Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas aeruginosa Chronic Lung Infection
Abstract
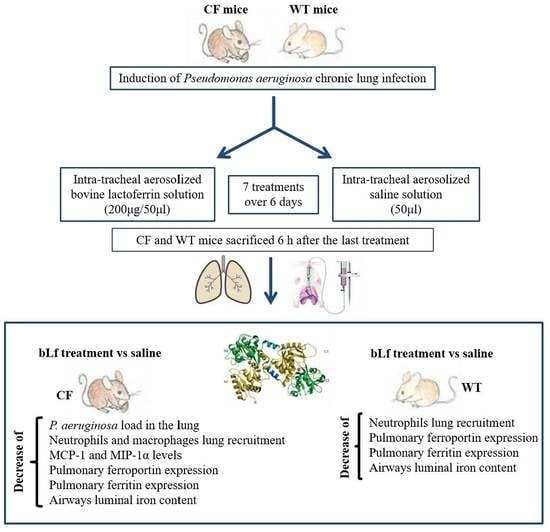
:1. Introduction
2. Results
2.1. Effect of Aerosolized bLf on Body Weight in Mice Infected by P. aeruginosa
2.2. Effect of Aerosolized bLf on Bacterial Growth in Murine Lung Infected by P. aeruginosa
2.3. Effect of Aerosolized bLf on Inflammatory Response in Murine Lung Infected by P. aeruginosa
2.4. Effect of Aerosolized bLf on Proteins of the Iron Homeostasis System of Mice Infected by P. aeruginosa
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain, Media, and Culture Conditions
4.2. Lactoferrin
4.3. Animals, Chronic Infection and Treatments
4.4. Broncho-alveolar Lavage (BAL) Fluid Collection and Analysis
4.5. Lung Homogenates
4.6. Cytokine Analysis
4.7. Western Blot Analysis
4.8. Determination of Total Iron in BALF
4.9. Statistical Analysis
4.10. Ethic Statement
Author Contributions
Funding
Conflicts of Interest
References
- Ranganathan, S.C.; Parsons, F.; Gangell, C.; Brennan, B.; Stick, S.M.; Sly, P.D. Australian Respiratory Early Surveillance Team for Cystic Fibrosis. Evolution of pulmonary inflammation and nutritional status in infants and young children with cystic fibrosis. Thorax 2011, 66, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Salsgiver, E.L.; Fink, A.K.; Knapp, E.A.; LiPuma, J.J.; Olivier, K.N.; Marshall, B.C.; Saiman, L. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest 2016, 149, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Lipuma, J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef]
- Hogardt, M.; Heesemann, J. Microevolution of Pseudomonas aeruginosa to a chronic pathogen of the cystic fibrosis lung. Curr. Top Microbiol. Immunol. 2013, 358, 91–118. [Google Scholar] [PubMed]
- Emerson, J.; Rosenfeld, M.; McNamara, S.; Ramsey, B.; Gibson, R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 91–100. [Google Scholar] [CrossRef]
- Dakin, C.J.; Numa, A.H.; Wang, H.; Morton, J.R.; Vertzyas, C.C.; Henry, R.L. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002, 165, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, C.; Remouchamps, C.; Hennuy, B.; Vanderplasschen, A.; Chariot, A.; Tabruyn, S.P.; Oury, C.; Bours, V. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochem. Pharmacol. 2007, 73, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Bragonzi, A.; Horati, H.; Kerrigan, L.; Lorè, N.I.; Scholte, B.J.; Weldon, S. Inflammation and host-pathogen interaction: Cause and consequence in cystic fibrosis lung disease. J. Cyst. Fibros. 2017, 17, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Chmiel, J.; Berger, M. Chronic inflammation in the cystic fibrosis lung: Alterations in inter- and intracellular signaling. Clin. Rev. Allergy Immunol. 2008, 34, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Cockx, M.; Gouwy, M.; Van Damme, J.; Struyf, S. Chemoattractants and cytokines in primary ciliary dyskinesia and cystic fibrosis: Key players in chronic respiratory diseases. Cell Mol. Immunol. 2018, 15, 312–323. [Google Scholar] [CrossRef]
- Reid, D.W.; Withers, N.J.; Francis, L.; Wilson, J.W.; Kotsimbos, T.C. Iron deficiency in cystic fibrosis: Relationship to lung disease severity and chronic Pseudomonas aeruginosa infection. Chest 2002, 121, 48–54. [Google Scholar] [CrossRef]
