Electrical Stimulation of the Mesencephalic Locomotor Region Attenuates Neuronal Loss and Cytokine Expression in the Perifocal Region of Photothrombotic Stroke in Rats
Abstract
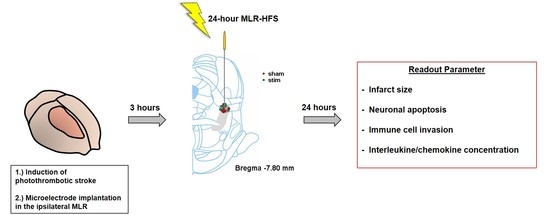
:1. Introduction
2. Results
2.1. No Change of Infarct Size due to MLR-HFS
2.2. Reduction of Perilesional Neuronal Apoptosis after MLR-HFS
2.3. Attenuation of Perilesional Interleukine/Chemokine Concentration after MLR-HFS
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of Photothrombotic Stroke
4.3. Microelectrode Implantation
4.4. High-Frequency Stimulation of the Mesencephalic Locomotor Region
4.5. Collection of Cerebral Tissue
4.6. Brain Cell Separation
4.7. Immunohistochemistry
4.8. Cytokine/Chemokine Quantification
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CxCL-1 | chemokine (C-X-C motive) ligand 1 |
| DBS | deep brain stimulation |
| HFS | high-frequency stimulation |
| IL | interleukin |
| MCP-1 | monocyte chemotactic protein-1 |
| MLR | mesencephalic locomotor region |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| tDCS | transcranial direct-current stimulation |
| TMS | transcranial magnetic stimulation |
References
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Abu-Raddad, L.J.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- Rathore, S.S.; Hinn, A.R.; Cooper, L.S.; Tyroler, H.A.; Rosamond, W.D. Characterization of Incident Stroke Signs and Symptoms: Findings from the Atherosclerosis Risk in Communities Study. Stroke 2002, 33, 2718–2721. [Google Scholar] [CrossRef] [PubMed]
- Fluri, F.; Malzahn, U.; Homola, G.A.; Schuhmann, M.K.; Kleinschnitz, C.; Volkmann, J. Stimulation of the mesencephalic locomotor region for gait recovery after stroke. Ann. Neurol. 2017, 82, 828–840. [Google Scholar] [CrossRef]
- Shik, M.L.; Severin, F.V.; Orlovskiĭ, G.N. Control of walking and running by means of electric stimulation of the midbrain. Biofizika 1966, 11, 659–666. [Google Scholar]
- Skinner, R.D.; Garcia-Rill, E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984, 323, 385–389. [Google Scholar] [CrossRef]
- Eidelberg, E.; Walden, J.G.; Nguyen, L.H. Locomotor Control in Macaque Monkeys. Brain 1981, 104, 647–663. [Google Scholar] [CrossRef]
- Golestanirad, L.; Elahi, B.; Graham, S.J.; Das, S.; Wald, L.L. Efficacy and Safety of Pedunculopontine Nuclei (PPN) Deep Brain Stimulation in the Treatment of Gait Disorders: A Meta-Analysis of Clinical Studies. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2016, 43, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nosko, D.; Ferraye, M.U.; Fraix, V.; Goetz, L.; Chabardès, S.; Pollak, P.; Debû, B. Low-frequency versus high-frequency stimulation of the pedunculopontine nucleus area in Parkinson’s disease: A randomised controlled trial. J. Neurol. Neurosurg. Psychiatry 2015, 86, 674–679. [Google Scholar] [CrossRef]
- Welter, M.-L.; Demain, A.; Ewenczyk, C.; Czernecki, V.; Lau, B.; Helou, A.E.; Belaid, H.; Yelnik, J.; François, C.; Bardinet, E.; et al. PPNa-DBS for gait and balance disorders in Parkinson’s disease: A double-blind, randomised study. J. Neurol. 2015, 262, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Dubuc, R. The Multifunctional Mesencephalic Locomotor Region. Curr. Pharm. Des. 2013, 19, 4448–4470. [Google Scholar] [CrossRef]
- Liang, N.; Mitchell, J.H.; Smith, S.A.; Mizuno, M. Exaggerated sympathetic and cardiovascular responses to stimulation of the mesencephalic locomotor region in spontaneously hypertensive rats. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H123–H131. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Cambiaghi, M.; Bacigaluppi, M.; Gallizioli, M.; Gaude, E.; Mari, S.; Sandrone, S.; Cursi, M.; Teneud, L.; Comi, G.; et al. Safety and Efficacy of Transcranial Direct Current Stimulation in Acute Experimental Ischemic Stroke. Stroke 2013, 44, 3166–3174. [Google Scholar] [Green Version]
