Measurement of renal tissue oxygenation with blood oxygen level-dependent MRI and oxygen transit modeling. Am. J. Physiol. Ren. Physiol. 2014, 306, F579–F587. [Google Scholar] [CrossRef]
- Welch, W.J.; Baumgartl, H.; Lubbers, D.; Wilcox, C.S. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 2001, 59, 230–237. [Google Scholar] [CrossRef]
- Carlsson, P.O.; Palm, F.; Andersson, A.; Liss, P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 2001, 50, 489–495. [Google Scholar] [CrossRef]
- Carlsson, P.O.; Liss, P.; Andersson, A.; Jansson, L. Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes 1998, 47, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.J.; Sherry, K.M.; Thorpe, J.A. Changes in gastric tissue oxygenation during mobilisation for oesophageal replacement. Eur. J. Cardio-Thorac. 1995, 9, 158–160; discussion 160. [Google Scholar] [CrossRef]
- Sheridan, W.G.; Lowndes, R.H.; Young, H.L. Intraoperative tissue oximetry in the human gastrointestinal tract. Am. J. Surg. 1990, 159, 314–319. [Google Scholar] [CrossRef]
- Korsback, C.; Hockerstedt, K. Small bowel and liver pO2 during vasopressin infusion into the superior mesenteric artery. Ann. Chir. Gynaecol. 1984, 73, 50–53. [Google Scholar]
- Lind Due, V.; Bonde, J.; Kann, T.; Perner, A. Extremely low oxygen tension in the rectal lumen of human subjects. Acta Anaesthesiol. Scand. 2003, 47, 372. [Google Scholar] [CrossRef] [PubMed]
- Ottosen, L.D.; Hindkaer, J.; Husth, M.; Petersen, D.E.; Kirk, J.; Ingerslev, H.J. Observations on intrauterine oxygen tension measured by fibre-optic microsensors. Reprod. Biomed. Online 2006, 13, 380–385. [Google Scholar] [CrossRef]
- Hirai, D.M.; Colburn, T.D.; Craig, J.C.; Hotta, K.; Kano, Y.; Musch, T.I.; Poole, D.C. Skeletal muscle interstitial O2 pressures: Bridging the gap between the capillary and myocyte. Microcirculation 2018, e12497. [Google Scholar] [CrossRef] [PubMed]
- Mole, P.A.; Chung, Y.; Tran, T.K.; Sailasuta, N.; Hurd, R.; Jue, T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am. J. Physiol. 1999, 277, R173–R180. [Google Scholar] [CrossRef]
- Richardson, R.S.; Duteil, S.; Wary, C.; Wray, D.W.; Hoff, J.; Carlier, P.G. Human skeletal muscle intracellular oxygenation: The impact of ambient oxygen availability. J. Physiol. 2006, 571, 415–424. [Google Scholar] [CrossRef]
- Grant, J.L.; Smith, B. Bone marrow gas tensions, bone marrow blood flow, and erythropoiesis in man. Ann. Intern. Med. 1963, 58, 801–809. [Google Scholar] [CrossRef]
- Fiegl, M.; Samudio, I.; Clise-Dwyer, K.; Burks, J.K.; Mnjoyan, Z.; Andreeff, M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood 2009, 113, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Ito, T. Kinetics of hemopoietic stem cells in a hypoxic culture. Eur. J. Haematol. 1988, 40, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.C.; Wenning, L.A.; Miller, W.M.; Papoutsakis, E.T. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh’s model. Biophys. J. 2001, 81, 675–684. [Google Scholar] [CrossRef]
- Chow, D.C.; Wenning, L.A.; Miller, W.M.; Papoutsakis, E.T. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys. J. 2001, 81, 685–696. [Google Scholar] [CrossRef]
- Harrison, J.S.; Rameshwar, P.; Chang, V.; Bandari, P. Oxygen saturation in the bone marrow of healthy volunteers. Blood 2002, 99, 394. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Reuther, M.S.; Briggs, K.K.; Schumacher, B.L.; Masuda, K.; Sah, R.L.; Watson, D. In vivo oxygen tension in human septal cartilage increases with age. Laryngoscope 2012, 122, 2407–2410. [Google Scholar] [CrossRef]
- Buerk, D.G.; Shonat, R.D.; Riva, C.E.; Cranstoun, S.D. O2 gradients and countercurrent exchange in the cat vitreous humor near retinal arterioles and venules. Microvasc. Res. 1993, 45, 134–148. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Scadden, D.T. The stem-cell niche as an entity of action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Gao, X.; Xue, Y.; Randell, S.H.; Kong, Y.Y.; Hogan, B.L. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011, 8, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Tata, P.R.; Mou, H.; Pardo-Saganta, A.; Zhao, R.; Prabhu, M.; Law, B.M.; Vinarsky, V.; Cho, J.L.; Breton, S.; Sahay, A.; et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 2013, 503, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.A.; Hu, Y.; Yamamoto, Y.; Hoe, N.B.; Wei, T.S.; Mu, D.; Sun, Y.; Joo, L.S.; Dagher, R.; Zielonka, E.M.; et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 2011, 147, 525–538. [Google Scholar] [CrossRef]
- Zuo, W.; Zhang, T.; Wu, D.Z.; Guan, S.P.; Liew, A.A.; Yamamoto, Y.; Wang, X.; Lim, S.J.; Vincent, M.; Lessard, M.; et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature 2015, 517, 616–620. [Google Scholar] [CrossRef]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Levy, V.; Lindon, C.; Harfe, B.D.; Morgan, B.A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 2005, 9, 855–861. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Rodeheffer, M.S.; Rosen, C.J.; Horowitz, M.C. Adipose Tissue Residing Progenitors (Adipocyte Lineage Progenitors and Adipose Derived Stem Cells (ADSC). Curr. Mol. Biol. Rep. 2015, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Rodeheffer, M.S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 2013, 15, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Psaltis, P.J.; Simari, R.D. Vascular wall progenitor cells in health and disease. Circ. Res. 2015, 116, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.E., 3rd; Kelley, R.W.; Patterson, C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Moore, D.B.; Woodard, W.; Fenoglio, A.; Yoder, M.C. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 2005, 105, 2783–2786. [Google Scholar] [CrossRef] [PubMed]
