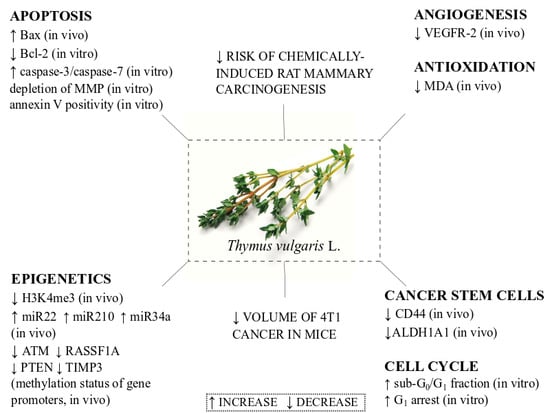
Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma In Vivo and In Vitro
Abstract
:1. Introduction
2. Results
2.1. Rat Mammary Carcinogenesis and Histopathology of Tumors
2.2. Mouse 4T1 Model
2.3. Immunohistochemistry of Rat Tumors
2.4. miRNA Expression
2.5. Quantitative Methylation Analysis
2.6. Physiological in Vivo Effects
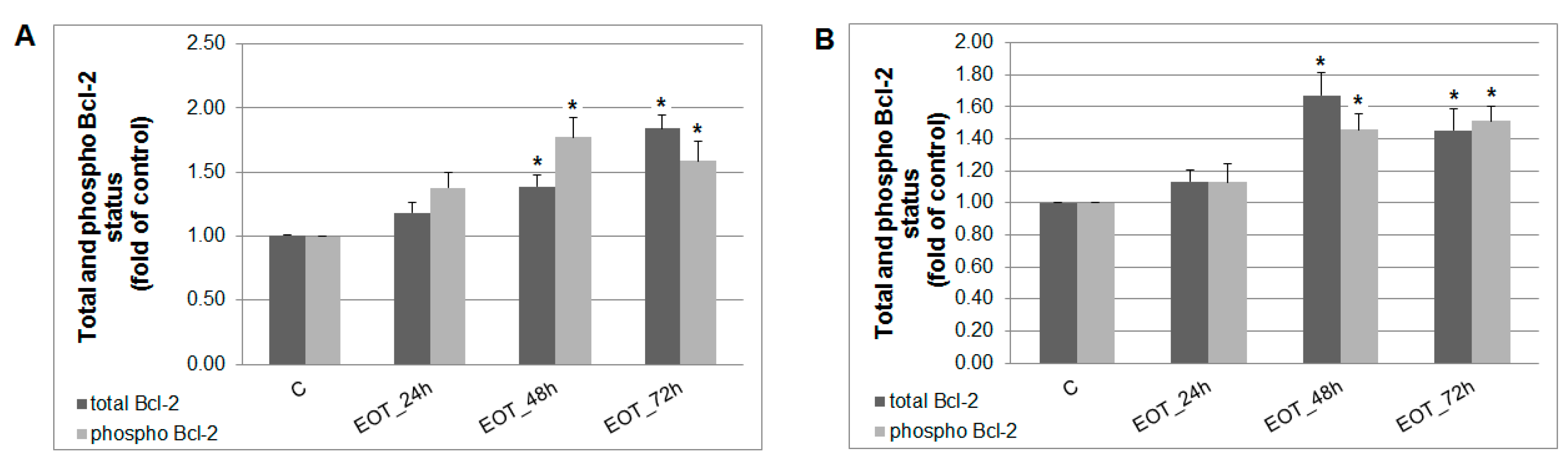
2.7. In vitro Analyses in MCF-7 and MDA-MB-231 Cells
2.8. Plant Secondary Metabolites in T. vulgaris
3. Discussion
4. Materials and Methods
4.1. Animals and Induction of Mammary Carcinogenesis, and Design of Experiment
4.2. Histopathological and Immunohistochemical Analysis of Rat and Mouse Tumors
4.3. miRNA Expression Analysis
4.4. Nucleic Acids Extraction and Bisulfite Conversion
4.5. Quantitative Methylation Analysis (Pyrosequencing)
4.6. Cell Culture and Experimental Design
4.7. Cytotoxicity Assay
4.8. 5-Bromo-20-deoxyuridine (BrdU) Cell Proliferation Assay
4.9. Flow Cytometry Analyses Protocol
4.10. Examination of Plant Secondary Metabolites in Essential Oil of T. vulgaris
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Kano, M.; Kaga, C. Possibility of breast cancer prevention: Use of soy isoflavones and fermented soy beverage produced using probiotics. Int. J. Mol. Sci. 2015, 16, 10907–10920. [Google Scholar] [CrossRef] [PubMed]
- Shapira, N. The potential contribution of dietary factors to breast cancer prevention. Eur. J. Cancer Prev. 2017, 2, 385–395. [Google Scholar] [CrossRef]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Gatenby, P.; Gerbi, V.; Janssens, J.; Johnston, B.; Kas, K.; La Vecchia, C.; Mainguet, P.; et al. Cancer prevention in Europe: The Mediterranean diet as a protective choice. Eur. J. Cancer Prev. 2013, 22, 90–95. [Google Scholar] [PubMed]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [PubMed]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [PubMed]
- Akhavan-Niaki, H.; Samadani, A.A. DNA methylation and cancer development: Molecular mechanism. Cell Biochem. Biophys. 2013, 2, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Hon, G.C.; Hawkins, R.D.; Caballero, O.L.; Lo, C.; Lister, R.; Pelizzola, M.; Valsesia, A.; Ye, Z.; Kuan, S.; Edsall, L.E.; et al. Global DNA hypomethylation coupled to repressive chromatin domain formation and gene silencing in breast cancer. Genome Res. 2012, 22, 246–258. [Google Scholar]
- Ng, J.M.; Yu, J. Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. Int. J. Mol. Sci. 2015, 16, 2472–2496. [Google Scholar] [PubMed]
- Sangaramoorthy, M.; Koo, J.; John, E.M. Intake of bean fiber, beans, and grains and reduced risk of hormone receptor-negative breast cancer: The San Francisco Bay Area Breast Cancer Study. Cancer Med. 2018, 7, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Dandamudi, A.; Tommie, J.; Nommsen-Rivers, L.; Couch, S. Dietary Patterns and Breast Cancer Risk: A Systematic Review. Anticancer Res. 2018, 38, 3209–3222. [Google Scholar] [CrossRef]