- Sasso, V.; Bisicchia, E.; Latini, L.; Ghiglieri, V.; Cacace, F.; Carola, V.; Molinari, M.; Viscomi, M.T. Repetitive transcranial magnetic stimulation reduces remote apoptotic cell death and inflammation after focal brain injury. J. Neuroinflamm. 2016, 13, 150. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Morrison, H.W.; Filosa, J.A. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J. Neuroinflamm. 2013, 10, 782. [Google Scholar] [CrossRef]
- Gelderblom, M.; Leypoldt, F.; Steinbach, K.; Behrens, D.; Choe, C.-U.; Siler, D.A.; Arumugam, T.V.; Orthey, E.; Gerloff, C.; Tolosa, E.; et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009, 40, 1849–1857. [Google Scholar] [CrossRef]
- Braun, J.S.; Jander, S.; Schroeter, M.; Witte, O.W.; Stoll, G. Spatiotemporal relationship of apoptotic cell death to lymphomonocytic infiltration in photochemically induced focal ischemia of the rat cerebral cortex. Acta Neuropathol. 1996, 92, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Jander, S.; Kraemer, M.; Schroeter, M.; Witte, O.W.; Stoll, G. Lymphocytic Infiltration and Expression of Intercellular Adhesion Molecule-1 in Photochemically Induced Ischemia of the Rat Cortex. J. Cereb. Blood Flow Metab. 1995, 15, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeter, M.; Jander, S.; Witte, O.; Stoll, G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J. Neuroimmunol. 1994, 55, 195–203. [Google Scholar] [CrossRef]
- Smith, C.J.; Hulme, S.; Vail, A.; Heal, C.; Parry-Jones, A.R.; Scarth, S.; Hopkins, K.; Hoadley, M.; Allan, S.M.; Rothwell, N.J.; et al. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke): A Randomized Controlled Phase 2 Trial. Stroke 2018, 49, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Chang, I.; Muralidharan, K.; et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): A randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017, 16, 217–226. [Google Scholar] [CrossRef]
- Wang, J.; Dong, W.-W.; Zhang, W.-H.; Zheng, J.; Wang, X. Electrical Stimulation of Cerebellar Fastigial Nucleus: Mechanism of Neuroprotection and Prospects for Clinical Application against Cerebral Ischemia. CNS Neurosci. Ther. 2014, 20, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Kameda, M.; Yasuhara, T.; Morimoto, T.; Kondo, A.; Shingo, T.; Tajiri, N.; Wang, F.; Miyoshi, Y.; Borlongan, C.V.; et al. Electrical Stimulation of the Cerebral Cortex Exerts Antiapoptotic, Angiogenic, and Anti-Inflammatory Effects in Ischemic Stroke Rats Through Phosphoinositide 3-Kinase/Akt Signaling Pathway. Stroke 2009, 40, e598–e605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Abulafia, D.P.; de Rivero Vaccari, J.P.; Lozano, J.D.; Lotocki, G.; Keane, R.W.; Dietrich, W.D. Inhibition of the Inflammasome Complex Reduces the Inflammatory Response after Thromboembolic Stroke in Mice. J. Cereb. Blood Flow Metab. 2009, 29, 534–544. [Google Scholar] [CrossRef]
- Ramos-Cejudo, J.; Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Expósito Alcaide, M.; Sánchez-Cabo, F.; Dopazo, A.; Díez-Tejedor, E. Spatial and Temporal Gene Expression Differences in Core and Periinfarct Areas in Experimental Stroke: A Microarray Analysis. PLoS ONE 2012, 7, e52121. [Google Scholar] [CrossRef]
- Gelderblom, M.; Weymar, A.; Bernreuther, C.; Velden, J.; Arunachalam, P.; Steinbach, K.; Orthey, E.; Arumugam, T.V.; Leypoldt, F.; Simova, O.; et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 2012, 120, 3793–3802. [Google Scholar] [CrossRef] [Green Version]
- Musacchio, T.; Rebenstorff, M.; Fluri, F.; Brotchie, J.M.; Volkmann, J.; Koprich, J.B.; Ip, C.W. Subthalamic nucleus deep brain stimulation is neuroprotective in the A53T α-synuclein Parkinson’s disease rat model. Ann. Neurol. 2017, 81, 825–836. [Google Scholar] [CrossRef]