- Passman, J.N.; Dong, X.R.; Wu, S.P.; Maguire, C.T.; Hogan, K.A.; Bautch, V.L.; Majesky, M.W. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9349–9354. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, A.; Yuan, F.; Yan, Z.; Liu, B.; Chu, J.S.; Helms, J.A.; Li, S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat. Commun. 2012, 3, 875. [Google Scholar] [CrossRef]
- Klein, D.; Weisshardt, P.; Kleff, V.; Jastrow, H.; Jakob, H.G.; Ergun, S. Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS ONE 2011, 6, e20540. [Google Scholar] [CrossRef]
- Psaltis, P.J.; Puranik, A.S.; Spoon, D.B.; Chue, C.D.; Hoffman, S.J.; Witt, T.A.; Delacroix, S.; Kleppe, L.S.; Mueske, C.S.; Pan, S.; et al. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ. Res. 2014, 115, 364–375. [Google Scholar] [CrossRef]
- Di Meglio, F.; Castaldo, C.; Nurzynska, D.; Miraglia, R.; Romano, V.; Russolillo, V.; Giuseppina, L.; Vosa, C.; Montagnani, S. Localization and origin of cardiac CD117-positive cells: Identification of a population of epicardially-derived cells in adult human heart. Ital. J. Anat. Embryol. 2010, 115, 71–78. [Google Scholar] [PubMed]
- Castaldo, C.; Di Meglio, F.; Nurzynska, D.; Romano, G.; Maiello, C.; Bancone, C.; Muller, P.; Bohm, M.; Cotrufo, M.; Montagnani, S. CD117-positive cells in adult human heart are localized in the subepicardium, and their activation is associated with laminin-1 and alpha6 integrin expression. Stem Cells 2008, 26, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, F.; Mahmoud, A.I.; Sosic, D.; Porrello, E.R.; Chen, R.; Garcia, J.A.; DeBerardinis, R.J.; Sadek, H.A. The hypoxic epicardial and subepicardial microenvironment. J. Cardiovasc. Transl. Res. 2012, 5, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Limana, F.; Zacheo, A.; Mocini, D.; Mangoni, A.; Borsellino, G.; Diamantini, A.; De Mori, R.; Battistini, L.; Vigna, E.; Santini, M.; et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ. Res. 2007, 101, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Bollini, S.; Dube, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef]
- Gonzalez, A.; Rota, M.; Nurzynska, D.; Misao, Y.; Tillmanns, J.; Ojaimi, C.; Padin-Iruegas, M.E.; Muller, P.; Esposito, G.; Bearzi, C.; et al. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ. Res. 2008, 102, 597–606. [Google Scholar] [CrossRef]
- Sanada, F.; Kim, J.; Czarna, A.; Chan, N.Y.; Signore, S.; Ogorek, B.; Isobe, K.; Wybieralska, E.; Borghetti, G.; Pesapane, A.; et al. c-Kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circ. Res. 2014, 114, 41–55. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Huang, X.; Bhaloo, S.I.; Zhao, H.; Zhang, S.; Pu, W.; Tian, X.; Li, Y.; Liu, Q.; et al. Genetic Lineage Tracing of Nonmyocyte Population by Dual Recombinases. Circulation 2018, 138, 793–805. [Google Scholar] [CrossRef]
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Palmer, T.D.; Takahashi, J.; Gage, F.H. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 1997, 8, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Goderie, S.K.; Jin, L.; Karanth, N.; Sun, Y.; Abramova, N.; Vincent, P.; Pumiglia, K.; Temple, S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004, 304, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, J.; Lin, Y.; Gaeta, X.; Meng, X.; Wisidagama, D.R.; Cinkornpumin, J.; Koehler, C.M.; Malone, C.S.; Teitell, M.A.; et al. Defining the role of oxygen tension in human neural progenitor fate. Stem Cell Rep. 2014, 3, 743–757. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.; Desmet, V. Ultrastructural characteristics of novel epithelial cell types identified in human pathologic liver specimens with chronic ductular reaction. Am. J. Pathol. 1992, 140, 1441–1450. [Google Scholar] [PubMed]
- Kordes, C.; Haussinger, D. Hepatic stem cell niches. J. Clin. Investig. 2013, 123, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.X.; Morahan, G. Pancreatic stem cells remain unresolved. Stem Cells Dev. 2014, 23, 2803–2812. [Google Scholar] [CrossRef]
- Smukler, S.R.; Arntfield, M.E.; Razavi, R.; Bikopoulos, G.; Karpowicz, P.; Seaberg, R.; Dai, F.; Lee, S.; Ahrens, R.; Fraser, P.E.; et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 2011, 8, 281–293. [Google Scholar] [CrossRef]
- Lysy, P.A.; Weir, G.C.; Bonner-Weir, S. Making beta cells from adult cells within the pancreas. Curr. Diabetes Rep. 2013, 13, 695–703. [Google Scholar] [CrossRef]
- Lanzoni, G.; Cardinale, V.; Carpino, G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatology 2016, 64, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sagrinati, C.; Netti, G.S.; Mazzinghi, B.; Lazzeri, E.; Liotta, F.; Frosali, F.; Ronconi, E.; Meini, C.; Gacci, M.; Squecco, R.; et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J. Am. Soc. Nephrol. 2006, 17, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, E.; Sagrinati, C.; Angelotti, M.L.; Lazzeri, E.; Mazzinghi, B.; Ballerini, L.; Parente, E.; Becherucci, F.; Gacci, M.; Carini, M.; et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 2009, 20, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Brittan, M.; Wright, N.A. Gastrointestinal stem cells. J. Pathol. 2002, 197, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Bartfeld, S.; Koo, B.K. Adult gastric stem cells and their niches. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Date, S.; Sato, T. Mini-gut organoids: Reconstitution of the stem cell niche. Annu. Rev. Cell Dev. Biol. 2015, 31, 269–289. [Google Scholar] [CrossRef]