- He, J.; Gu, Y.; Zhang, S. Consumption of vegetables and fruits and breast cancersurvival: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 599. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Chiuve, S.E.; Willett, W.C.; Hankinson, S.E.; Hu, F.B.; Holmes, M.D. Intake of specific fruits and vegetables in relation to risk of estrogen receptor-negative breast cancer among postmenopausal women. Breast Cancer Res. Treat. 2013, 138, 925–930. [Google Scholar] [CrossRef]
- Noroozisharaf, A.; Kaviani, M. Effect of soil application of humic acid on nutrients uptake, essential oil and chemical compositions of garden thyme (Thymus vulgaris L.) under greenhouse conditions. Physiol. Mol. Biol. Plants 2018, 24, 423–431. [Google Scholar] [CrossRef]
- Pérez López, L.A.; de la Torre, Y.C.; Cirio, A.T.; de Torres, N.W.; Flores Suárez, A.E.; Aranda, R.S. Essential oils from Zanthoxylum fagara Wild Lime, Ruta chalepensis L. and Thymus vulgaris L.: Composition and activity against Aedes aegypti larvae. Pak. J. Pharm. Sci. 2015, 28, 1911–1915. [Google Scholar]
- Vila, R. Flavonoids and Further Polyphenols in the Genus Thymus. In Thyme: The Genus Thymus; Stahl-Biskup, E., Saez, F., Eds.; CRC Press: London, UK, 2002; pp. 144–177. ISBN 9780203216859. [Google Scholar]
- Bentayeb, K.; Vera, P.; Rubio, C.; Nerín, C. The additive properties of Oxygen Radical Absorbance Capacity (ORAC) assay: The case of essential oils. Food Chem. 2014, 148, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Salehzadeh, A.; Sadat Shandiz, S.A.; Tajdoost, S. Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech. 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Al-Menhali, A.; Al-Rumaihi, A.; Al-Mohammed, H.; Al-Mazrooey, H.; Al-Shamlan, M.; AlJassim, M.; Al-Korbi, N.; Eid, A.H. Thymus vulgaris (thyme) inhibits proliferation, adhesion, migration, and invasion of human colorectal cancer cells. J. Med. Food 2015, 18, 54–59. [Google Scholar] [CrossRef]
- Ayesh, B.M.; Abed, A.A.; Faris, D.M. In vitro inhibition of human leukemia THP-1 cells by Origanum syriacum L. and Thymus vulgaris L. extracts. BMC Res. Notes 2014, 7, 612. [Google Scholar]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Cytotoxicity of Thymus vulgaris essential oil towards human oral cavity squamous cell carcinoma. Anticancer Res. 2011, 31, 81–87. [Google Scholar]
- Kubatka, P.; Kapinová, A.; Kružliak, P.; Kello, M.; Výbohová, D.; Kajo, K.; Novák, M.; Chripková, M.; Adamkov, M.; Péč, M.; et al. Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition 2015, 31, 560–569. [Google Scholar] [CrossRef]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Výbohová, D.; Šmejkal, K.; Maršík, P.; Zulli, A.; Gönciová, G.; Mojžiš, J.; et al. Young Barley Indicates Antitumor Effects in Experimental Breast Cancer In Vivo and In Vitro. Nutr. Cancer 2016, 68, 611–621. [Google Scholar] [CrossRef]
