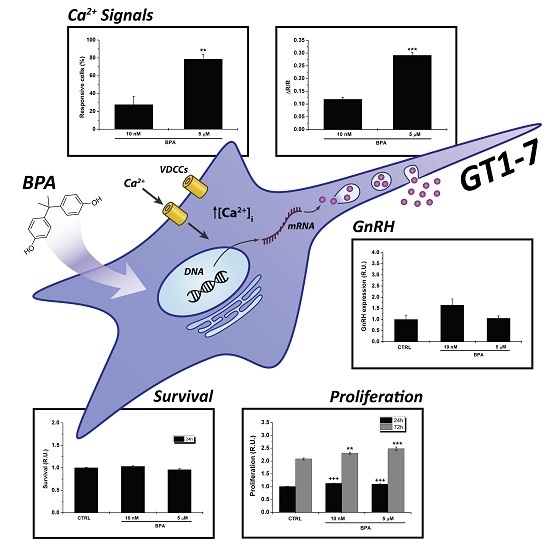
Bisphenol A Activates Calcium Influx in Immortalized GnRH Neurons
Abstract
:1. Introduction
2. Results
2.1. Effects of BPA on Proliferation of GT1–7 Cells
2.2. BPA Does Not Affect GnRH Expression
2.3. BPA Induces Changes in [Ca2+]i
2.4. Changes in [Ca2+]i Induced by E2
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Survival and Proliferation Assays
4.4. GnRH Expression Analysis
4.5. Calcium Imaging
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Matthews, J.B.; Twomey, K.; Zacharewski, T.R. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem. Res. Toxicol. 2001, 14, 149–157. [Google Scholar] [CrossRef]
- Welshons, W.V.; Nagel, S.C.; vom Saal, F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006, 147, S56–S69. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef]
- Shelnutt, S.; Kind, J.; Allaben, W. Bisphenol A: Update on newly developed data and ho w they address NTP’s 2008 finding of “Some Concern”. Food Chem. Toxicol. 2013, 57, 284–295. [Google Scholar] [CrossRef]
- Hines, E.P.; Mendola, P.; von Ehrenstein, O.S.; Ye, X.; Calafat, A.M.; Fenton, S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2014, 54, 120–128. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Akingbemi, B.T.; Belcher, S.M.; Birnbaum, L.S.; Crain, D.A.; Eriksen, M.; Farabollini, F.; Guillette, L.J., Jr.; Hauser, R.; Heindel, J.J.; et al. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007, 24, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, L.N.; Hunt, P.A.; Myers, J.P.; Vom Saal, F.S. Human exposures to bisphenol A: Mismatches between data and assumptions. Rev. Environ. Health 2013, 28, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.S.; Leung, A.O.W.; Wong, M.H. The association of environmental toxicants and autism spectrum disorders in children. Environ. Pollut. 2017, 227, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D. Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas 2010, 66, 236–241. [Google Scholar] [CrossRef]
- Braun, J.M.; Hauser, R. Bisphenol A and children’s health. Curr. Opin. Pediatr. 2011, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Inadera, H. Neurological Effects of Bisphenol A and its Analogues. Int. J. Med. Sci. 2015, 12, 926–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Hajszan, T.; Leranth, C. Bisphenol A interferes with synaptic remodeling. Front. Neuroendocrinol. 2010, 31, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Matsuyoshi, C.; Miyazaki, W.; Benner, S.; Hosokawa, M.; Yokoyama, K.; Kakeyama, M.; Tohyama, C. Prenatal exposure to bisphenol A impacts neuronal morphology in the hippocampal CA1 region in developing and aged mice. Arch. Toxicol. 2016, 90, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Endo, T.; Kubo, K.; Nakajima, K.; Kakeyama, M.; Tohyama, C. In Utero Bisphenol A Exposure Induces Abnormal Neuronal Migration in the Cerebral Cortex of Mice. Front. Endocrinol. 2016, 7, 7. [Google Scholar] [CrossRef]
- Liu, Z.H.; Ding, J.J.; Yang, Q.Q.; Song, H.Z.; Chen, X.T.; Xu, Y.; Xiao, G.R.; Wang, H.L. Early developmental bisphenol-A exposure sex-independently impairs spatial memory by remodeling hippocampal dendritic architecture and synaptic transmission in rats. Sci. Rep. 2016, 6, 32492. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Li, T.; Gong, H.; Chen, Z.; Jin, Y.; Xu, G.; Wang, M. Bisphenol A Impairs Synaptic Plasticity by Both Pre- and Postsynaptic Mechanisms. Adv. Sci. (Weinh) 2017, 4, 1600493. [Google Scholar] [CrossRef]
- Inagaki, T.; Smith, N.; Lee, E.K.; Ramakrishnan, S. Low dose exposure to Bisphenol A alters development of gonadotropin-releasing hormone 3 neurons and larval locomotor behavior in Japanese Medaka. Neurotoxicology 2016, 52, 188–197. [Google Scholar] [CrossRef]