- Darzi, S.; Werkmeister, J.A.; Deane, J.A.; Gargett, C.E. Identification and Characterization of Human Endometrial Mesenchymal Stem/Stromal Cells and Their Potential for Cellular Therapy. Stem Cells Transl. Med. 2016, 5, 1127–1132. [Google Scholar] [CrossRef]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niu, C.; Ye, L.; Huang, H.; He, X.; Tong, W.G.; Ross, J.; Haug, J.; Johnson, T.; Feng, J.Q.; et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.J.; Yilmaz, O.H.; Iwashita, T.; Yilmaz, O.H.; Terhorst, C.; Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Sipkins, D.A.; Wei, X.; Wu, J.W.; Runnels, J.M.; Cote, D.; Means, T.K.; Luster, A.D.; Scadden, D.T.; Lin, C.P. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005, 435, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Fehrer, C.; Brunauer, R.; Laschober, G.; Unterluggauer, H.; Reitinger, S.; Kloss, F.; Gully, C.; Gassner, R.; Lepperdinger, G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 2007, 6, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Gorski, B. Gingiva as a new and the most accessible source of mesenchymal stem cells from the oral cavity to be used in regenerative therapies. Postepy Hig. I Med. Dosw. (Online) 2016, 70, 858–871. [Google Scholar] [CrossRef]
- Rodesch, F.; Simon, P.; Donner, C.; Jauniaux, E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 1992, 80, 283–285. [Google Scholar]
- Harvey, A.J.; Rathjen, J.; Yu, L.J.; Gardner, D.K. Oxygen modulates human embryonic stem cell metabolism in the absence of changes in self-renewal. Reprod. Fertil. Dev. 2016, 28, 446–458. [Google Scholar] [CrossRef]
- Catt, J.W.; Henman, M. Toxic effects of oxygen on human embryo development. Hum. Reprod. 2000, 15 (Suppl. 2), 199–206. [Google Scholar] [CrossRef]
- Nanassy, L.; Peterson, C.A.; Wilcox, A.L.; Peterson, C.M.; Hammoud, A.; Carrell, D.T. Comparison of 5% and ambient oxygen during days 3–5 of in vitro culture of human embryos. Fertil. Steril. 2010, 93, 579–585. [Google Scholar] [CrossRef]
- Christensen, D.R.; Calder, P.C.; Houghton, F.D. Effect of oxygen tension on the amino acid utilisation of human embryonic stem cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Cipolleschi, M.G.; Dello Sbarba, P.; Olivotto, M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 1993, 82, 2031–2037. [Google Scholar] [PubMed]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Klass, M.; Harris, C.; Csete, M. A reducing redox environment promotes C2C12 myogenesis: Implications for regeneration in aged muscle. Cell Biol. Int. 2007, 31, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cai, H.; Yang, S.; Yan, L.; Tan, W. Comparison between the effects of normoxia and hypoxia on antioxidant enzymes and glutathione redox state in ex vivo culture of CD34(+) cells. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Bruge, F.; Cirilli, I.; Marcheggiani, F.; Olivieri, F.; Armeni, T.; Cianfruglia, L.; Giuliani, A.; Orlando, P.; Tiano, L. Modulation of Oxidative Status by Normoxia and Hypoxia on Cultures of Human Dermal Fibroblasts: How Does It Affect Cell Aging? Oxid. Med. Cell. Longev. 2018, 2018, 5469159. [Google Scholar] [CrossRef] [PubMed]
- El Alami, M.; Vina-Almunia, J.; Gambini, J.; Mas-Bargues, C.; Siow, R.C.; Penarrocha, M.; Mann, G.E.; Borras, C.; Vina, J. Activation of p38, p21, and NRF-2 mediates decreased proliferation of human dental pulp stem cells cultured under 21% O2. Stem Cell Rep. 2014, 3, 566–573. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Vina-Almunia, J.; Ingles, M.; Sanz-Ros, J.; Gambini, J.; Ibanez-Cabellos, J.S.; Garcia-Gimenez, J.L.; Vina, J.; Borras, C. Role of p16INK4a and BMI-1 in oxidative stress-induced premature senescence in human dental pulp stem cells. Redox Biol. 2017, 12, 690–698. [Google Scholar] [CrossRef]
- Bell, E.L.; Chandel, N.S. Genetics of mitochondrial electron transport chain in regulating oxygen sensing. Methods Enzymol. 2007, 435, 447–461. [Google Scholar] [CrossRef]
- Ito, K.; Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzon-Muvdi, T.; Quinones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Shyh-Chang, N.; Daley, G.Q.; Cantley, L.C. Stem cell metabolism in tissue development and aging. Development 2013, 140, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takubo, K.; Semenza, G.L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011, 9, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Takubo, K.; Nagamatsu, G.; Kobayashi, C.I.; Nakamura-Ishizu, A.; Kobayashi, H.; Ikeda, E.; Goda, N.; Rahimi, Y.; Johnson, R.S.; Soga, T.; et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 2013, 12, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Warr, M.R.; Passegue, E. Metabolic makeover for HSCs. Cell Stem Cell 2013, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.M.; Liu, X.; Shen, J.; Jovanovic, O.; Pohl, E.E.; Gerson, S.L.; Finkel, T.; Broxmeyer, H.E.; Qu, C.K. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 2013, 12, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Simsek, T.; Kocabas, F.; Zheng, J.; Deberardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef]