- Kubatka, P.; Kapinová, A.; Kello, M.; Kruzliak, P.; Kajo, K.; Výbohová, D.; Mahmood, S.; Murin, R.; Viera, T.; Mojžiš, J.; et al. Fruit peel polyphenols demonstrate substantial anti-tumour effects in the model of breast cancer. Eur. J. Nutr. 2016, 55, 955–965. [Google Scholar] [CrossRef]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Výbohová, D.; Mojžiš, J.; Adamkov, M.; Fialová, S.; Veizerová, L.; Zulli, A.; et al. Oregano demonstrates distinct tumour-suppressive effects in the breast carcinoma model. Eur. J. Nutr. 2017, 56, 1303–1316. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Kruzliak, P.; Mojzis, J.; Vybohova, D.; Adamkov, M.; Jasek, K.; Lasabova, Z.; et al. Antineoplastic effects of clove buds (Syzygium aromaticum L.) in the model of breast carcinoma. J. Cell. Mol. Med. 2017, 21, 2837–2851. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Solár, P.; Sačková, V.; Hrčková, G.; Demečková, V.; Kassayová, M.; Bojková, B.; Mudroňová, D.; Gancarčíková, S.; Jendželovský, R.; Fedoročko, P. Antitumor effect of the combination of manumycin A and Immodin is associated with antiplatelet activity and increased granulocyte tumor infiltration in a 4T1 breast tumor model. Oncol. Rep. 2017, 37, 368–378. [Google Scholar] [CrossRef]
- Demečková, V.; Solár, P.; Hrčková, G.; Mudroňová, D.; Bojková, B.; Kassayová, M.; Gancarčiková, S. Immodin and its immune system supportive role in paclitaxel therapy of 4T1 mouse breast cancer. Biomed. Pharmacother. 2017, 89, 245–256. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Safavi, M.; Ardestani, S.K. In-vitro evaluation of apoptotic effect of OEO and thymol in 2D and 3D cell cultures and the study of their interaction mode with DNA. Sci. Rep. 2018, 25, 15787. [Google Scholar] [CrossRef]
- Montani, M.; Pazmay, G.V.B.; Hysi, A.; Lupidi, G.; Pettinari, R.; Gambini, V.; Tilio, M.; Marchetti, F.; Pettinari, C.; Ferraro, S.; et al. The water soluble ruthenium(II) organometallic compound [Ru(p-cymene)(bis(3,5 dimethylpyrazol-1-yl)methane)Cl]Cl suppresses triple negative breast cancer growth by inhibiting tumor infiltration of regulatory T cells. Pharmacol. Res. 2016, 107, 282–290. [Google Scholar] [CrossRef]
- Rahman, F.U.; Bhatti, M.Z.; Ali, A.; Duong, H.Q.; Zhang, Y.; Ji, X.; Lin, Y.; Wang, H.; Li, Z.T.; Zhang, D.W. Dimetallic Ru(II) arene complexes appended on bis-salicylaldimine induce cancer cell death and suppress invasion via p53-dependent signaling. Eur. J. Med. Chem. 2018, 157, 1480–1490. [Google Scholar] [CrossRef]
- Jeyabalan, J.; Aqil, F.; Munagala, R.; Annamalai, L.; Vadhanam, M.V.; Gupta, R.C. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. J. Agric. Food Chem. 2014, 62, 3963–3971. [Google Scholar] [CrossRef]
- Ravoori, S.; Vadhanam, M.V.; Aqil, F.; Gupta, R.C. Inhibition of estrogen-mediated mammary tumorigenesis by blueberry and black raspberry. J. Agric. Food Chem. 2012, 60, 5547–5555. [Google Scholar] [CrossRef]
- Singletary, K.; MacDonald, C.; Wallig, M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996, 104, 43–48. [Google Scholar] [CrossRef]
- Bishayee, A.; Mandal, A.; Bhattacharyya, P.; Bhatia, D. Pomegranate exerts chemoprevention of experimentally induced mammary tumorigenesis by suppression of cell proliferation and induction of apoptosis. Nutr. Cancer 2016, 68, 120–130. [Google Scholar] [CrossRef]
- Salakou, S.; Kardamakis, D.; Tsamandas, A.C.; Zolota, V.; Apostolakis, E.; Tzelepi, V.; Papathanasopoulos, P.; Bonikos, D.S.; Papapetropoulos, T.; Petsas, T.; et al. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo 2007, 21, 123–132. [Google Scholar]
