Extracellular l-arginine Enhances Relaxations Induced by Opening of Calcium-Activated SKCa Channels in Porcine Retinal Arteriole
Abstract
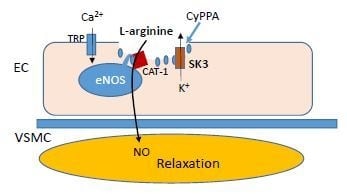
:1. Introduction
2. Results
2.1. l-arginine Uptake in Rat Valve Endothelial Cells
2.2. Localization of eNOS, SKCa3 and CAT-1 Protein
2.3. Pharmacological Activation of SKCa Channels in Porcine Arterial Endothelial Cells
2.4. Effect of l-arginine on Endothelium-Dependent Relaxation
3. Discussion
4. Materials and Methods
4.1. l-arginine Uptake
4.2. Isolation of Pig Eyes
4.3. Immunohistochemistry
4.4. Patch Clamp Experiments in Endothelial Cells
4.5. Functional Studies in Porcine Retinal Arterioles
4.6. Drugs and Solutions
4.7. Data and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| CAT-1 | cationic amino acid transporter |
| CyPPA | cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine |
| EDH | Endothelium-derived hyperpolarization |
| eNOS | Endothelial nitric oxide synthase |
| IKCa | Ca2+-activated K channels with intermediate conductance (KCa3.1) |
| NO | Nitric oxide |
| SKCa | Ca2+-activated K channels with small conductance (KCa2.1-3) |
| UCL1684 | Blocker of Ca2+-activated K channels (KCa2.1-3) |
Appendix A


References
- Bek, T. Inner retinal ischaemia: Current understanding and needs for further investigations. Acta Ophthalmol 2009, 87, 362–367. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Harris, A.; Martin, B.J. Ocular perfusion and age-related macular degeneration. Acta Ophthalmol. Scand. 2001, 79, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohner, E.M.; Patel, V.; Rassam, S.M.B. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes 1995, 44, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.; Bek, T. Preserved Pressure Autoregulation but Disturbed Cyclo-Oxygenase and Nitric Oxide Effects on Retinal Arterioles during Acute Hypoxia in Diabetic Patients without Retinopathy. Ophthalmologica 2016, 235, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Delles, C.; Michelson, G.; Harazny, J.; Oehmer, M.; Hilgers, K.F.; Schmieder, R.E. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke 2004, 35, 1289–1293. [Google Scholar] [CrossRef]
- Resch, H.G.; Garhofer, G.; Fuchsjäger-Mayrl, G.; Hommer, A.; Schmetterer, L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009, 87, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Schmetterer, L.; Findl, O.; Fasching, P.; Ferber, W.; Strenn, K.; Breiteneder, H.; Adam, H.; Eichler, H.G.; Wolzt, M. Nitric oxide and ocular blood flow in patients with IDDM. Diabetes 1997, 46, 653–658. [Google Scholar] [CrossRef]
- Burnham, M.P.; Bychkov, R.; Félétou, M.; Richards, G.R.; Vanhoutte, P.M.; Weston, A.H.; Edwards, G. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: Relevance to EDHF. Br. J. Pharmacol. 2002, 135, 1133–1143. [Google Scholar] [CrossRef]
- Bychkov, R.; Burnham, M.P.; Richards, G.R.; Edwards, G.; Weston, A.H.; Félétou, M.; Vanhoutte, P.M. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+channel in porcine coronary endothelium: Relevance to EDHF. Br. J. Pharmacol. 2002, 137, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Eichler, I.; Wibawa, J.; Grgic, I.; Knorr, A.; Brakemeier, S.; Pries, A.R.; Hoyer, J.; Köhler, R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br. J. Pharmacol. 2003, 138, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.P.H.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Other ion channels. Br. J. Pharmacol. 2017, 174, S195–S207. [Google Scholar] [CrossRef] [Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease – a 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Kroigaard, C.; Bek, T.; Simonsen, U. Role of calcium-activated potassium channels with small conductance in bradykinin-induced vasodilation of porcine retinal arterioles. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3819–3825. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Kroigaard, C.; Misfeldt, M.; Bek, T.; Simonsen, U. Openers of small conductance calcium-activated potassium channels selectively enhance NO-mediated bradykinin vasodilatation in porcine retinal arterioles. Br. J. Pharmacol. 2010, 160, 1496–1508. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, P.; Aalkjaer, C.; Bek, T. Bradykinin relaxation in small porcine retinal arterioles. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1891–1896. [Google Scholar]
- Kulkarni, P.; Cai, J.; Hurst, H.E. Lipids and nitric oxide in porcine retinal and choroidal blood vessels. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2002, 18, 265–275. [Google Scholar] [CrossRef]