- Reid, D.W.; Lam, Q.T.; Schneider, H.; Walters, E.H. Airway iron and iron-regulatory cytokines in cystic fibrosis. Eur. Respir. J. 2004, 24, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, D.W.; Carroll, V.; O’May, C.; Champion, A.; Kirov, S.M. Increased airway iron as a potential factor in the persistence of Pseudomonas aeruginosa infection in cystic fibrosis. Eur. Respir. J. 2007, 30, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Anderson, G.J.; Bell, S.C.; Reid, D.W. Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity. J. Cyst. Fibros. 2014, 13, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, D.M.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Roggli, V.L.; Soukup, J.M.; Richards, J.H.; Randell, S.H.; Muhlebach, M.S. Iron accumulates in the lavage and explanted lungs of cystic fibrosis patients. J. Cyst. Fibros. 2013, 12, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Morea, C.; Battistoni, A.; Sarli, S.; Cipriani, P.; Superti, F.; Ammendolia, M.G.; Valenti, P. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2005, 18, 661–670. [Google Scholar] [CrossRef]
- Gómez, M.I.; Prince, A. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr. Opin. Pharmacol. 2007, 7, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Gifford, A.H.; Moulton, L.A.; Dorman, D.B.; Olbina, G.; Westerman, M.; Parker, H.W.; Stanton, B.A.; O’Toole, G.A. Iron homeostasis during cystic fibrosis pulmonary exacerbation. Clin. Transl. Sci. 2012, 5, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Catizone, A.; Pantanella, F.; Frioni, A.; Natalizi, T.; Tendini, M.; Berlutti, F. Lactoferrin decreases inflammatory response by cystic fibrosis bronchial cells invaded with Burkholderia cenocepacia iron-modulated biofilm. Int. J. Immunopathol. Pharmacol. 2011, 24, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin differently modulates the inflammatory response in epithelial models mimicking human inflammatory and infectious diseases. Biometals 2014, 27, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Latorre, D.; Berlutti, F.; Valenti, P.; Gessani, S.; Puddu, P. LF immuno-modulatory strategies: Mastering bacterial endotoxin. Biochem. Cell Biol. 2012, 90, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Schippa, S.; Morea, C.; Sarli, S.; Perfetto, B.; Donnarumma, G.; Valenti, P. Lactoferrin down-regulates pro-inflammatory cytokines upexpressed in intestinal epithelial cells infected with invasive or noninvasive Escherichia coli strains. Biochem. Cell. Biol. 2006, 84, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Superti, F.; Nicoletti, M.; Morea, C.; Frioni, A.; Ammendolia, M.G.; Battistoni, A.; Valenti, P. Bovine lactoferrin inhibits the efficiency of invasion of respiratory A549 cells of different iron-regulated morphological forms of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2008, 21, 51–59. [Google Scholar] [CrossRef]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Rosa, L.; Cutone, A.; Frioni, A.; Berlutti, F.; Paesano, R.; Valenti, P. Effect of bovine lactoferrin on Chlamydia trachomatis infection and inflammation. Biochem. Cell Biol. 2017, 95, 34–40. [Google Scholar] [CrossRef]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef]
- Puddu, P.; Latorre, D.; Carollo, M.; Catizone, A.; Ricci, G.; Valenti, P.; Gessani, S. Bovine lactoferrin counteracts Toll-like receptor mediated activation signals in antigen presenting cells. PLoS ONE 2011, 6, e22504. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi di Patti, M.C.; Cutone, A.; Polticelli, F.; Rosa, L.; Lepanto, M.S.; Valenti, P.; Musci, G. The ferroportin-ceruloplasmin system and the mammalian iron homeostasis machine: Regulatory pathways and the role of lactoferrin. Biometals 2018, 31, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Frioni, A.; Berlutti, F.; Valenti, P.; Musci, G.; Bonaccorsi di Patti, M.C. Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals 2014, 27, 807–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin efficiently counteracts the inflammation-induced changes of the iron homeostasis system in macrophages. Front. Immunol. 2017, 15, 705. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Frioni, A.; Rossi, A.; Ranucci, S.; De Fino, I.; Cutone, A.; Luigi, R.; Bragonzi, A.; Berlutti, F. Aerosolized bovine lactoferrin reduces neutrophils and pro-inflammatory cytokines in mouse models of Pseudomonas aeruginosa lung infections. Biochem. Cell Biol. 2017, 95, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Facchini, M.; De Fino, I.; Riva, C.; Bragonzi, A. Long term chronic Pseudomonas aeruginosa airway infection in mice. J. Vis. Exp. 2014, 85. [Google Scholar] [CrossRef]
- van Heeckeren, A.M.; Schluchter, M.D.; Xue, W.; Davis, P.B. Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am. J. Respir. Crit. Care Med. 2006, 173, 288–296. [Google Scholar] [CrossRef]
- Waters, V.; Smyth, A. Cystic fibrosis microbiology: Advances in antimicrobial therapy. J. Cyst. Fibros. 2015, 14, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef]
- Vidya, P.; Smith, L.; Beaudoin, T.; Yau, Y.C.; Clark, S.; Coburn, B.; Guttman, D.S.; Hwang, D.M.; Waters, V. Chronic infection phenotypes of Pseudomonas aeruginosa are associated with failure of eradication in children with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 67–74. [Google Scholar] [CrossRef]
- Ghio, A.J.; Fracica, P.J.; Young, S.L.; Piantadosi, C.A. Synthetic surfactant scavenges oxidants and protects against hyperoxic lung injury. J. Appl. Physiol. (1985) 1994, 77, 1217–1223. [Google Scholar] [CrossRef]
- Louie, S.; Arata, M.A.; Offerdahl, S.D.; Halliwell, B. Effect of tracheal insufflation of deferoxamine on acute ozone toxicity in rats. J. Lab. Clin. Med. 1993, 121, 502–509. [Google Scholar]
- Manzoni, P.; Meyer, M.; Stolfi, I.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Decembrino, L.; Laforgia, N.; et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial. Early Hum. Dev. 2014, 1, S60–S65. [Google Scholar] [CrossRef]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [CrossRef]
- Paesano, R.; Pietropaoli, M.; Berlutti, F.; Valenti, P. Bovine lactoferrin in preventing preterm delivery associated with sterile inflammation. Biochem. Cell Biol. 2012, 90, 468–475. [Google Scholar] [CrossRef]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: An interventional study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of lactoferrin oral administration in the treatment of anemia and anemia of inflammation in pregnant and non-pregnant women: An interventional study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Standiford, T.J.; Kunkel, S.L.; Lukacs, N.W.; Greenberger, M.J.; Danforth, J.M.; Kunkel, R.G.; Strieter, R.M. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J. Immunol. 1995, 155, 1515–1524. [Google Scholar]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Rao, S.; Wright, A.K.A.; Montiero, W.; Ziegler-Heitbrock, L.; Grigg, J. Monocyte chemoattractant chemokines in cystic fibrosis. J. Cyst. Fibros. 2009, 8, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Ghio, A.J. Disruption of iron homeostasis and lung disease. Biochim. Biophys. Acta 2009, 1790, 731–739. [Google Scholar] [CrossRef]
- Heilig, E.A.; Thompson, K.J.; Molina, R.M.; Ivanov, A.R.; Brain, J.D.; Wessling-Resnick, M. Manganese and iron transport across pulmonary epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L1247–L1259. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Peng, H.; Gelbart, T.; Wang, L.; Beutler, E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc. Natl. Acad. Sci. USA 2005, 102, 1906–1910. [Google Scholar] [CrossRef] [Green Version]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef] [Green Version]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef]