- Pal, M.K.; Jaiswar, S.P.; Srivastav, A.K.; Goyal, S.; Dwivedi, A.; Verma, A.; Singh, J.; Pathak, A.K.; Sankhwar, P.L.; Ray, R.S. Synergistic effect of piperine and paclitaxel on cell fate via cyt-c, Bax/Bcl-2-caspase-3 pathway in ovarian adenocarcinomas SKOV-3 cells. Eur. J. Pharmacol. 2016, 791, 751–762. [Google Scholar] [CrossRef]
- Zhang, J.; Park, H.S.; Kim, J.A.; Hong, G.E.; Nagappan, A.; Park, K.I.; Kim, G.S. Flavonoids identified from korean Scutellaria baicalensis induce apoptosis by ROS generation and caspase activation on human fibrosarcoma cells. Am. J. Chin. Med. 2014, 42, 465–483. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Hsu, C.P.; Shih, Y.T.; Lin, B.R.; Chiu, C.F.; Lin, C.C. Inhibitory effect and mechanisms of an anthocyanins- and anthocyanidins-rich extract from purple-shoot tea on colorectal carcinoma cell proliferation. J. Agric. Food Chem. 2012, 60, 3686–3692. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) Shells Extract: Phenolic Composition, Antioxidant Effect and Cytotoxic Activity on Human Cancer Cell Lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar]
- Li, J.; Gong, X.; Jiang, R.; Lin, D.; Zhou, T.; Zhang, A.; Li, H.; Zhang, X.; Wan, J.; Kuang, G.; et al. Fisetin Inhibited Growth and Metastasis of Triple-Negative Breast Cancer by Reversing Epithelial-to-Mesenchymal Transition via PTEN/Akt/GSK3β Signal Pathway. Front. Pharmacol. 2018, 9, 772. [Google Scholar] [CrossRef]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Kim, D.H.; Park, J.H.; Lee, H.G.; Kim, H.J.; Darvin, P.; Park, Y.M.; Yang, Y.M. Nobiletin Inhibits CD36-Dependent Tumor Angiogenesis, Migration, Invasion, and Sphere Formation through the Cd36/Stat3/Nf-Κb Signaling Axis. Nutrients 2018, 10, 772. [Google Scholar] [CrossRef]
- Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Mohamad, J.; Abdul Kadir, H. Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 110. [Google Scholar]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Lee, J.H.; Kong, A.N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Elisia, I.; Popovich, D.G.; Hu, C.; Kitts, D.D. Evaluation of viability assays for anthocyanins in cultured cells. Phytochem. Anal. 2008, 19, 479–486. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Kadioglu, O.; Seo, E.J.; Efferth, T. Targeting angiogenesis by phytochemicals. Med. Aromat. Plants 2013, 2, 134. [Google Scholar]
- Ye, Z.W.; Zhang, J.; Townsend, D.M.; Tew, K.D. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta 2014, 1850, 1607–1621. [Google Scholar] [CrossRef]
- Pan, Y.; Deng, Z.Y.; Zheng, S.L.; Chen, X.; Zhang, B.; Li, H. Daily Dietary Antioxidant Interactions Are Due to Not Only the Quantity but Also the Ratios of Hydrophilic and Lipophilic Phytochemicals. J. Agric. Food Chem. 2018, 66, 9107–9120. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Ko, Y.S.; Jin, H.; Lee, J.S.; Park, S.W.; Chang, K.C.; Kang, K.M.; Jeong, B.K.; Kim, H.J. Radioresistant breast cancer cells exhibit increased resistance to chemotherapy and enhanced invasive properties due to cancer stem cells. Oncol. Rep. 2018, 40, 3752–3762. [Google Scholar] [CrossRef]