- Xu, G.; Hu, F.; Wang, X.; Zhang, B.; Zhou, Y. Bisphenol A exposure perturbs visual function of adult cats by remodeling the neuronal activity in the primary visual pathway. Arch. Toxicol. 2018, 92, 455–468. [Google Scholar] [CrossRef]
- Elsworth, J.D.; Jentsch, J.D.; Groman, S.M.; Roth, R.H.; Redmond, E.D., Jr.; Leranth, C. Low circulating levels of bisphenol-A induce cognitive deficits and loss of asymmetric spine synapses in dorsolateral prefrontal cortex and hippocampus of adult male monkeys. J. Comp. Neurol. 2015, 523, 1248–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Ning, S.; Zhang, Q.; Chen, A.; Jiang, C.; Cui, Y.; Hu, J.; Li, H.; Fan, G.; Qin, L.; et al. Bisphenol A Represses Dopaminergic Neuron Differentiation from Human Embryonic Stem Cells through Downregulating the Expression of Insulin-like Growth Factor 1. Mol. Neurobiol. 2016, 54, 3798–3812. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.K.; Heger, S. Endocrine disrupting chemicals affect the gonadotropin releasing hormone neuronal network. Reprod. Toxicol. 2014, 44, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Uppu, S.; Claville, M.O.; Uppu, R.M. Prooxidant actions of bisphenol A (BPA) phenoxyl radicals: Implications to BPA-related oxidative stress and toxicity. Toxicol. Mech. Methods 2013, 23, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Franssen, D.; Gérard, A.; Hennuy, B.; Donneau, A.F.; Bourguignon, J.P.; Parent, A.S. Delayed Neuroendocrine Sexual Maturation in Female Rats After a Very Low Dose of Bisphenol A Through Altered GABAergic Neurotransmission and Opposing Effects of a High Dose. Endocrinology 2016, 157, 1740–1750. [Google Scholar] [CrossRef]
- Heindel, J.J.; Newbold, R.R.; Bucher, J.R.; Camacho, L.; Delclos, K.B.; Lewis, S.M.; Vanlandingham, M.; Churchwell, M.I.; Twaddle, N.C.; McLellen, M.; et al. NIEHS/FDA CLARITY-BPA research program update. Reprod. Toxicol. 2015, 58, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Patisaul, H.B.; Todd, K.L.; Mickens, J.A.; Adewale, H.B. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 2009, 30, 350–357. [Google Scholar] [CrossRef]
- Bai, Y.; Chang, F.; Zhou, R.; Jin, P.P.; Matsumoto, H.; Sokabe, M.; Chen, L. Increase of anteroventral periventricular kisspeptin neurons and generation of E2-induced LH-surge system in male rats exposed perinatally to environmental dose of bisphenol-A. Endocrinology 2011, 152, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, F.; Bai, Y.; Chen, F.; Zhang, J.; Chen, L. Bisphenol A enhances kisspeptin neurons in anteroventral periventricular nucleus of female mice. J. Endocrinol. 2014, 221, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Naulé, L.; Picot, M.; Martini, M.; Parmentier, C.; Hardin-Pouzet, H.; Keller, M.; Franceschini, I.; Mhaouty-Kodja, S. Neuroendocrine and behavioral effects of maternal exposure to oral bisphenol A in female mice. J. Endocrinol. 2014, 220, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adewale, H.B.; Jefferson, W.N.; Newbold, R.R.; Patisaul, H.B. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol. Reprod. 2009, 81, 690–699. [Google Scholar] [CrossRef]
- Soriano, S.; Ripoll, C.; Alonso-Magdalena, P.; Fuentes, E.; Quesada, I.; Nadal, A.; Martinez-Pinna, J. Effects of Bisphenol A on ion channels: Experimental evidence and molecular mechanisms. Steroids 2016, 111, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.C.; Huang, J.K.; Chou, C.T.; Cheng, J.S.; Tsai, J.Y.; Fang, Y.C.; Hsu, S.S.; Liao, W.C.; Chang, H.T.; Ho, C.M.; et al. Effect of bisphenol A on Ca(2+) fluxes and viability in Madin-Darby canine renal tubular cells. Drug Chem. Toxicol. 2011, 34, 454–461. [Google Scholar] [CrossRef]
- Deutschmann, A.; Hans, M.; Meyer, R.; Häberlein, H.; Swandulla, D. Bisphenol A inhibits voltage-activated Ca(2+) channels in vitro: Mechanisms and structural requirements. Mol. Pharmacol. 2013, 83, 501–511. [Google Scholar] [CrossRef]