- Inoue, S.; Noda, S.; Kashima, K.; Nakada, K.; Hayashi, J.; Miyoshi, H. Mitochondrial respiration defects modulate differentiation but not proliferation of hematopoietic stem and progenitor cells. FEBS Lett. 2010, 584, 3402–3409. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Takubo, K.; Goda, N.; Yamada, W.; Iriuchishima, H.; Ikeda, E.; Kubota, Y.; Shima, H.; Johnson, R.S.; Hirao, A.; Suematsu, M.; et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 2010, 7, 391–402. [Google Scholar] [CrossRef]
- Estrada, J.C.; Albo, C.; Benguria, A.; Dopazo, A.; Lopez-Romero, P.; Carrera-Quintanar, L.; Roche, E.; Clemente, E.P.; Enriquez, J.A.; Bernad, A.; et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012, 19, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Ghourichaee, S.S.; Powell, E.M.; Leach, J.B. Enhancement of human neural stem cell self-renewal in 3D hypoxic culture. Biotechnol. Bioeng. 2017, 114, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.; Andrade, P.Z.; Boura, J.S.; Abecasis, M.M.; da Silva, C.L.; Cabral, J.M. Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol. 2010, 223, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.P.; Ho, J.H.; Shih, Y.R.; Lo, T.; Lee, O.K. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010, 8, 18. [Google Scholar] [CrossRef]
- Efimenko, A.; Starostina, E.; Kalinina, N.; Stolzing, A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 2011, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Song, S.Y.; Park, S.G.; Song, S.U.; Xia, Y.; Sung, J.H. Primary involvement of NADPH oxidase 4 in hypoxia-induced generation of reactive oxygen species in adipose-derived stem cells. Stem Cells Dev. 2012, 21, 2212–2221. [Google Scholar] [CrossRef]
- Csete, M.; Walikonis, J.; Slawny, N.; Wei, Y.; Korsnes, S.; Doyle, J.C.; Wold, B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J. Cell. Physiol. 2001, 189, 189–196. [Google Scholar] [CrossRef]
- Lees, S.J.; Childs, T.E.; Booth, F.W. p21(Cip1) expression is increased in ambient oxygen, compared to estimated physiological (5%) levels in rat muscle precursor cell culture. Cell Prolif. 2008, 41, 193–207. [Google Scholar] [CrossRef]
- von Zglinicki, T.; Saretzki, G.; Docke, W.; Lotze, C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: A model for senescence? Exp. Cell Res. 1995, 220, 186–193. [Google Scholar] [CrossRef]
- Parrinello, S.; Samper, E.; Krtolica, A.; Goldstein, J.; Melov, S.; Campisi, J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003, 5, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Csete, M. Oxygen in the cultivation of stem cells. Ann. N. Y. Acad. Sci. 2005, 1049, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Woo, R.A.; Poon, R.Y. Activated oncogenes promote and cooperate with chromosomal instability for neoplastic transformation. Genes Dev. 2004, 18, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.H.; Boura, J.S.; Abecasis, M.M.; Gimble, J.M.; da Silva, C.L.; Cabral, J.M. Impact of hypoxia and long-term cultivation on the genomic stability and mitochondrial performance of ex vivo expanded human stem/stromal cells. Stem Cell Res. 2012, 9, 225–236. [Google Scholar] [CrossRef] [PubMed]
- el-Deiry, W.S.; Tokino, T.; Waldman, T.; Oliner, J.D.; Velculescu, V.E.; Burrell, M.; Hill, D.E.; Healy, E.; Rees, J.L.; Hamilton, S.R.; et al. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995, 55, 2910–2919. [Google Scholar] [PubMed]
- Giono, L.E.; Manfredi, J.J. The p53 tumor suppressor participates in multiple cell cycle checkpoints. J. Cell. Physiol. 2006, 209, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Pistollato, F.; Hoeppner, D.J.; Ni, H.T.; McKay, R.D.; Panchision, D.M. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells 2007, 25, 2291–2301. [Google Scholar] [CrossRef]
- Pistollato, F.; Chen, H.L.; Schwartz, P.H.; Basso, G.; Panchision, D.M. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol. Cell. Neurosci. 2007, 35, 424–435. [Google Scholar] [CrossRef]
- O’Reilly, M.A. Redox activation of p21Cip1/WAF1/Sdi1: A multifunctional regulator of cell survival and death. Antioxid. Redox Signal. 2005, 7, 108–118. [Google Scholar] [CrossRef]
- Kim, G.Y.; Mercer, S.E.; Ewton, D.Z.; Yan, Z.; Jin, K.; Friedman, E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J. Biol. Chem. 2002, 277, 29792–29802. [Google Scholar] [CrossRef]
- Ciria, M.; Garcia, N.A.; Ontoria-Oviedo, I.; Gonzalez-King, H.; Carrero, R.; De La Pompa, J.L.; Montero, J.A.; Sepulveda, P. Mesenchymal Stem Cell Migration and Proliferation Are Mediated by Hypoxia-Inducible Factor-1alpha Upstream of Notch and SUMO Pathways. Stem Cells Dev. 2017, 26, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, S.M.; Sung, J.H. Cellular and molecular stimulation of adipose-derived stem cells under hypoxia. Cell Biol. Int. 2014, 38, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kwon, S.J.; Jin, H.J.; Jeong, S.Y.; Choi, S.J.; Oh, W.; Yang, Y.S.; Jeon, H.B.; Jeon, E.S. Optimization of culture conditions for rapid clinical-scale expansion of human umbilical cord blood-derived mesenchymal stem cells. Clin. Transl. Med. 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Mohd Ali, N.; Boo, L.; Yeap, S.K.; Ky, H.; Satharasinghe, D.A.; Liew, W.C.; Ong, H.K.; Cheong, S.K.; Kamarul, T. Probable impact of age and hypoxia on proliferation and microRNA expression profile of bone marrow-derived human mesenchymal stem cells. PeerJ 2016, 4, e1536. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.F.; Wang, H.; Xiao, F.J.; Yin, Y.; Xu, Q.Q.; Ge, R.L.; Wang, L.S. MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem. Biophys. Res. Commun. 2016, 470, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, L.; Zhan, Y.; Yu, S.; Kang, T. Adipose-derived stem cell-derived microvesicle-released miR-210 promoted proliferation, migration and invasion of endothelial cells by regulating RUNX3. Cell Cycle 2018, 17, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wen, Y.; Bi, P.; Lai, X.; Liu, X.S.; Liu, X.; Kuang, S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 2012, 139, 2857–2865. [Google Scholar] [CrossRef]