- Shima, H.; Yamada, A.; Ishikawa, T.; Endo, I. Are breast cancer stem cells the key to resolving clinical issues in breast cancer therapy? Gland Surg. 2017, 6, 82–88. [Google Scholar] [CrossRef]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44+/CD24−) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Wise, R.; Zolkiewska, A. Metalloprotease-dependent activation of EGFR modulates CD44+/CD24− populations in triple negative breast cancer cells through the MEK/ERK pathway. Breast Cancer Res. Treat. 2017, 166, 421–433. [Google Scholar]
- Ryu, D.; Ryoo, I.G.; Kwak, M.K. Overexpression of CD44 Standard Isoform Upregulates HIF-1α Signaling in Hypoxic Breast Cancer Cells. Biomol. Ther. 2018, 26, 487–493. [Google Scholar] [CrossRef]
- Sankpal, N.V.; Fleming, T.P.; Gillanders, W.E. EpCAM modulates NF-κB signaling and interleukin-8 expression in breast cancer. Mol. Cancer Res. 2013, 11, 418–426. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, Y.; Oh, E.; Lee, N.; An, H.; Sung, D.; Cho, T.M.; Seo, J.H. Disulfiram targets cancer stem-like properties and the HER2/Akt signaling pathway in HER2-positive breast cancer. Cancer Lett. 2016, 379, 39–48. [Google Scholar] [CrossRef]
- Alam, M.; Ahmad, R.; Rajabi, H.; Kharbanda, A.; Kufe, D. MUC1-C oncoprotein activates ERK→C/EBPβ signaling and induction of aldehyde dehydrogenase 1A1 in breast cancer cells. J. Biol. Chem. 2013, 288, 30892–30903. [Google Scholar] [CrossRef]
- Zhao, D.; Mo, Y.; Li, M.T.; Zou, S.W.; Cheng, Z.L.; Sun, Y.P.; Xiong, Y.; Guan, K.L.; Lei, Q.Y. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J. Clin. Investig. 2014, 124, 5453–5465. [Google Scholar] [CrossRef]
- Rennó, A.L.; Alves-Júnior, M.J.; Rocha, R.M.; De Souza, P.C.; de Souza, V.B.; Jampietro, J.; Vassallo, J.; Hyslop, S.; Anhê, G.F.; de Moraes Schenka, N.G.; et al. Decreased expression of stem cell markers by simvastatin in 7,12-dimethylbenz(a)anthracene (DMBA)-induced breast cancer. Toxicol. Pathol. 2015, 43, 400–410. [Google Scholar] [CrossRef]
- Levi, E.; Misra, S.; Du, J.; Patel, B.B.; Majumdar, A.P. Combination of aging and dimethylhydrazine treatment causes an increase in cancer-stem cell population of rat colonic crypts. Biochem. Biophys. Res. Commun. 2009, 385, 430–433. [Google Scholar] [CrossRef]
- Khan, S.; Shukla, S.; Sinha, S.; Meeran, S.M. Epigenetic targets in cancer and aging: Dietary and therapeutic interventions. Expert Opin. Ther. Targets 2016, 20, 689–703. [Google Scholar] [CrossRef]
- Uramova, S.; Kubatka, P.; Dankova, Z.; Kapinova, A.; Zolakova, B.; Samec, M.; Zubor, P.; Zulli, A.; Valentova, V.; Kwon, T.K.; et al. Plant natural modulators in breast cancer prevention: Status quo and future perspectives reinforced by predictive, preventive and personalised medical approach. EPMA J. 2018, 9, 403–419. [Google Scholar] [CrossRef]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef]
- Altonsy, M.O.; Habib, T.N.; Andrews, S.C. Diallyl disulfide-induced apoptosis in a breast-cancer cell line (MCF-7) may be caused by inhibition of histone deacetylation. Nutr. Cancer 2012, 64, 1251–1260. [Google Scholar] [CrossRef]