- Nakazawa, T.; Kaneko, Y.; Mori, A.; Saito, M.; Sakamoto, K.; Nakahara, T.; Ishii, K. Attenuation of nitric oxide- and prostaglandin-independent vasodilation of retinal arterioles induced by acetylcholine in streptozotocin-treated rats. Vascul. Pharmacol. 2007, 46, 153–159. [Google Scholar] [CrossRef]
- Hein, T.W.; Rosa, R.H.; Yuan, Z.; Roberts, E.; Kuo, L. Divergent roles of nitric oxide and rho kinase in vasomotor regulation of human retinal arterioles. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1583–1590. [Google Scholar] [CrossRef]
- Pournaras, C.J.; Rungger-Brändle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef]
- Sheng, J.Z.; Ella, S.; Davis, M.J.; Hill, M.A.; Braun, A.P. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009, 23, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Stankevičius, E.; Lopez-Valverde, V.; Rivera, L.; Hughes, A.D.; Mulvany, M.J.; Simonsen, U. Combination of Ca 2+-activated K + channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery. Br. J. Pharmacol. 2006, 149, 560–572. [Google Scholar] [CrossRef]
- Stankevicius, E.; Dalsgaard, T.; Kroigaard, C.; Beck, L.; Boedtkjer, E.; Misfeldt, M.W.; Nielsen, G.; Schjorring, O.; Hughes, A.; Simonsen, U. Opening of Small and Intermediate Calcium-Activated Potassium Channels Induces Relaxation Mainly Mediated by Nitric-Oxide Release in Large Arteries and Endothelium-Derived Hyperpolarizing Factor in Small Arteries from Rat. J. Pharmacol. Exp. Ther. 2011, 339, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Gaete, P.S.; Lillo, M.A.; Ardiles, N.M.; Pérez, F.R.; Figueroa, X.F. Ca2+-activated K+channels of small and intermediate conductance control eNOS activation through NAD(P)H oxidase. Free Radic. Biol. Med. 2012, 52, 860–870. [Google Scholar] [CrossRef]
- Parnell, M.M.; Chin-Dusting, J.P.F.; Starr, J.; Kaye, D.M. In vivo and in vitro evidence for ACh-stimulated 3H-arginine uptake. Am. J. Physiol. Circ. Physiol. 2004, 287, H395–H400. [Google Scholar] [CrossRef]
- Shin, S.; Mohan, S.; Fung, H.L. Intracellular l-arginine concentration does not determine NO production in endothelial cells: Implications on the l-arginine paradox’. Biochem. Biophys. Res. Commun. 2011, 414, 660–663. [Google Scholar] [CrossRef]
- Delgado, E.; Marques-Neves, C.; Rocha, I.; Sales-Luís, J.; Silva-Carvalho, L. L-Arginine and L-Nitroarginine Methylester Effects on Vasomotion in Isolated Rabbit Eyes. Ophthalmic. Res. 2009, 43, 113–121. [Google Scholar] [CrossRef]
- Chin-Dusting, J.P.F.; Willems, L.; Kaye, D.M. l-Arginine transporters in cardiovascular disease: A novel therapeutic target. Pharmacol. Ther. 2007, 116, 428–436. [Google Scholar] [CrossRef]
- Zani, B.G.; Bohlen, H.G. Transport of extracellular 3H-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am. J. Physiol. Circ. Physiol. 2005, 289, H1381–H1390. [Google Scholar] [CrossRef]
- Bogle, R.G.; Coade, S.B.; Moncada, S.; Pearson, J.D.; Mann, G.E. Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells. Biochem. Biophys. Res. Commun. 1991, 180, 926–932. [Google Scholar] [CrossRef]
- Zharikov, S.I.; Herrera, H.; Block, E.R. Role of membrane potential in hypoxic inhibition of L-arginine uptake by lung endothelial cells. Am. J. Physiol. 1997, 272, L78–L84. [Google Scholar] [CrossRef]
- Hougaard, C.; Eriksen, B.L.; Jørgensen, S.; Johansen, T.H.; Dyhring, T.; Madsen, L.S.; Strøbæk, D.; Christophersen, P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+channels. Br. J. Pharmacol. 2007, 151, 655–665. [Google Scholar] [CrossRef]
- Campos Rosa, J.; Galanakis, D.; Piergentili, A.; Bhandari, K.; Ganellin, C.R.; Dunn, P.M.; Jenkinson, D.H. Synthesis, molecular modeling, and pharmacological testing of bis-quinolinium cyclophanes: Potent, non-peptidic blockers of the apamin-sensitive Ca(2+)-activated K(+) channel. J. Med. Chem. 2000, 43, 420–431. [Google Scholar] [CrossRef]
- Garcia-Cardena, G.; Oh, P.; Liu, J.; Schnitzer, J.E.; Sessa, W.C. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling. Proc. Natl. Acad. Sci. USA 1996, 93, 6448–6453. [Google Scholar] [CrossRef]
- García-Cardeña, G.; Martasek, P.; Masters, B.S.S.; Skidd, P.M.; Couet, J.; Li, S.; Lisanti, M.P.; Sessa, W.C. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997, 272, 25437–25440. [Google Scholar] [CrossRef]
- Shaul, P.W.; Smart, E.J.; Robinson, L.J.; German, Z.; Yuhanna, I.S.; Ying, Y.; Anderson, R.G.W.; Michel, T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996, 271, 6518–6522. [Google Scholar] [CrossRef]