- De Donatis, A.; Ranaldi, F.; Cirri, P. Reciprocal control of cell proliferation and migration. Cell Commun. Signal. 2010, 8, 20. [Google Scholar] [CrossRef]
- Bellio, M.A.; Rodrigues, C.O.; Landin, A.M.; Hatzistergos, K.E.; Kuznetsov, J.; Florea, V.; Valasaki, K.; Khan, A.; Hare, J.M.; Schulman, I.H. Physiological and hypoxic oxygen concentration differentially regulates human c-Kit+ cardiac stem cell proliferation and migration. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1509–H1519. [Google Scholar] [CrossRef]
- Fuchs, E.; Weber, K. Intermediate filaments: Structure, dynamics, function, and disease. Annu. Rev. Biochem. 1994, 63, 345–382. [Google Scholar] [CrossRef]
- Cox, B.D.; Natarajan, M.; Stettner, M.R.; Gladson, C.L. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J. Cell. Biochem. 2006, 99, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, Y.J.; Song, C.H.; Ahn, Y.K.; Han, H.J. Role of FAK phosphorylation in hypoxia-induced hMSCS migration: Involvement of VEGF as well as MAPKS and eNOS pathways. Am. J. Physiol. Cell Physiol. 2010, 298, C847–C856. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Lee, M.Y.; Ryu, J.M.; Song, C.H.; Han, H.J. Role of HIF-1alpha and VEGF in human mesenchymal stem cell proliferation by 17beta-estradiol: Involvement of PKC, PI3K/Akt, and MAPKs. Am. J. Physiol. Cell Physiol. 2009, 296, C317–C326. [Google Scholar] [CrossRef] [PubMed]
- Ezashi, T.; Das, P.; Roberts, R.M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4783–4788. [Google Scholar] [CrossRef]
- Westfall, S.D.; Sachdev, S.; Das, P.; Hearne, L.B.; Hannink, M.; Roberts, R.M.; Ezashi, T. Identification of oxygen-sensitive transcriptional programs in human embryonic stem cells. Stem Cells Dev. 2008, 17, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Csete, M.; Groves, A.K.; Melega, W.; Wold, B.; Anderson, D.J. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 2000, 20, 7370–7376. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Diabira, S.; Howard, G.A.; Roos, B.A.; Schiller, P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 2006, 39, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Hansson, E.M.; Lendahl, U.; Chapman, G. Notch signaling in development and disease. Semin. Cancer Biol. 2004, 14, 320–328. [Google Scholar] [CrossRef]
- Dahlqvist, C.; Blokzijl, A.; Chapman, G.; Falk, A.; Dannaeus, K.; Ibanez, C.F.; Lendahl, U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development 2003, 130, 6089–6099. [Google Scholar] [CrossRef]
- de la Pompa, J.L.; Wakeham, A.; Correia, K.M.; Samper, E.; Brown, S.; Aguilera, R.J.; Nakano, T.; Honjo, T.; Mak, T.W.; Rossant, J.; et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 1997, 124, 1139–1148. [Google Scholar] [PubMed]
- Nofziger, D.; Miyamoto, A.; Lyons, K.M.; Weinmaster, G. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development 1999, 126, 1689–1702. [Google Scholar]
- Varnum-Finney, B.; Xu, L.; Brashem-Stein, C.; Nourigat, C.; Flowers, D.; Bakkour, S.; Pear, W.S.; Bernstein, I.D. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 2000, 6, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.V.; Zheng, X.; Pereira, T.; Gradin, K.; Jin, S.; Lundkvist, J.; Ruas, J.L.; Poellinger, L.; Lendahl, U.; Bondesson, M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 2005, 9, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Cheng, D.; Ma, Y.; Zhou, W.; Wang, Y. The relationship between oxygen concentration, reactive oxygen species and the biological characteristics of human bone marrow hematopoietic stem cells. Transplant. Proc. 2011, 43, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Wagegg, M.; Gaber, T.; Lohanatha, F.L.; Hahne, M.; Strehl, C.; Fangradt, M.; Tran, C.L.; Schonbeck, K.; Hoff, P.; Ode, A.; et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS ONE 2012, 7, e46483. [Google Scholar] [CrossRef] [PubMed]
- Volkmer, E.; Kallukalam, B.C.; Maertz, J.; Otto, S.; Drosse, I.; Polzer, H.; Bocker, W.; Stengele, M.; Docheva, D.; Mutschler, W.; et al. Hypoxic preconditioning of human mesenchymal stem cells overcomes hypoxia-induced inhibition of osteogenic differentiation. Tissue Eng. Part A 2010, 16, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Sun, J.; Dai, Y.; Cao, P.; Zhang, L.; Peng, S.; Zhou, Y.; Li, G.; Tang, J.; Xiang, J. HIF-1A and C/EBPs transcriptionally regulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia. Stem Cell Res. Ther. 2015, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Abildtrup, L.; Fogd, K.; Abdallah, B.M.; Kassem, M.; Ebbesen, P.; Zachar, V. Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells 2004, 22, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef]