- Fischer, D.L.; Kemp, C.J.; Cole-Strauss, A.; Polinski, N.K.; Paumier, K.L.; Lipton, J.W.; Steece-Collier, K.; Collier, T.J.; Buhlinger, D.J.; Sortwell, C.E. Subthalamic Nucleus Deep Brain Stimulation Employs trkB Signaling for Neuroprotection and Functional Restoration. J. Neurosci. 2017, 37, 6786–6796. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, J.; Yasuhara, T.; Kameda, M.; Umakoshi, M.; Kin, I.; Kuwahara, K.; Kin, K.; Okazaki, M.; Takeuchi, H.; Sasaki, T.; et al. Electrical Stimulation Enhances Migratory Ability of Transplanted Bone Marrow Stromal Cells in a Rodent Ischemic Stroke Model. Cell. Physiol. Biochem. 2018, 46, 57–68. [Google Scholar] [Green Version]
- Morimoto, T.; Yasuhara, T.; Kameda, M.; Baba, T.; Kuramoto, S.; Kondo, A.; Takahashi, K.; Tajiri, N.; Wang, F.; Meng, J.; et al. Striatal Stimulation Nurtures Endogenous Neurogenesis and Angiogenesis in Chronic-Phase Ischemic Stroke Rats. Cell Transplant. 2011, 20, 1049–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-C.; Shi, L.; Zhu, G.-Y.; Wang, X.; Liu, D.; Liu, Y.; Jiang, Y.; Zhang, X.; Zhang, J.-G. Effects of anterior thalamic nuclei deep brain stimulation on neurogenesis in epileptic and healthy rats. Brain Res. 2017, 1672, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cooperrider, J.; Furmaga, H.; Plow, E.; Park, H.-J.; Chen, Z.; Kidd, G.; Baker, K.B.; Gale, J.T.; Machado, A.G. Chronic Deep Cerebellar Stimulation Promotes Long-Term Potentiation, Microstructural Plasticity, and Reorganization of Perilesional Cortical Representation in a Rodent Model. J. Neurosci. 2014, 34, 9040–9050. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gonzalez, C.; Bolam, J.P.; Mena-Segovia, J. Topographical Organization of the Pedunculopontine Nucleus. Front. Neuroanat. 2011, 5, 22. [Google Scholar] [CrossRef]
- Nielsen, R.K.; Jensen, W. Low-Frequency Intracortical Electrical Stimulation Decreases Sensorimotor Cortex Hyperexcitability in the Acute Phase of Ischemic Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1287–1296. [Google Scholar]
- Fong, W.-H.; Tsai, H.-D.; Chen, Y.-C.; Wu, J.-S.; Lin, T.-N. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol. Neurobiol. 2010, 41, 180–186. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Jiang, Y.; Li, L.; Li, C.; Li, J. Electrical stimulation of cerebellar fastigial nucleus protects against cerebral ischemic injury by PPARγ upregulation. Neurol. Res. 2017, 39, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.-M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease: The role of inflammation in CNS. Br. J. Pharmacol. 2009, 147, S232–S240. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Meisel, A.; Planas, A.M.; Urra, X.; van de Beek, D.; Veltkamp, R. The immunology of acute stroke. Nat. Rev. Neurol. 2012, 8, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinschnitz, C.; Schwab, N.; Kraft, P.; Hagedorn, I.; Dreykluft, A.; Schwarz, T.; Austinat, M.; Nieswandt, B.; Wiendl, H.; Stoll, G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 2010, 115, 3835–3842. [Google Scholar] [CrossRef] [Green Version]
- Beurrier, C.; Bioulac, B.; Audin, J.; Hammond, C. High-Frequency Stimulation Produces a Transient Blockade of Voltage-Gated Currents in Subthalamic Neurons. J. Neurophysiol. 2001, 85, 1351–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, M.T.H.; Bean, B.P. Subthreshold sodium currents and pacemaking of subthalamic neurons: Modulation by slow inactivation. Neuron 2003, 39, 109–120. [Google Scholar] [CrossRef]
- Black, J.A.; Waxman, S.G. Noncanonical Roles of Voltage-Gated Sodium Channels. Neuron 2013, 80, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Fraser, S.P.; Diss, J.K.; Lloyd, L.J.; Pani, F.; Chioni, A.-M.; George, A.J.; Djamgoz, M.B. T-lymphocyte invasiveness: Control by voltage-gated Na + channel activity. FEBS Lett. 2004, 569, 191–194. [Google Scholar] [CrossRef]