- Attoub, S.; Hassan, A.H.; Vanhoecke, B.; Iratni, R.; Takahashi, T.; Gaben, A.M.; Bracke, M.; Awad, S.; John, A.; Kamalboor, H.A.; et al. Inhibition of cell survival, invasion, tumor growth and histone deacetylase activity by the dietary flavonoid luteolin in human epithelioid cancer cells. Eur. J. Pharmacol. 2011, 651, 18–25. [Google Scholar] [CrossRef]
- Dagdemir, A.; Durif, J.; Ngollo, M.; Bignon, Y.J.; Bernard-Gallon, D. Histone lysine trimethylation or acetylation can be modulated by phytoestrogen, estrogen or anti-HDAC in breast cancer cell lines. Epigenomics 2013, 5, 51–63. [Google Scholar] [CrossRef]
- Collins, H.M.; Abdelghany, M.K.; Messmer, M.; Yue, B.; Deeves, S.E.; Kindle, K.B.; Mantelingu, K.; Aslam, A.; Winkler, G.S.; Kundu, T.K.; et al. Differential effects of garcinol and curcumin on histone and p53 modifications in tumour cells. BMC Cancer 2013, 13, 37. [Google Scholar] [CrossRef]
- McAnena, P.; Brown, J.A.; Kerin, M.J. Circulating Nucleosomes and Nucleosome Modifications as Biomarkers in Cancer. Cancers 2017, 9, 5. [Google Scholar] [CrossRef]
- Chen, X.; Hu, H.; He, L.; Yu, X.; Liu, X.; Zhong, R.; Shu, M. A novel subtype classification and risk of breast cancer by histone modification profiling. Breast Cancer Res. Treat. 2016, 157, 267–279. [Google Scholar] [CrossRef]
- Rodríguez-Miguel, C.; Moral, R.; Escrich, R.; Vela, E.; Solanas, M.; Escrich, E. The Role of Dietary Extra Virgin Olive Oil and Corn Oil on the Alteration of Epigenetic Patterns in the Rat DMBA-Induced Breast Cancer Model. PLoS ONE 2015, 10, e0138980. [Google Scholar] [CrossRef]
- Messier, T.L.; Gordon, J.A.; Boyd, J.R.; Tye, C.E.; Browne, G.; Stein, J.L.; Lian, J.B.; Stein, G.S. Histone H3 lysine 4 acetylation and methylation dynamics define breast cancer subtypes. Oncotarget 2016, 7, 5094–5109. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Ding, M.; Zhang, H.; Xu, X.; Tang, J. Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer (Review). Int. J. Oncol. 2016, 50, 345–355. [Google Scholar] [CrossRef]
- Imani, S.; Zhang, X.; Hosseinifard, H.; Fu, S.; Fu, J. The diagnostic role of microRNA-34a in breast cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 23177–23187. [Google Scholar] [CrossRef]
- Venturutti, L.; Romero, L.V.; Urtreger, A.J.; Chervo, M.F.; Cordo Russo, R.I.; Mercogliano, M.F.; Inurrigarro, G.; Pereyra, M.G.; Proietti, C.J.; Izzo, F.; et al. Stat3regulatesErbB-2expression and co-opts ErbB-2 nuclear function to induce miR-21 expression, PDCD4 downregulation and breast cancer metastasis. Oncogene 2016, 35, 2208–2222. [Google Scholar] [CrossRef]
- Jung, D.E.; Park, S.B.; Kim, K.; Kim, C.; Song, S.Y. CG200745, an HDAC inhibitor, induces anti-tumour effects in cholangiocarcinoma cell lines via miRNAs targeting the Hippo pathway. Sci. Rep. 2017, 7, 10921. [Google Scholar] [CrossRef]
- Wang, H.; Bian, S.; Yang, C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis 2011, 32, 1881–1889. [Google Scholar] [CrossRef]
- Stefansson, O.A.; Esteller, M. Epigenetic modifications in breast cancer and their role in personalized medicine. Am. J. Pathol. 2013, 183, 1052–1063. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Flammer, J. Individualised patient profile: Clinical utility of Flammer syndrome phenotype and general lessons for predictive, preventive and personalised medicine. EPMA J. 2018, 9, 15–20. [Google Scholar] [CrossRef]