- Frank, P.G.; Woodman, S.E.; Park, D.S.; Lisanti, M.P. Caveolin, caveolae, and endothelial cell function. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1161–1168. [Google Scholar] [CrossRef]
- Mann, G.E.; Yudilevich, D.L.; Sobrevia, L. Regulation of Amino Acid and Glucose Transporters in Endothelial and Smooth Muscle Cells. Physiol. Rev. 2003, 83, 183–252. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Huang, W.; Harris, M.B.; Goolsby, J.M.; Venema, R.C. Interaction of the endothelial nitric oxide synthase with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport. Biochem. J. 2005, 386, 567–574. [Google Scholar] [CrossRef] [Green Version]
- McDonald, K.K.; Zharikov, S.; Block, E.R.; Kilberg, M.S. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the ‘arginine paradox’. J. Biol. Chem. 1997, 272, 31213–31216. [Google Scholar] [CrossRef]
- Hucks, D.; Khan, N.M.; Ward, J.P.T. Essential role of L-arginine uptake and protein tyrosine kinase activity for NO-dependent vasorelaxation induced by stretch, isometric tension and cyclic AMP in rat pulmonary arteries. Br. J. Pharmacol. 2000, 131, 1475–1481. [Google Scholar] [CrossRef] [Green Version]
- Hucks, D.; Ward, J.P.T. Critical dependence of the NO-mediated component of cyclic AMP-induced vasorelaxation on extracellular L-arginine in pulmonary arteries of the rat. Br. J. Pharmacol. 2000, 130, 997–1004. [Google Scholar] [CrossRef] [Green Version]
- Goetz, R.M.; Thatte, H.S.; Prabhakar, P.; Cho, M.R.; Michel, T.; Golan, D.E. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 2788–2793. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, P.; Thatte, H.S.; Goetz, R.M.; Cho, M.R.; Golan, D.E.; Michel, T. Receptor-regulated translocation of endothelial nitric-oxide synthase. J. Biol. Chem. 1998, 273, 27383–27388. [Google Scholar] [CrossRef]
- Böger, R.H. L-Arginine therapy in cardiovascular pathologies: Beneficial or dangerous? Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Stankevicius, E.; Martinez, A.C.; Mulvany, M.J.; Simonsen, U. Blunted acetylcholine relaxation and nitric oxide release in arteries from renal hypertensive rats. J. Hypertens. 2002, 20, 1571–1579. [Google Scholar] [CrossRef]
- Kaya, M.Y.; Petersen, L.; Bek, T. Lack of effect of nitroglycerin on the diameter response of larger retinal arterioles in normal persons during hypoxia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 277–283. [Google Scholar] [CrossRef]
- Andersen, M.R.; Simonsen, U.; Uldbjerg, N.; Aalkjær, C.; Stender, S. Smoking cessation early in pregnancy and birth weight, length, head circumference, and endothelial nitric oxide synthase activity in umbilical and chorionic vessels. An observational study of healthy singleton pregnancies. Circulation 2009, 119, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Oliván-Viguera, A.O.; Valero, M.S.; Murillo, M.D.; Wulff, H.; García-Otín, A.L.; Arbonés-Mainar, J.M.; Köhler, R. Novel phenolic inhibitors of small/intermediate-conductance Ca2+-activated K+ channels, KCa3.1 and KCa2.3. PLoS ONE 2013, 8, e58614. [Google Scholar] [CrossRef]
- Hessellund, A.; Jeppesen, P.; Aalkjaer, C.; Bek, T. Characterization of vasomotion in porcine retinal arterioles. Acta Ophthalmol. Scand. 2003, 81, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernanz, R.; Alonso, M.J.; Zibrandtsen, H.; Alvarez, Y.; Salaices, M.; Simonsen, U. Measurements of nitric oxide concentration and hyporeactivity in rat superior mesenteric artery exposed to endotoxin. Cardiovasc. Res. 2004, 62, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonsen, U.; Winther, A.K.; Oliván-Viguera, A.; Comerma-Steffensen, S.; Köhler, R.; Bek, T. Extracellular l-arginine Enhances Relaxations Induced by Opening of Calcium-Activated SKCa Channels in Porcine Retinal Arteriole. Int. J. Mol. Sci. 2019, 20, 2032. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20082032
Simonsen U, Winther AK, Oliván-Viguera A, Comerma-Steffensen S, Köhler R, Bek T. Extracellular l-arginine Enhances Relaxations Induced by Opening of Calcium-Activated SKCa Channels in Porcine Retinal Arteriole. International Journal of Molecular Sciences. 2019; 20(8):2032. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20082032
Chicago/Turabian StyleSimonsen, Ulf, Anna K. Winther, Aida Oliván-Viguera, Simon Comerma-Steffensen, Ralf Köhler, and Toke Bek. 2019. "Extracellular l-arginine Enhances Relaxations Induced by Opening of Calcium-Activated SKCa Channels in Porcine Retinal Arteriole" International Journal of Molecular Sciences 20, no. 8: 2032. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20082032