- Jeukens, J.; Boyle, B.; Bianconi, I.; Kukavica-Ibrulj, I.; Tümmler, B.; Bragonzi, A.; Levesque, R.C. Complete Genome Sequence of Persistent Cystic Fibrosis Isolate Pseudomonas aeruginosa Strain RP73. Genome Announc. 2013, 1. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Scotti, M.J.; Conte, M.P.; Paesano, R.; Valenti, P. Physico-chemical properties influence the functions and efficacy of commercial bovine lactoferrins. Biometals 2018, 31, 301–312. [Google Scholar] [CrossRef]
- Bianconi, I.; Jeukens, J.; Freschi, L.; Alcalá-Franco, B.; Facchini, M.; Boyle, B.; Molinaro, A.; Kukavica-Ibrulj, I.; Tümmler, B.; Levesque, R.C.; et al. Comparative genomics and biological characterization of sequential Pseudomonas aeruginosa isolates from persistent airways infection. BMC Genomics 2015, 16, 1105. [Google Scholar] [CrossRef]
- Cigana, C.; Lorè, N.I.; Riva, C.; De Fino, I.; Spagnuolo, L.; Sipione, B.; Rossi, G.; Nonis, A.; Cabrini, G.; Bragonzi, A. Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections. Sci Rep. 2016, 6, 21465. [Google Scholar] [CrossRef]
- van Heeckeren, A.M. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 2002, 36, 291–312. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.L.; Tran, L.; Winters, A.; Lee, K.J.; Plewa, C.; Foltz, I.; King, C.; Miranda, L.P.; Allen, J.; Beckman, H.; et al. Molecular mechanism of hepcidin-mediated ferroportin internalization requires ferroportin lysines, not tyrosines or JAK-STAT. Cell Metab. 2012, 15, 905–917. [Google Scholar] [CrossRef]






| Cytokine/chemokine (pg/mL) | WT | WT bLf | CF | CF bLf |
|---|---|---|---|---|
| IL-1α | 95 ± 22 | 78±45 | 167 ± 93 | 110 ± 82 |
| IL-1β | 130 ± 32 | 119 ± 40 | 175 ± 65 | 124 ± 66 |
| IL-2 | ND | ND | ND | ND |
| IL-3 | 3 ± 1 | 3 ± 2 | 3 ± 1 | 3 ± 2 |
| IL-4 | ND | ND | ND | ND |
| IL-5 | 3 ± 1 | 6 ± 2 | 5 ± 2 | 5 ± 1 |
| IL-6 | 9 ± 5 | 6 ± 4 | 11 ± 4 | 9 ± 4 |
| IL-9 | ND | ND | ND | ND |
| IL-10 | 12 ± 3 | 10 ± 4 | 17 ± 4 | 12 ± 4 |
| IL-12(p40) | 10 ± 6 | 16 ± 9 | 7 ± 1 | 9 ± 4 |
| IL-12(p70) | 69 ± 14 | 64 ± 18 | 105 ± 35 | 71 ± 43 |
| IL-13 | 77 ± 15 | 83 ± 17 | 92 ± 13 | 78 ± 10 |
| IL-17 | 11 ± 3 | 14 ± 8 | 5 ± 2 | 11 ± 6 |
| Eotaxin | 540 ± 167 | 417 ± 93 | 612 ± 149 | 390 ± 120 |
| G-CSF | 301 ± 216 | 134 ± 162 | 479 ± 252 | 283 ± 225 |
| GM-CSF | ND | ND | ND | ND |
| IFN-γ | 11 ± 1 | 12 ± 5 | 11 ± 2 | 11 ± 5 |
| KC | 90 ± 50 | 67 ± 29 | 131 ± 63 | 79 ± 41 |
| RANTES | 42 ± 44 | 63 ± 26 | 20 ± 21 | 40 ± 14 |
| TNF-α | 35 ± 14 | 33 ± 9 | 52 ± 22 | 38 ± 23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.J.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas aeruginosa Chronic Lung Infection. Int. J. Mol. Sci. 2019, 20, 2128. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092128
Cutone A, Lepanto MS, Rosa L, Scotti MJ, Rossi A, Ranucci S, De Fino I, Bragonzi A, Valenti P, Musci G, et al. Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas aeruginosa Chronic Lung Infection. International Journal of Molecular Sciences. 2019; 20(9):2128. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092128
Chicago/Turabian StyleCutone, Antimo, Maria Stefania Lepanto, Luigi Rosa, Mellani Jinnett Scotti, Alice Rossi, Serena Ranucci, Ida De Fino, Alessandra Bragonzi, Piera Valenti, Giovanni Musci, and et al. 2019. "Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas aeruginosa Chronic Lung Infection" International Journal of Molecular Sciences 20, no. 9: 2128. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092128