- Polivka, J., Jr.; Altun, I.; Golubnitschaja, O. Pregnancy Associated Breast Cancer: The Risky Status Quo and New Concepts of Predictive Medicine. EPMA J. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Golubnitschaja, O. Feeling cold and other underestimated symptoms in breast cancer: Anecdotes or individual profiles for advanced patient stratification? EPMA J. 2017, 8, 17–22. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Baban, B.; Boniolo, G.; Wang, W.; Bubnov, R.; Kapalla, M.; Krapfenbauer, K.; Mozaffari, M.; Costigliola, V. Medicine in the early twenty-first century: Paradigm and anticipation—EPMA position paper 2016. EPMA J. 2016, 7, 23. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Debald, M.; Yeghiazaryan, K.; Kuhn, W.; Pešta, M.; Costigliola, V.; Grech, G. Breast cancer epidemic in the early 21st century: Evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumor Biol. 2016, 37, 12941–12957. [Google Scholar] [CrossRef]
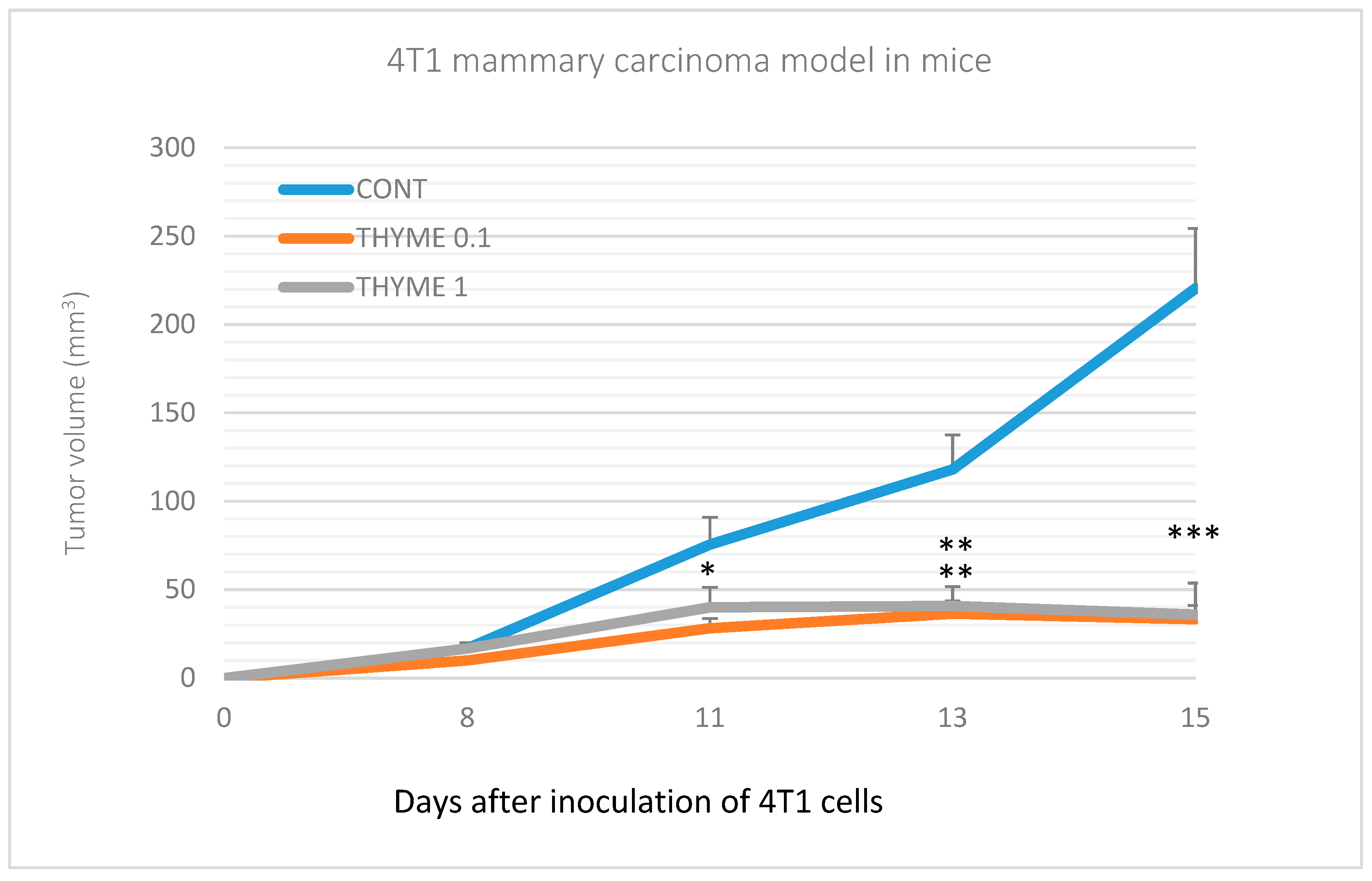

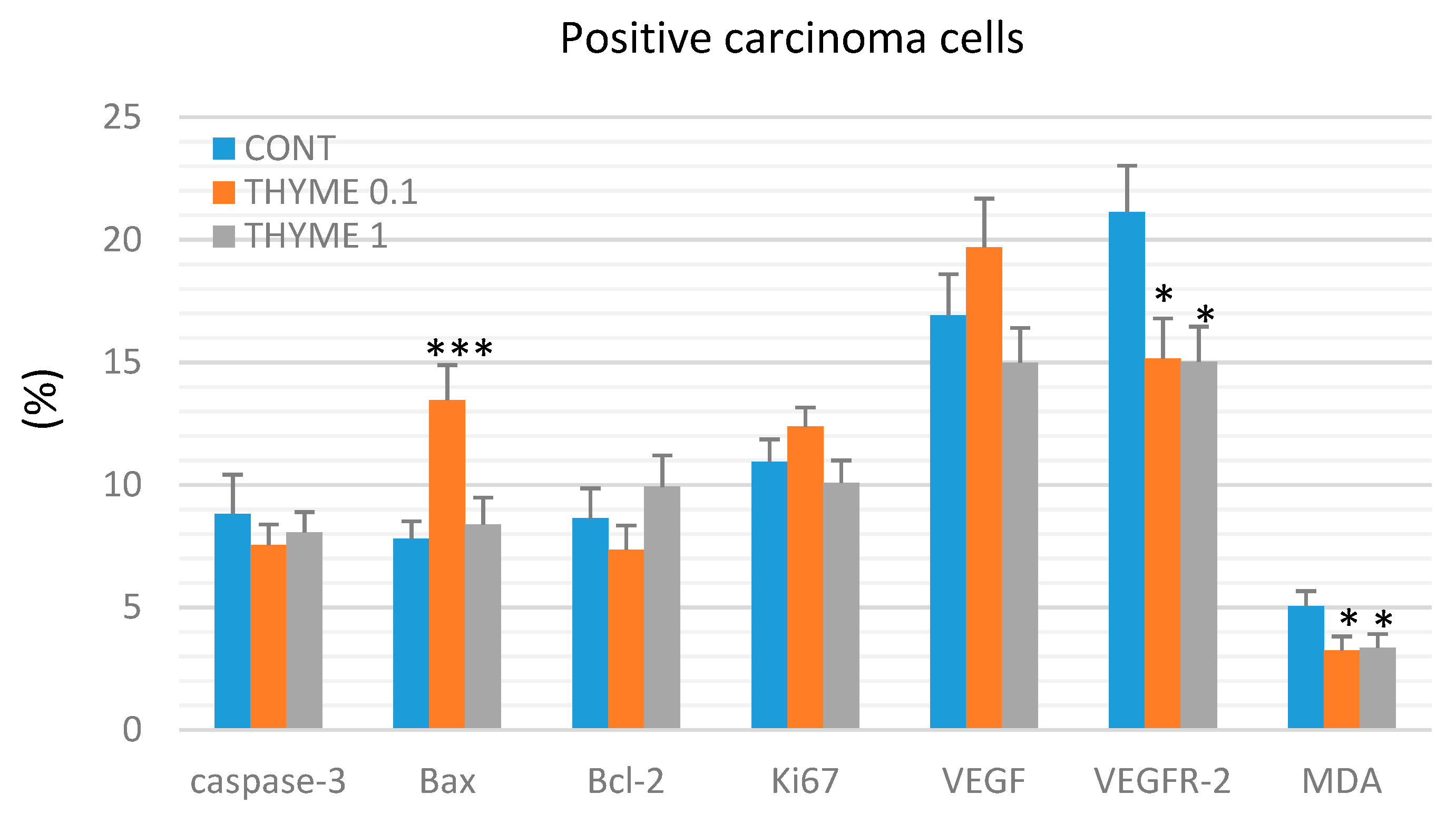

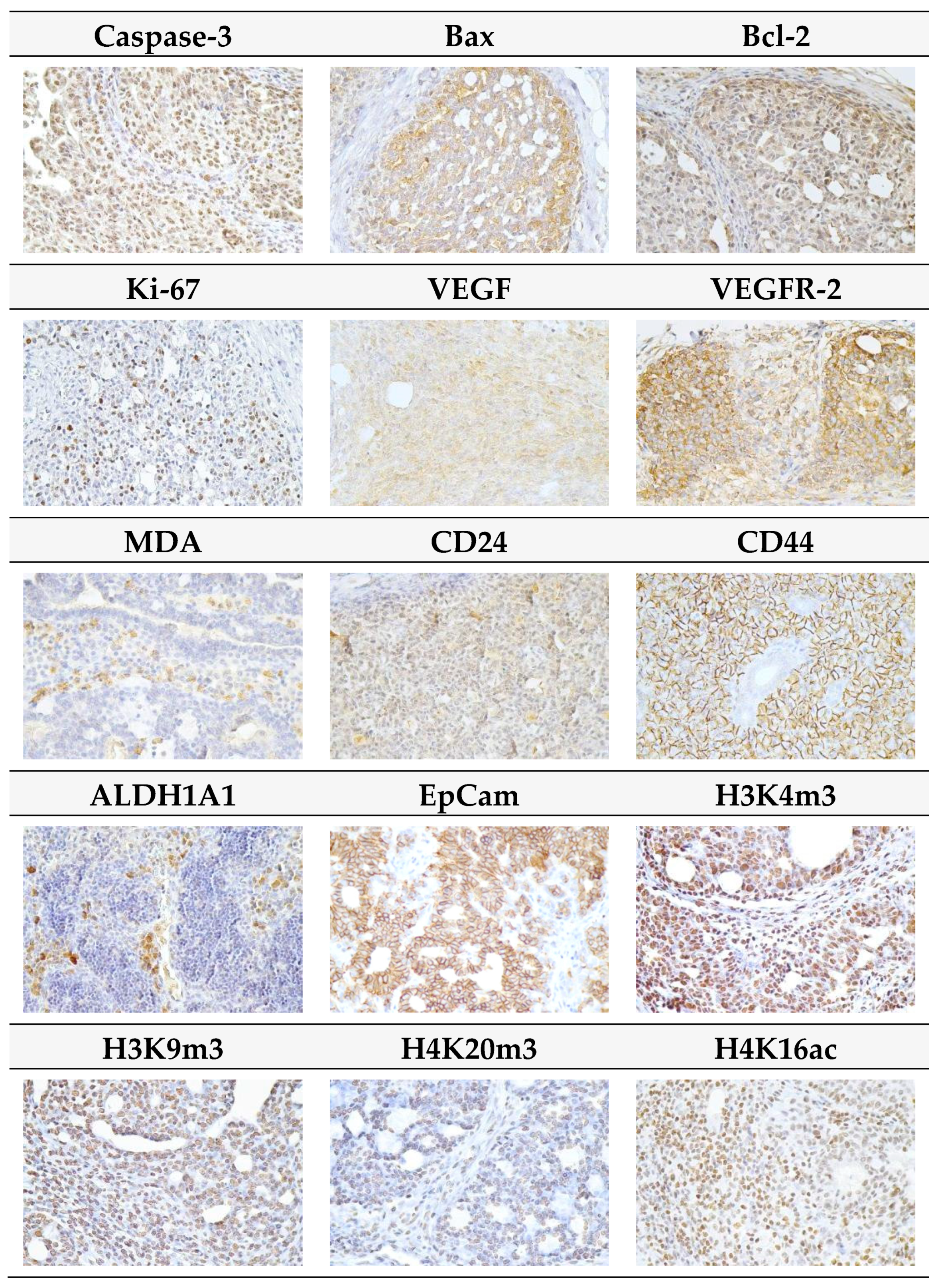
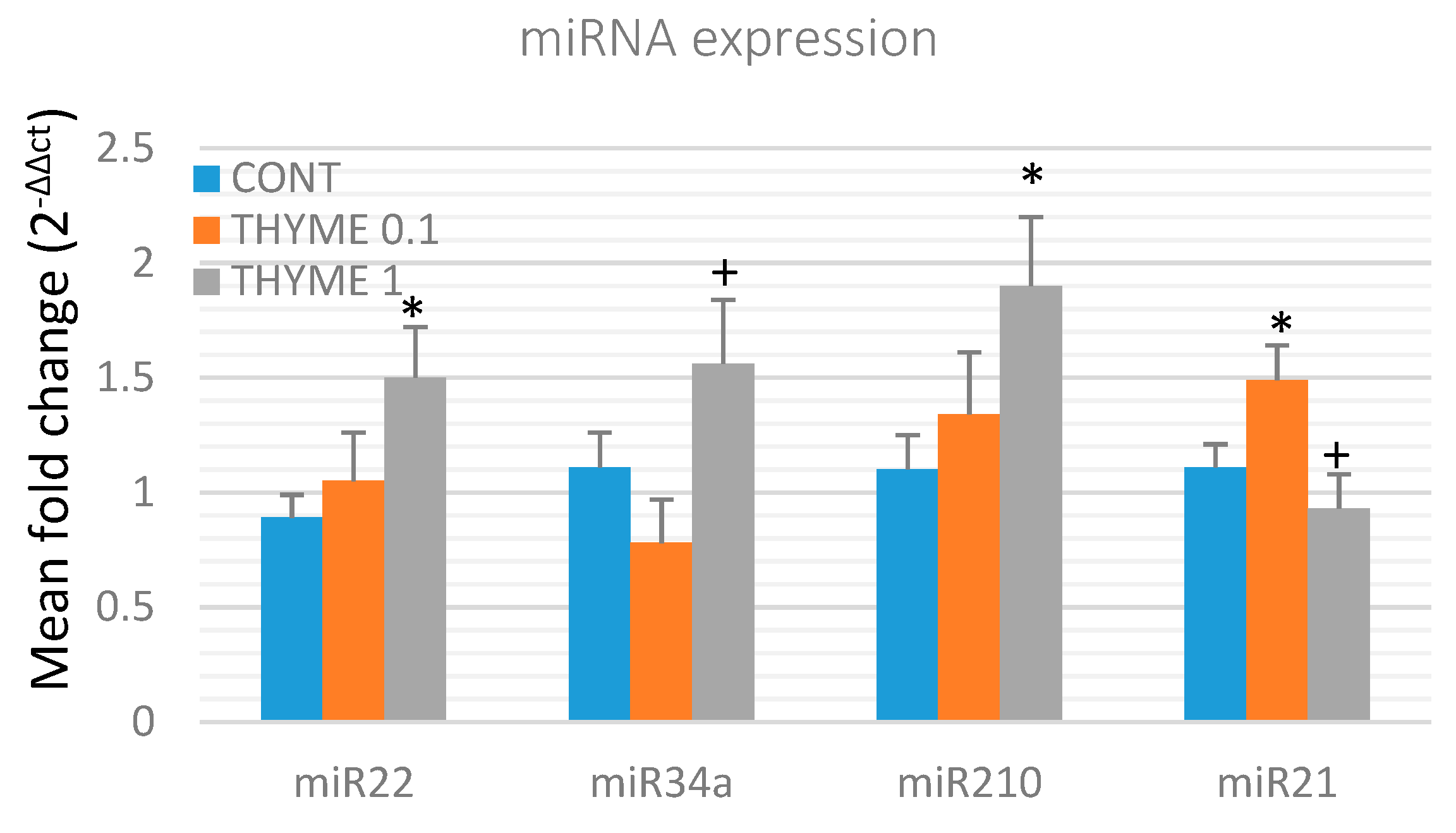





| Group | CONT | THYME 0.1 | THYME 1 |
|---|---|---|---|
| Tumor bearing/all animals | 16/24 | 17/25 | 13/25 |
| Tumor frequency per group * | 1.54 ± 0.38 | 2.00 ± 0.43 (+30%) | 0.72 ± 0.19 a,b (−53%) |
| Tumor latency * (days) | 80.29 ± 5.34 | 88.28 ± 3.89 (+8 days) | 86.15 ± 6.65 (+6 days) |
| Tumor incidence (%) | 66.7 | 68.0 (+2%) | 52.0 (−22%) |
| Average tumor volume * (cm3) | 0.46 ± 0.14 | 0.36 ± 0.09 (−21.5%) | 0.52 ± 0.16 (+13%) |
| Cumulative tumor volume ** (cm3) | 15.81 | 18.19 (+15%) | 8.90 (−43.5%) |
| Parameter | CONT | THYME 0.1 | THYME 1 |
|---|---|---|---|
| Necrosis/all tumor area | 8.71 ± 3.08 | 1.99 ± 1.11 ** | 1.66 ± 1.16 ** |
| Mitotic activity index | 25.15 ± 2.32 | 17.26 ± 1.82 * | 18.82 ± 1.99 * |
| Time (h) | 24 | 48 | 72 | |||
|---|---|---|---|---|---|---|
| Treatment | CONT | EOT | CONT | EOT | CONT | EOT |
| Sub-G0/G1 | 1.59 ± 0.41 | 10.86 ± 0.36 * | 1.73 ± 0.41 | 19.69 ± 1.38 ** | 2.93 ± 0.43 | 23.51 ± 2.75 ** |
| G1 | 70.47 ± 1.55 | 62.53 ± 2.54 * | 68.83 ± 3.73 | 47.80 ± 3.24 ** | 70.53 ± 0.44 | 50.20 ± 3.41 ** |
| S | 14.90 ± 1.41 | 13.83 ± 0.28 | 15.60 ± 1.62 | 18.25 ± 1.35 | 13.50 ± 0.78 | 12.87 ± 1.02 |
| G2/M | 13.03 ± 2.34 | 12.77 ± 0.27 | 13.83 ± 1.00 | 14.25 ± 0.43 | 13.03 ± 2.18 | 13.39 ± 0.41 |
| Time (h) | 24 | 48 | 72 | |||
|---|---|---|---|---|---|---|
| Treatment | CONT | EOT | CONT | EOT | CONT | EOT |
| Sub-G0/G1 | 1.13 ± 0.20 | 2.46 ± 0.43 | 0.54 ± 0.12 | 3.13 ± 0.33 * | 0.70 ± 0.11 | 7.47 ± 0.55 * |
| G1 | 51.60 ± 2.56 | 60.70 ± 0.90 * | 58.80 ± 0.33 | 60.67 ± 3.26 | 64.83 ± 1.84 | 58.25 ± 2.21 |
| S | 21.55 ± 1.38 | 14.10 ± 2.40 * | 19.80 ± 0.85 | 17.57 ± 1.19 | 15.27 ± 0.78 | 17.75 ± 2.07 |
| G2/M | 25.75 ± 1.13 | 22.75 ± 3.75 | 20.87 ± 1.09 | 18.63 ± 1.15 | 19.20 ± 2.25 | 16.55 ± 1.03 |
| Time (h) | 24 | 48 | 72 | |||
|---|---|---|---|---|---|---|
| Treatment | CONT | EOT | CONT | EOT | CONT | EOT |
| An−/PI− | 86.67 ± 2.25 | 61.75 ± 0.42 ** | 71.07 ± 3.01 | 46.90 ± 3.76 ** | 88.73 ± 3.47 | 32.47 ± 3.90 *** |