- Markway, B.D.; Tan, G.K.; Brooke, G.; Hudson, J.E.; Cooper-White, J.J.; Doran, M.R. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010, 19, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Yang, M.H.; Tsai, C.C.; Huang, T.F.; Chen, Y.H.; Hung, S.C. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS ONE 2011, 6, e23965. [Google Scholar] [CrossRef] [PubMed]
- Nekanti, U.; Dastidar, S.; Venugopal, P.; Totey, S.; Ta, M. Increased proliferation and analysis of differential gene expression in human Wharton’s jelly-derived mesenchymal stromal cells under hypoxia. Int. J. Biol. Sci. 2010, 6, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Fotia, C.; Massa, A.; Boriani, F.; Baldini, N.; Granchi, D. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology 2015, 67, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Heinis, M.; Simon, M.T.; Ilc, K.; Mazure, N.M.; Pouyssegur, J.; Scharfmann, R.; Duvillie, B. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 2010, 59, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Vieira, H.L.; Alves, P.M.; Vercelli, A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog. Neurobiol. 2011, 93, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Covello, K.L.; Kehler, J.; Yu, H.; Gordan, J.D.; Arsham, A.M.; Hu, C.J.; Labosky, P.A.; Simon, M.C.; Keith, B. HIF-2alpha regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006, 20, 557–570. [Google Scholar] [CrossRef]
- Forristal, C.E.; Wright, K.L.; Hanley, N.A.; Oreffo, R.O.; Houghton, F.D. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 2010, 139, 85–97. [Google Scholar] [CrossRef]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Koshiji, M.; Kageyama, Y.; Pete, E.A.; Horikawa, I.; Barrett, J.C.; Huang, L.E. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004, 23, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, P.; Fukuda, R.; Kumar, G.; Krishnamachary, B.; Zeller, K.I.; Dang, C.V.; Semenza, G.L. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 2007, 11, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Bertout, J.A.; Hu, C.J.; Diehl, J.A.; Simon, M.C. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 2007, 11, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Kim, K.H.; Choi, H.K.; Shim, J.S.; Whang, S.Y.; Hahn, S.J.; Kwon, O.J.; Oh, I.H. Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp. Mol. Med. 2013, 45, e44. [Google Scholar] [CrossRef] [PubMed]
- Drela, K.; Sarnowska, A.; Siedlecka, P.; Szablowska-Gadomska, I.; Wielgos, M.; Jurga, M.; Lukomska, B.; Domanska-Janik, K. Low oxygen atmosphere facilitates proliferation and maintains undifferentiated state of umbilical cord mesenchymal stem cells in an hypoxia inducible factor-dependent manner. Cytotherapy 2014, 16, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Basciano, L.; Nemos, C.; Foliguet, B.; de Isla, N.; de Carvalho, M.; Tran, N.; Dalloul, A. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011, 12, 12. [Google Scholar] [CrossRef]
- Yoshida, Y.; Takahashi, K.; Okita, K.; Ichisaka, T.; Yamanaka, S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 2009, 5, 237–241. [Google Scholar] [CrossRef]
- Mathieu, J.; Zhou, W.; Xing, Y.; Sperber, H.; Ferreccio, A.; Agoston, Z.; Kuppusamy, K.T.; Moon, R.T.; Ruohola-Baker, H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 2014, 14, 592–605. [Google Scholar] [CrossRef]
- Mathieu, J.; Zhang, Z.; Nelson, A.; Lamba, D.A.; Reh, T.A.; Ware, C.; Ruohola-Baker, H. Hypoxia induces re-entry of committed cells into pluripotency. Stem Cells 2013, 31, 1737–1748. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Seluanov, A.; Gorbunova, V.; Falcovitz, A.; Sigal, A.; Milyavsky, M.; Zurer, I.; Shohat, G.; Goldfinger, N.; Rotter, V. Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53. Mol. Cell. Biol. 2001, 21, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kanaseki, T.; Mizushima, N.; Mizuta, T.; Arakawa-Kobayashi, S.; Thompson, C.B.; Tsujimoto, Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 2004, 6, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, J.M.; Galluzzi, L.; Tajeddine, N.; Ortiz, C.; Criollo, A.; Tasdemir, E.; Morselli, E.; Ben Younes, A.; Maiuri, M.C.; Lavandero, S.; et al. Senescence, apoptosis or autophagy? When a damaged cell must decide its path--a mini-review. Gerontology 2008, 54, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, F.V.; Cortini, M.; Avnet, S.; Perut, F.; Columbaro, M.; De Milito, A.; Baldini, N. The Role of Autophagy in the Maintenance of Stemness and Differentiation of Mesenchymal Stem Cells. Stem Cell Rev. 2016, 12, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jung, J.; Cho, K.J.; Lee, S.K.; Park, J.W.; Oh, I.H.; Kim, G.J. Increased SCF/c-kit by hypoxia promotes autophagy of human placental chorionic plate-derived mesenchymal stem cells via regulating the phosphorylation of mTOR. J. Cell. Biochem. 2013, 114, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sotthibundhu, A.; Promjuntuek, W.; Liu, M.; Shen, S.; Noisa, P. Roles of autophagy in controlling stem cell identity: A perspective of self-renewal and differentiation. Cell Tissue Res. 2018, 374, 205–216. [Google Scholar] [CrossRef]
