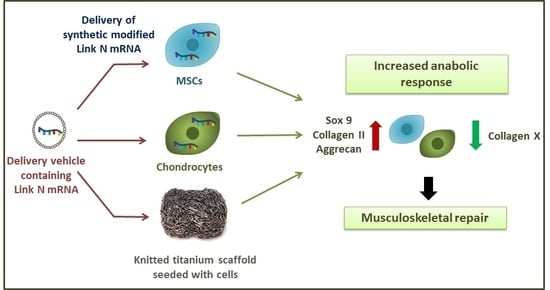
Exogenous Delivery of Link N mRNA into Chondrocytes and MSCs—The Potential Role in Increasing Anabolic Response
Abstract
:1. Introduction
2. Results
2.1. Generation of Synthetic Modified Link N mRNA
2.2. Analysis of Transfectability of Human Primary Chondrocytes and SCP1 Cells with Synthetic mRNA
2.3. Exogenous Delivery of Link N mRNA Does Not Affect Cell Viability
2.4. Link N mRNA Transfection Augments Anabolic Effects in Human Primary Chondrocytes
2.5. Link N mRNA Transfection Upholds the Expression of ECM-Related Genes in SCP1 Cells
2.6. Transfectability of Cells Seeded on Knitted Titanium Scaffold with eGFP mRNA
2.7. Link N mRNA Transfection of Cells Seeded on Knitted Titanium Scaffold Triggers the Expression of Chondrocyte-Specific and ECM-Related Genes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Generation of Modified Synthetic mRNA
4.3. Cy3 Labeling of Link N mRNA
4.4. Analysis of Transfection Efficiency Using Cy3 Labeled Link N mRNA
4.5. Analysis of Cell Viability
4.6. Cultivation of Cells
4.7. Transfection of Cells with Modified Synthetic Link N mRNA in 2D Cell Culture
4.8. Semi-Quantitative RT-PCR
4.9. Histochemical Analysis of ECM Production
4.10. Dot Blot Assay for Quantifying Link N Protein Post mRNA Transfection
4.11. Transient Cell Infections and Gene Reporter Assay
4.12. Preparation of Knitted Titanium Scaffolds and Cell Seeding
4.13. Transfection of Cells Seeded on Knitted Titanium Scaffold
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fontana, G.; See, E.; Pandit, A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv. Drug Deliv. Rev. 2015, 84, 146–158. [Google Scholar] [CrossRef]
- Khan, A.; Jacobsen, H.; Khan, J.; Filippi, C.; Levine, M.; Lehman, R.; Riew, K.; Lenke, L.; Chahine, N. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Kawai, A.; Wollan, P.; Yawn, B. Adverse impacts of chronic pain on health-related quality of life, work productivity, depression and anxiety in a community-based study. Fam. Pract. 2017, 34, 656–661. [Google Scholar] [CrossRef]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [Green Version]
- Freemont, A. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology 2009, 48, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Mobasheri, A.; Freemont, A.; Hoyland, J. Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol. Histopathol. 2007, 22, 1033–1041. [Google Scholar] [PubMed]
- Urban, J.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Clouet, J.; Fusellier, M.; Camus, A.; Le Visage, C.; Guicheux, J. Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef]
- Henry, N.; Clouet, J.; Le Bideau, J.; Le Visage, C.; Guioheux, J. Innovative strategies for intervertebral disc regenerative medicine: From cell therapies to multiscale delivery systems. Biotechnol. Adv. 2018, 36, 281–294. [Google Scholar] [CrossRef]