- Lee, S.; Suk, K.; Kim, I.K.; Jang, I.S.; Park, J.W.; Johnson, V.J.; Kwon, T.K.; Choi, B.J.; Kim, S.H. Signaling pathways of bisphenol A-induced apoptosis in hippocampal neuronal cells: Role of calcium-induced reactive oxygen species, mitogen-activated protein kinases, and nuclear factor-kappaB. J. Neurosci. Res. 2008, 86, 2932–2942. [Google Scholar] [CrossRef]
- Tanabe, N.; Kimoto, T.; Kawato, S. Rapid Ca(2+) signaling induced by Bisphenol A in cultured rat hippocampal neurons. Neuro Endocrinol. Lett. 2006, 27, 97–104. [Google Scholar]
- Kochukov, M.Y.; Jeng, Y.J.; Watson, C.S. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 somatomammotropes. Environ. Health Perspect. 2009, 117, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Klenke, U.; Constantin, S.; Wray, S. BPA Directly Decreases GnRH Neuronal Activity via Noncanonical Pathway. Endocrinology 2016, 157, 1980–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmetz, R.; Brown, N.G.; Allen, D.L.; Bigsby, R.M.; Ben-Jonathan, N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology 1997, 138, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Gilardino, A.; Catalano, F.; Ruffinatti, F.A.; Alberto, G.; Nilius, B.; Antoniotti, S.; Martra, G.; Lovisolo, D. Interaction of SiO2 nanoparticles with neuronal cells: Ionic mechanisms involved in the perturbation of calcium homeostasis. Int. J. Biochem. Cell Biol. 2015, 66, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Romanò, N.; Herbison, A.E. Activity-dependent modulation of gonadotrophin-releasing hormone neurone activity by acute oestradiol. J. Neuroendocrinol. 2012, 24, 1296–1303. [Google Scholar] [CrossRef]
- Rønnekleiv, O.K.; Bosch, M.A.; Zhang, C. 17β-oestradiol regulation of gonadotrophin-releasing hormone neuronal excitability. J. Neuroendocrinol. 2012, 24, 122–130. [Google Scholar] [CrossRef]
- Rønnekleiv, O.K.; Zhang, C.; Bosch, M.A.; Kelly, M.J. Kisspeptin and Gonadotropin-Releasing Hormone Neuronal Excitability: Molecular Mechanisms Driven by 17β-Estradiol. Neuroendocrinology 2015, 102, 184–193. [Google Scholar] [CrossRef]
- Terasawa, E.; Kenealy, B.P. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front. Neuroendocrinol. 2012, 33, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Laing, E.; Sunder, A.; Wray, S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J. Neurosci. 2004, 24, 6326–6333. [Google Scholar] [CrossRef] [PubMed]
- Mellon, P.L.; Windle, J.J.; Goldsmith, P.C.; Padula, C.A.; Roberts, J.L.; Weiner, R.I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 1990, 5, 1–10. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiroi, T.; Osada, M.; Asada, A.; Funae, Y. Non-genomic modulation of dopamine release by bisphenol-A in PC12 cells. J. Neurochem. 2003, 87, 1499–1508. [Google Scholar] [CrossRef] [Green Version]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Ruffinatti, F.A.; Gilardino, A.; Lovisolo, D.; Ferraro, M. Spatial wavelet analysis of calcium oscillations in developing neurons. PLoS ONE 2013, 8, e75986. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffinatti, F.A.; Gilardino, A.; Secchi, V.; Cottone, E.; Lovisolo, D.; Bovolin, P. Bisphenol A Activates Calcium Influx in Immortalized GnRH Neurons. Int. J. Mol. Sci. 2019, 20, 2160. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092160
Ruffinatti FA, Gilardino A, Secchi V, Cottone E, Lovisolo D, Bovolin P. Bisphenol A Activates Calcium Influx in Immortalized GnRH Neurons. International Journal of Molecular Sciences. 2019; 20(9):2160. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092160
Chicago/Turabian StyleRuffinatti, Federico Alessandro, Alessandra Gilardino, Valter Secchi, Erika Cottone, Davide Lovisolo, and Patrizia Bovolin. 2019. "Bisphenol A Activates Calcium Influx in Immortalized GnRH Neurons" International Journal of Molecular Sciences 20, no. 9: 2160. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092160