- Liu, J.; Hao, H.; Huang, H.; Tong, C.; Ti, D.; Dong, L.; Chen, D.; Zhao, Y.; Liu, H.; Han, W.; et al. Hypoxia regulates the therapeutic potential of mesenchymal stem cells through enhanced autophagy. Int. J. Low. Extrem. Wounds 2015, 14, 63–72. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.; Wang, Y.; Wang, L.; Jin, Z.; Ding, L.; Zhang, L.; Zhang, L.; Jiang, W.; Gao, G.; et al. Autophagy regulates the apoptosis of bone marrow-derived mesenchymal stem cells under hypoxic condition via AMP-activated protein kinase/mammalian target of rapamycin pathway. Cell Biol. Int. 2016, 40, 671–685. [Google Scholar] [CrossRef]
- Gottlieb, R.A.; Carreira, R.S. Autophagy in health and disease. 5. Mitophagy as a way of life. Am. J. Physiol. Cell Physiol. 2010, 299, C203–C210. [Google Scholar] [CrossRef]
- Garcia-Prat, L.; Martinez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodriguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy maintains stemness by preventing senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Cai, N.; Li, M.; Liu, G.H.; Izpisua Belmonte, J.C. Autophagic control of cell ‘stemness’. EMBO Mol. Med. 2013, 5, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, I.; Johnstone, R.W.; Smyth, M.J. SnapShot: Extrinsic apoptosis pathways. Cell 2010, 143, 1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiong, L.; Tang, W.; Tang, L.; Wang, B. Hypoxic culture enhances the expansion of rat bone marrow-derived mesenchymal stem cells via the regulatory pathways of cell division and apoptosis. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Liu, J.; Tao, J.; Liu, J.; Yang, S. Influence of caspase-3 silencing on the proliferation and apoptosis of rat bone marrow mesenchymal stem cells under hypoxia. Int. J. Clin. Exp. Med. 2015, 8, 1624–1633. [Google Scholar] [PubMed]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chiang, C.H.; Hung, S.C.; Chian, C.F.; Tsai, C.L.; Chen, W.C.; Zhang, H. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS ONE 2017, 12, e0187637. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Weinberg, R.A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef]
- Yeh, C.K. Cellular senescence and aging. Oral Dis. 2016, 22, 587–590. [Google Scholar] [CrossRef]
- Martinez-Zamudio, R.I.; Robinson, L.; Roux, P.F.; Bischof, O. SnapShot: Cellular Senescence Pathways. Cell 2017, 170, 816–816.e1. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Rufini, A.; Tucci, P.; Celardo, I.; Melino, G. Senescence and aging: The critical roles of p53. Oncogene 2013, 32, 5129–5143. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Ko, Y.J.; Lee, M.W.; Park, H.J.; Park, Y.J.; Kim, D.I.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Effect of low oxygen tension on the biological characteristics of human bone marrow mesenchymal stem cells. Cell Stress Chaperones 2016, 21, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chen, Y.J.; Yew, T.L.; Chen, L.L.; Wang, J.Y.; Chiu, C.H.; Hung, S.C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 2011, 117, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Vono, R.; Jover Garcia, E.; Spinetti, G.; Madeddu, P. Oxidative Stress in Mesenchymal Stem Cell Senescence: Regulation by Coding and Noncoding RNAs. Antioxid. Redox Signal. 2018, 29, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tang, Q.; Zhang, Y.; Yu, M.; Jing, W.; Tian, W. Physioxia: A more effective approach for culturing human adipose-derived stem cells for cell transplantation. Stem Cell Res. Ther. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Ratushnyy, A.; Lobanova, M.; Buravkova, L.B. Expansion of adipose tissue-derived stromal cells at “physiologic” hypoxia attenuates replicative senescence. Cell Biochem. Funct. 2017, 35, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.; Yong, K.W.; Poon, C.T.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Xu, F.; Wan Safwani, W.K. In situ normoxia enhances survival and proliferation rate of human adipose tissue-derived stromal cells without increasing the risk of tumourigenesis. PLoS ONE 2015, 10, e0115034. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kato, T.; Furu, M.; Nasu, A.; Kajita, Y.; Mitsui, H.; Ueda, M.; Aoyama, T.; Nakayama, T.; Nakamura, T.; et al. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem. Biophys. Res. Commun. 2010, 391, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Vina-Almunia, J.; Mas-Bargues, C.; Borras, C.; Gambini, J.; El Alami, M.; Sanz-Ros, J.; Penarrocha, M.; Vina, J. Influence of Partial O(2) Pressure on the Adhesion, Proliferation, and Osteogenic Differentiation of Human Dental Pulp Stem Cells on beta-Tricalcium Phosphate Scaffold. Int. J. Oral Maxillofac. Implant. 2017, 32, 1251–1256. [Google Scholar] [CrossRef] [PubMed]


| Cell Type | Oxygen Conditions | Duration | Affected Parameters | Ref. |
|---|---|---|---|---|
| C2C12 myoblasts | 6% vs. 21% | 72 h | ROS production, differentiation | [134] |
| HSCs (CD34+ cells) | 5% vs. 21% | 7 days | ROS levels, antioxidant enzymes (SOD, CAT and GPx), glutathione redox state | [135] |
| Human Dermal Fibroblasts (HDFs) | 5% vs. 21% | 72 h | ROS production, enzymatic and non-enzymatic antioxidant response system, DNA damage, extracellular matrix (ECM) proteins | [136] |
| DPSCs | 3% vs. 21% | Up to passage 25 | Oxidative stress parameters (ROS, MDA, carbonylation, antioxidant defenses), proliferation, stemness (OSKM) | [138] |
| MSCs from adipose tissue | 3% vs. 20% | Up to 22 passages | Genetic stability, glycolytic function, cell differentiation and ROS production and targets (Protein carbonylation and MDA) | [151] |
| NSCs | 3% vs. 21% | 10 days | Survival, renewal potential and differentiation | [152] |