- Rustenburg, C.M.E.; Emanuel, K.S.; Peeters, M.; Lems, W.F.; Vergroesen, P.P.A.; Smit, T.H. Osteoarthritis and intervertebral disc degeneration: Quite different, quite similar. JOR Spine 2018, 1, e1033. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Ghivizzani, S.C.; Evans, C.H. Gene transfer approaches to the healing of bone and cartilage. Mol. Ther. 2002, 6, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Fargnoli, A.; Williams, R.; Bridges, C. Gene Therapy Delivery Systems for Enhancing Viral and Nonviral Vectors for Cardiac Diseases: Current Concepts and Future Applications. Hum. Gene Ther. 2013, 24, 914–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinle, H.; Behring, A.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Concise Review: Application of In Vitro Transcribed Messenger RNA for Cellular Engineering and Reprogramming: Progress and Challenges. Stem Cells 2017, 35, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Aini, H.; Itaka, K.; Fujisawa, A.; Uchida, H.; Uchida, S.; Fukushima, S.; Kataoka, K.; Saito, T.; Chung, U.I.; Ohba, S. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Sci. Rep. 2016, 6, 18743. [Google Scholar] [CrossRef] [Green Version]
- Bach, F.C.; Laagland, L.T.; Grant, M.P.; Creemers, L.B.; Ito, K.; Meij, B.P.; Mwale, F.; Tryfonidou, M.A. Link-N: The missing link towards intervertebral disc repair is species-specific. PLoS ONE 2017, 12, e0187831. [Google Scholar] [CrossRef] [PubMed]
- Gawri, R.; Antoniou, J.; Ouellet, J.; Awwad, W.; Steffen, T.; Roughley, P.; Haglund, L.; Mwale, F. Link-N can stimulate proteoglycan synthesis in the degenerated human intervertebral discs. Eur. Cells Mater. 2013, 26, 107–119. [Google Scholar] [CrossRef]
- Gawri, R.; Ouellet, J.; Onnerfjord, P.; Alkhatib, B.; Steffen, T.; Heinegard, D.; Roughley, P.; Antoniou, J.; Mwale, F.; Haglund, L. Link N is Cleaved by Human Annulus Fibrosus Cells Generating a Fragment with Retained Biological Activity. J. Orthop. Res. 2014, 32, 1189–1197. [Google Scholar] [CrossRef]
- Wang, Z.; Hutton, W.C.; Yoon, S.T. ISSLS Prize winner: Effect of link protein peptide on human intervertebral disc cells. Spine 2013, 38, 1501–1507. [Google Scholar] [CrossRef]
- Mwale, F.; Wang, H.; Roughley, P.; Antoniou, J.; Haglund, L. Link N and Mesenchymal Stem Cells Can Induce Regeneration of the Early Degenerate Intervertebral Disc. Tissue Eng. Part A 2014, 20, 2942–2949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, J.; Wang, H.T.; Alaseem, A.M.; Haglund, L.; Roughley, P.J.; Mwale, F. The effect of Link N on differentiation of human bone marrow-derived mesenchymal stem cells. Arthritis Res. Ther. 2012, 14, R267. [Google Scholar] [CrossRef]
- Mwale, F.; Masuda, K.; Pichika, R.; Epure, L.M.; Yoshikawa, T.; Hemmad, A.; Roughley, P.J.; Antoniou, J. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res. Ther. 2011, 13, R120. [Google Scholar] [CrossRef]
- Tendulkar, G.; Grau, P.; Ziegler, P.; Buck, A.; Badke, A.; Kaps, H.P.; Ehnert, S.; Nussler, A.K. Imaging Cell Viability on Non-transparent Scaffolds—Using the Example of a Novel Knitted Titanium Implant. J. Vis. Exp. 2016. [Google Scholar] [CrossRef]
- Tendulkar, G.; Sreekumar, V.; Rupp, F.; Teotia, A.K.; Athanasopulu, K.; Kemkemer, R.; Buck, A.; Kaps, H.P.; Geis-Gerstorfer, J.; Kumar, A.; et al. Characterisation of porous knitted titanium for replacement of intervertebral disc nucleus pulposus. Sci. Rep. 2017, 7, 16611. [Google Scholar] [CrossRef] [Green Version]
- Sampara, P.; Banala, R.R.; Vemuri, S.K.; Av, G.R.; Gpv, S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018, 25, 67–82. [Google Scholar] [CrossRef]
- Shimer, A.L.; Chadderdon, R.C.; Gilbertson, L.G.; Kang, J.D. Gene therapy approaches for intervertebral disc degeneration. Spine 2004, 29, 2770–2778. [Google Scholar] [CrossRef]