- Lo, W.-L.; Donermeyer, D.L.; Allen, P.M. A voltage-gated sodium channel is essential for the positive selection of CD4+ T cells. Nat. Immunol. 2012, 13, 880–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappalardo, L.W.; Black, J.A.; Waxman, S.G. Sodium channels in astroglia and microglia: Sodium Channels in Astroglia and Microglia. Glia 2016, 64, 1628–1645. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, Y.-C.; Ji, W.-J.; Zhang, L.; Dong, Y.; Ge, L.; Lu, R.-Y.; Sun, H.-Y.; Guo, Z.-Z.; Yang, G.-H.; et al. Modulation of Mononuclear Phagocyte Inflammatory Response by Liposome-Encapsulated Voltage Gated Sodium Channel Inhibitor Ameliorates Myocardial Ischemia/Reperfusion Injury in Rats. PLoS ONE 2013, 8, e74390. [Google Scholar] [CrossRef]
- Craner, M.J.; Damarjian, T.G.; Liu, S.; Hains, B.C.; Lo, A.C.; Black, J.A.; Newcombe, J.; Cuzner, M.L.; Waxman, S.G. Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia 2005, 49, 220–229. [Google Scholar] [CrossRef]
- Chen, N.; Gao, Y.; Yan, N.; Liu, C.; Zhang, J.-G.; Xing, W.-M.; Kong, D.-M.; Meng, F.-G. High-frequency stimulation of the hippocampus protects against seizure activity and hippocampal neuronal apoptosis induced by kainic acid administration in macaques. Neuroscience 2014, 256, 370–378. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Zhu, G.-Y.; Wang, X.; Shi, L.; Jiang, Y.; Zhang, X.; Zhang, J.-G. Deep brain stimulation of the anterior nucleus of the thalamus reverses the gene expression of cytokines and their receptors as well as neuronal degeneration in epileptic rats. Brain Res. 2017, 1657, 304–311. [Google Scholar] [CrossRef]
- Zhou, P.; Qian, L.; Zhou, T.; Iadecola, C. Mitochondria are involved in the neurogenic neuroprotection conferred by stimulation of cerebellar fastigial nucleus. J. Neurochem. 2005, 95, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Ranck, J.B., Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Res. 1975, 98, 417–440. [Google Scholar] [CrossRef]
- Bittner, S.; Ruck, T.; Schuhmann, M.K.; Herrmann, A.M.; ou Maati, H.M.; Bobak, N.; Göbel, K.; Langhauser, F.; Stegner, D.; Ehling, P.; et al. Endothelial TWIK-related potassium channel-1 (TREK1) regulates immune-cell trafficking into the CNS. Nat. Med. 2013, 19, 1161–1165. [Google Scholar] [CrossRef]
- Schuhmann, M.K.; Kraft, P.; Stoll, G.; Lorenz, K.; Meuth, S.G.; Wiendl, H.; Nieswandt, B.; Sparwasser, T.; Beyersdorf, N.; Kerkau, T.; et al. CD28 superagonist-mediated boost of regulatory T cells increases thrombo-inflammation and ischemic neurodegeneration during the acute phase of experimental stroke. J. Cereb. Blood Flow Metab. 2015, 35, 6–10. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuhmann, M.K.; Stoll, G.; Bohr, A.; Volkmann, J.; Fluri, F. Electrical Stimulation of the Mesencephalic Locomotor Region Attenuates Neuronal Loss and Cytokine Expression in the Perifocal Region of Photothrombotic Stroke in Rats. Int. J. Mol. Sci. 2019, 20, 2341. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092341
Schuhmann MK, Stoll G, Bohr A, Volkmann J, Fluri F. Electrical Stimulation of the Mesencephalic Locomotor Region Attenuates Neuronal Loss and Cytokine Expression in the Perifocal Region of Photothrombotic Stroke in Rats. International Journal of Molecular Sciences. 2019; 20(9):2341. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092341
Chicago/Turabian StyleSchuhmann, Michael K., Guido Stoll, Arne Bohr, Jens Volkmann, and Felix Fluri. 2019. "Electrical Stimulation of the Mesencephalic Locomotor Region Attenuates Neuronal Loss and Cytokine Expression in the Perifocal Region of Photothrombotic Stroke in Rats" International Journal of Molecular Sciences 20, no. 9: 2341. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092341