| An+/PI− | 4.64 ± 0.72 | 29.86 ± 3.75 ** | 8.23 ± 1.20 | 41.13 ± 4.45 *** | 3.57 ± 0.57 | 39.97 ± 2.20 *** |
| An+/PI+ | 3.00 ± 0.53 | 3.59 ± 0.88 | 10.27 ± 0.87 | 7.75 ± 0.73 | 2.53 ± 1.10 | 11.71 ± 2.21 * |
| An−/PI+ | 5.69 ± 0.41 | 4.79 ± 1.24 | 10.43 ± 1.12 | 4.21 ± 1.05 | 5.17 ± 1.06 | 15.84 ± 0.74 * |
| Time (h) | 24 | 48 | 72 | |||
|---|---|---|---|---|---|---|
| Treatment | CONT | EOT | CONT | EOT | CONT | EOT |
| An−/PI− | 88.07 ± 1.43 | 71.97 ± 2.62 * | 92.27 ± 1.40 | 72.60 ± 3.05 ** | 93.47 ± 2.21 | 62.47 ± 1.70 ** |
| An+/PI− | 5.78 ± 0.93 | 17.15 ± 0.91 * | 1.58 ± 0.44 | 19.40 ± 0.25 ** | 1.02 ± 0.12 | 27.15 ± 1.40 ** |
| An+/PI+ | 2.06 ± 0.33 | 4.06 ± 0.37 | 2.18 ± 0.06 | 4.34 ± 0.26 | 1.38 ± 0.17 | 5.35 ± 0.36 * |
| An−/PI+ | 4.12 ± 0.29 | 6.81 ± 1.77 | 3.98 ± 0.24 | 3.66 ± 0.67 | 4.13 ± 0.07 | 5.06 ± 0.85 |
| No. | Compound | Retention Time [min] | Relative Content [%] |
|---|---|---|---|
| 1 | α-Pinene | 9.423 | 2.855 |
| 2 | Camphene | 10.919 | 0.748 |
| 3 | β-Pinene | 12.424 | 0.579 |
| 4 | β-Myrcene | 14.752 | 1.035 |
| 5 | Limonene | 15.957 | 0.742 |
| 6 | Eucalyptol | 16.262 | 0.694 |
| 7 | p-Cymene | 18.875 | 43.108 |
| 8 | Linalool | 28.072 | 4.555 |
| 9 | β-Caryophyllene | 29.682 | 0.947 |
| 10 | α-Terpineol | 32.561 | 1.066 |
| 11 | Borneol | 32.695 | 0.745 |
| 12 | Thymol | 45.154 | 39.769 |
| 13 | Carvacrol | 45.787 | 0.681 |
| Total | - | 97.524 |
| Analyses | Staining Solution | Company |
|---|---|---|
| Cell cycle * | 10% Triton X-100 0.5 mg/ml ribonuclease A 0.025 mg/ml propidium iodide (PI) In 500 µL PBS | Sigma-Aldrich, Steinheim, Germany |
| Apoptosis | Annexin V-Alexa Fluor 647 1:100 | Thermo Scientific, Rockford, IL, USA |
| PI (5mg/ml) 1:500 | Sigma-Aldrich | |
| Mitochondrial membrane potential | TMRE (tetramethylrhodamine ethyl ester per chlorate) final conc. 0.1 µM | Molecular Probes, Eugene, OR, USA |
| Caspase activation | Cleaved Caspase-3 rabbit monoclonal antibody (mAb) phycoerythrin (PE) mAb PE conjugate 1:100 | Cell Signaling, Danvers, MA, USA |
| Cleaved Caspase-7 rabbit mAb PE conjugate 1:100 | ||
| Protein analysis | Cleaved PARP rabbit mAb PE conjugate 1:100 | |
| Bcl-2 mouse mAb PE conjugated 1:100 | ||
| Phospho-Bcl-2 (Ser 70) rabbit mAb Alexa Fluor 488 conjugate 1:200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma In Vivo and In Vitro. Int. J. Mol. Sci. 2019, 20, 1749. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071749
Kubatka P, Uramova S, Kello M, Kajo K, Samec M, Jasek K, Vybohova D, Liskova A, Mojzis J, Adamkov M, et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma In Vivo and In Vitro. International Journal of Molecular Sciences. 2019; 20(7):1749. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071749
Chicago/Turabian StyleKubatka, Peter, Sona Uramova, Martin Kello, Karol Kajo, Marek Samec, Karin Jasek, Desanka Vybohova, Alena Liskova, Jan Mojzis, Marian Adamkov, and et al. 2019. "Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma In Vivo and In Vitro" International Journal of Molecular Sciences 20, no. 7: 1749. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071749