- Evans, C.H.; Huard, J. Gene therapy approaches to regenerating the musculoskeletal system. Nat. Rev. Rheumatol. 2015, 11, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Ranganathan, P.; Diamond, S.L. Nuclear targeting peptide scaffolds for lipofection of nondividing mammalian cells. Nat. Biotechnol. 1999, 17, 873–877. [Google Scholar] [CrossRef]
- Goodrich, L.; Grieger, J.; Phillips, J.; Khan, N.; Gray, S.; McIlwraith, C.; Samulski, R. scAAVIL-1ra dosing trial in a large animal model and validation of long-term expression with repeat administration for osteoarthritis therapy. Gene Ther. 2015, 22, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Avci-Adali, M.; Behring, A.; Keller, T.; Krajewski, S.; Schlensak, C.; Wendel, H.P. Optimized conditions for successful transfection of human endothelial cells with in vitro synthesized and modified mRNA for induction of protein expression. J. Biol. Eng. 2014, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Roughley, P.; Antoniou, J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: A requisite for tissue engineering of intervertebral disc. Eur. Cell Mater. 2004, 8, 58–63; discussion 63–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Adkisson, H.D.; Wendland, M.; Seyedin, M.; Berven, S.; Lotz, J.C. Juvenile chondrocytes may facilitate disc repair. Open Tissue Eng. Regen. Med. J. 2010, 3, 28. [Google Scholar] [CrossRef]
- Adams, M.A.; Dolan, P. Intervertebral disc degeneration: Evidence for two distinct phenotypes. J. Anat. 2012, 221, 497–506. [Google Scholar] [CrossRef]
- Steck, E.; Bertram, H.; Abel, R.; Chen, B.; Winter, A.; Richter, W. Induction of intervertebral disc–like cells from adult mesenchymal stem cells. Stem Cells 2005, 23, 403–411. [Google Scholar] [CrossRef]
- Bougioukli, S.; Evans, C.H.; Alluri, R.K.; Ghivizzani, S.C.; Lieberman, J.R. Gene Therapy to Enhance Bone and Cartilage Repair in Orthopaedic Surgery. Curr. Gene Ther. 2018, 18, 154–170. [Google Scholar] [CrossRef]
- Otvos, L.; Wade, J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Baur, J.; Schmitt, A.; Neumaier, M.; Lucke, M.; Dooley, S.; Vester, H.; Wildemann, B.; Stöckle, U.; Nussler, A.K. TGF-β1 as possible link between loss of bone mineral density and chronic inflammation. PLoS ONE 2010, 5, e14073. [Google Scholar] [CrossRef]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.-H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef]
- Sahin, U.; Kariko, K.; Tureci, O. Mrna-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mrna delivery for potent cancer immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef]
- Steinle, H.; Ionescu, T.M.; Schenk, S.; Golombek, S.; Kunnakattu, S.J.; Ozbek, M.T.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Incorporation of synthetic mrna in injectable chitosan-alginate hybrid hydrogels for local and sustained expression of exogenous proteins in cells. Int. J. Mol. Sci. 2018, 19, 1313. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, Z.S.; Berezhanskyy, T.; Utzinger, M.; Aneja, M.K.; Emrich, D.; Erben, R.; Schuler, C.; Altpeter, P.; Ferizi, M.; Hasenpusch, G.; et al. Transcript-activated collagen matrix as sustained mrna delivery system for bone regeneration. J. Control. Release 2016, 239, 137–148. [Google Scholar] [CrossRef] [PubMed]