| BMSCs | 2% vs. 20% | 12 days | Proliferation kinetics, metabolism, differentiation potential | [153] |
| BMSCs | 1% vs. 21% | 7 days | Proliferation, migration, morphology, adhesion molecules, osteogenic differentiation | [154] |
| MSCs from umbilical cord | 1.5%, 2.5%, 5%, 21% | 70 h | Proliferation, metabolism, pH, oxygen consumption | [155] |
| ADSCs | 1% vs. 20% | 72 h | Proliferation, ROS generation, migration, OSKM | [157] |
| Muscle Precursor Cells (MPCs) | 5%, 10%, 15%, 20% | Up to passage 2 | Cell cycle regulation (p21 and p27), Proliferation | [159] |
| BM-MSCs and ADSCs | 2% vs. 21% | Up to passage 10 | Morphology, differentiation potential, genomic stability, telomere length, mitochondrial membrane potential, ATP content | [164] |
| Central Nervous System (CNS) Precursor Cells | 2%, 5%, 20% | Up to passage 2 (35 days) | Proliferation, HIF1α, apoptosis, multilineage differentiation potential | [167,168] |
| MSCs from umbilical cord | 3% vs. 21% | Up to passage 12 | Proliferation, HIF1α, ERK signalling pathway, stemness (OCT3/4 and Nanog), p21, p16, p53 | [173] |
| BM-MSCs | 5% vs. 21% | Up to passage 15 | Donor age, differentiation potential, SA-β-Gal, miRNA sequencing, KEGG signalling pathways | [174] |
| BM-MSCs | 1% vs. 21% | Up to passage 4 | Migration, proliferation, apoptosis, differentiation potential, PTEN-PI3K/AKT signalling pathway, miRNAs, HGF and VEGF | [175] |
| Satellite Cells | 1% vs. 21% | 48 h | Quiescence, self-renewal, miRNAs, Notch signalling pathway, transplantation efficiency | [177] |
| CSCs | 0.5%, 5%, 21% | Up to passage 10 | Proliferation, survival, migration, SA-β-Gal, apoptosis | [179] |
| MSCs from umbilical cord | 2.2% vs. 21% | 24 h | ROS levels, migration, HIF1α, VEGF | [182] |
| ESCs | 1–5% vs. 21% | Up to passage 50 | Morphology, colony growth, differentiation, hGC production, embryoid body formation | [184] |
| ESCs | 4% vs. 20% | Up to passage 50 | Morphological differentiation, microarray and transcriptome profiling, HIF, stemness | [185] |
| Neural Crest Stem Cells | 5% vs. 20% | 12 days | Survival, proliferation, multilineage differentiation | [186] |
| BM-MSCs | 1, 3, 5, 10% vs. 21% | 7 days | Viability, proliferation, self-renewal, osteogenic differentiation | [187] |
| C2C12 myoblasts, Satellite Cells and NSCs | 1% vs. 21% | 7 days | Notch signalling pathway, undifferentiated state maintenance | [194] |
| BM-MSCs and HSCs | 5, 12, 20% | 10 days | ROS content, proliferation, directional differentiation, apoptosis, cell cycle, migration | [195] |
| BM-MSCs | 2% vs. 18% | 2 weeks | Osteogenic and adipogenic differentiation, HIF1α, VEGF | [196] |
| BM-MSCs | 1% vs. 21% | 7 days/4 weeks | Proliferation, migration, stemness (OCT3/4, Nanog, SALL4, KLF4), differentiation | [154] |
| MSCs | 2% vs. 20% | 7 days | Proliferation, osteogenic differentiation | [197] |
| BM-MSCs | 0.2% vs. 21% | 7 or 14 days | Osteogenic and adipogenic differentiation, HIF1α | [198] |
| MSCs | 1, 2, 3, 4, 6% vs. 21% | 2, 4, 8, 24, 48, 72 h | Adipogenic differentiation | [199] |
| BM-MSCs | 3% vs. 21% | Isolation and expansion (4 weeks) | Chondrogenic differentiation, cell surface markers, ECM formation, expansion, HIFs | [200] |
| BM-MSCs | 2% vs. 20% | 14 days | Chondrogenic differentiation | [201] |
| MSCs | 1% vs. 21% | 21 days | Osteogenic differentiation, HIFs | [202] |
| WJ-MSCs | 3% vs. 21% | Up to passage 13 | Growth kinetics, SA-β-Gal, differentiation, HIFs, p16, p21, p53, karyotype | [203] |
| ADSCs | 1% vs. 21% | Up to passage 2 | Proliferation, multilineage differentiation, stemness (Nanog, SOX2) | [204] |
| ESCs (dorsal pancreatic bud) | 3%, 8%, 21% | 24h or 7 days | Cell differentiation, HIF1α gene and protein expression | [205] |
| ESCs | 3–5% vs. 20% | Up to passage 3 | Morphology, proliferation, pluripotency (SOX2, Nanog and OCT3/4), HIFs | [210] |
| BM-MSCs | 1% vs. 21% | 14 days | Proliferation, differentiation, self-renewal | [215] |
| WJ-MSCs | 5% vs. 21% | 2-4 weeks | Proliferation, stemness (OCT3/4, Nanog, REX1 and SOX2), HIFs, differentiation | [216] |
| BM-MSCs | 5% vs. 21% | Up to passage 2 | Morphology, differentiation, transcriptional profiling, metabolism, adhesion | [217] |
| Dermal Fibroblasts into IPSCs | 1%, 5%, 21% | 40 days | Efficiency of reprogramming into iPSCs (ESC markers, teratoma formation) | [218] |
| Fibroblasts, ESCs and IPSCs | 2%, 5%, 21% | 2 weeks | Reprogramming efficiency, HIFs, metabolism (OCR and ECAR) | [219,220] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Inglés, M.; Gimeno-Mallench, L.; El Alami, M.; Viña-Almunia, J.; Gambini, J.; Viña, J.; Borrás, C. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1195. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051195
Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Inglés M, Gimeno-Mallench L, El Alami M, Viña-Almunia J, Gambini J, Viña J, Borrás C. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. International Journal of Molecular Sciences. 2019; 20(5):1195. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051195
Chicago/Turabian StyleMas-Bargues, Cristina, Jorge Sanz-Ros, Aurora Román-Domínguez, Marta Inglés, Lucia Gimeno-Mallench, Marya El Alami, José Viña-Almunia, Juan Gambini, José Viña, and Consuelo Borrás. 2019. "Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine" International Journal of Molecular Sciences 20, no. 5: 1195. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051195