- van Uden, S.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Current strategies for treatment of intervertebral disc degeneration: Substitution and regeneration possibilities. Biomater. Res. 2017, 21, 22. [Google Scholar] [CrossRef]
- Lewis, G. Nucleus pulposus replacement and regeneration/repair technologies: Present status and future prospects. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1702–1720. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Elliott, D.M.; Mauck, R.L. Mechanical design criteria for intervertebral disc tissue engineering. J. Biomech. 2010, 43, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Zigler, J.E.; Delamarter, R.B. Five-year results of the prospective, randomized, multicenter, Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential arthrodesis for the treatment of single-level degenerative disc disease. J. Neurosurg. Spine 2012, 17, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Avci-Adali, M.; Behring, A.; Steinle, H.; Keller, T.; Krajeweski, S.; Schlensak, C.; Wendel, H.P. In vitro synthesis of modified mRNA for induction of protein expression in human cells. J. Vis. Exp. 2014, e51943. [Google Scholar] [CrossRef]
- Böcker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell. Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Bae, K.; Kim, S.-G.; Kwak, D.; Moon, Y.-J.; Choi, C.-H.; Kim, Y.-R.; Na, C.-S.; Kim, S.-J. Anti-osteoarthritic effects of ChondroT in a rat model of collagenase-induced osteoarthritis. BMC Complement. Altern. Med. 2018, 18, 131. [Google Scholar] [CrossRef]








| Gene Name | Accession Number | Forward Primer (5′-3′) | Reverse Primer (3′-5′) | Amplicon (bp) | Tm (°C) | Cycle No. |
|---|---|---|---|---|---|---|
| GAPDH | NM_002046 | GTCAGTGGTGGACCTGACCT | AGGGGTCTACATGGCAACTG | 420 | 56 | 30 |
| SOX9 | NM_000346 | GAAGGACCACCCGGATTACA | GCCTTGAAGATGGCGTTGG | 120 | 60 | 35 |
| ACAN | NM_001135 | CTTGGACTTGGGCAAACTGC | CACTAAAGTCAGGCAGGCCA | 143 | 60 | 35 |
| COL X | NM_000493 | AAACCTGGACAACAGGGACC | CGACCAGGAGCACCATATCC | 125 | 60 | 35 |
| COL II | NM_001844 | TGGATGCCACACTCAAGTCC | GCTGCTCCACCAGTTCTTCT | 254 | 60 | 35 |
| BMP2 | NM_001200 | CCCCCTACATGCTAGACCTGT | CACTCGTTTCTGGTAGTTCTTCC | 150 | 60 | 35 |
| BMP7 | NM_001719 | TAGCCATTTCCTCACCGACG | AGATCCGATTCCCTGCCCAA | 255 | 60 | 35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tendulkar, G.; Ehnert, S.; Sreekumar, V.; Chen, T.; Kaps, H.-P.; Golombek, S.; Wendel, H.-P.; Nüssler, A.K.; Avci-Adali, M. Exogenous Delivery of Link N mRNA into Chondrocytes and MSCs—The Potential Role in Increasing Anabolic Response. Int. J. Mol. Sci. 2019, 20, 1716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071716
Tendulkar G, Ehnert S, Sreekumar V, Chen T, Kaps H-P, Golombek S, Wendel H-P, Nüssler AK, Avci-Adali M. Exogenous Delivery of Link N mRNA into Chondrocytes and MSCs—The Potential Role in Increasing Anabolic Response. International Journal of Molecular Sciences. 2019; 20(7):1716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071716
Chicago/Turabian StyleTendulkar, Gauri, Sabrina Ehnert, Vrinda Sreekumar, Tao Chen, Hans-Peter Kaps, Sonia Golombek, Hans-Peter Wendel, Andreas K. Nüssler, and Meltem Avci-Adali. 2019. "Exogenous Delivery of Link N mRNA into Chondrocytes and MSCs—The Potential Role in Increasing Anabolic Response" International Journal of Molecular Sciences 20, no. 7: 1716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071716