Proteomic Analyses Reveal New Insights on the Antimicrobial Mechanisms of Chitosan Biopolymers and Their Nanosized Particles against Escherichia coli
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Nanoparticle Characterization
2.2. Growth Curve Analysis
2.3. Differential Proteome Analysis
2.4. E. coli Proteome Response to Challenge by Chitosan
2.5. E. coli Metabolic and Cellular Processes Globally Affected by Chitosan
2.6. Similarities and Dissimilarities of E. coli Proteome Responses to Chitosan Polymers and Nanoparticles
2.7. Chitosan, A Destabilizer of E. coli Outer Membrane
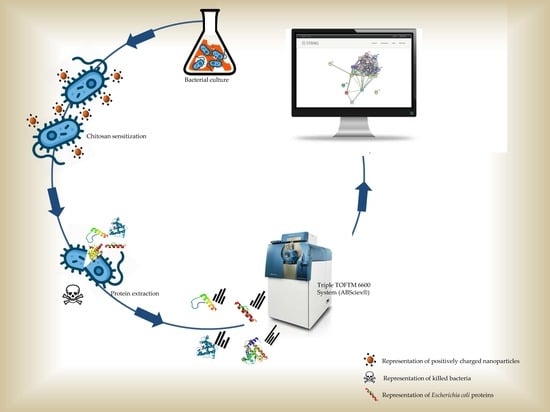
3. Material and Methods
3.1. Nanoparticle Preparation
3.2. Nanoparticle and Polymer Characterization
3.3. Bacterial Strain and Culture
3.4. Effects of Antimicrobial Agents on Bacteria
3.5. Total Protein Extraction
3.6. Tryptic Digestion of Total Soluble Protein Extracts
3.7. Liquid Chromatography Electrospray Ionization Mass Spectrometry (LC-ESI-MS/MS)
3.8. Proteome Data Processing
3.9. Statistical and Bioinformatics Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | Ammonia bicarbonate |
| CS | Chitosan |
| CV | Coefficient of variation |
| DTT | Ditiotreitol |
| FA | Formic acid |
| GO | Gene ontology |
| IDA | Information-dependent acquisition |
| LB | Luria-Bertani |
| LC-MS/MS | Liquid chromatography mass spectrometry |
| LPS | Lipopolysaccharides |
| MIC | Minimum inhibitory concentration |
| NP | Nanoparticle |
| OD | Optical density |
| OM | Outer membrane |
| OMP | Outer membrane protein |
| OPGs | Osmoregulated periplasmic glucans |
| PdI | Polydispersity index |
| PLS-DA | Partial Least Squares-Discriminant Analysis |
| Rh | Hydrodynamic radius |
| US | Ultrasonication |
| VIP | Variable Importance in Projection |
References
- Gomes, L.; Souza, H.; Campiña, J.; Andrade, C.; Silva, A.; Gonçalves, M.; Paschoalin, V. Edible chitosan films and their nanosized counterparts exhibit antimicrobial activity and enhanced mechanical and barrier properties. Molecules 2019, 24, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Kim, D.; Adesogan, A.T.; Ko, S.; Galvao, K.; Jeong, K.C. Chitosan microparticles exert broad-spectrum antimicrobial activity against antibiotic-resistant micro-organisms without increasing resistance. ACS Appl. Mater. Interfaces 2016, 8, 10700–10709. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Yang, C.; Li, B.; Ge, M.; Zhou, K.; Wang, Y.; Luo, J.; Ibrahim, M.; Xie, G.; Sun, G. Inhibitory effect and mode of action of chitosan solution against rice bacterial brown stripe pathogen Acidovorax avenae subsp. avenae RS-1. Carbohydr. Res. 2014, 391, 48–54. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.J.; Oh, M.; Yeo, W.-S.; Galvao, K.N.; Jeong, K.C. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS ONE 2014, 9, e92723. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Gomes, L.P.; Del Aguila, E.M.; Alexander, C.; Paschoalin, V.M.F.; de Andrade, C.T. Assessing the Antimicrobial Activity of Chitosan Nanoparticles by Fluorescence-Labeling. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2018, 12, 111–117. [Google Scholar]
- Gomes, L.P.; Souza, H.K.; Campiña, J.M.; Andrade, C.T.; Paschoalin, V.M.F.; Silva, A.; Gonçalves, M.P. Tweaking the Mechanical and Structural Properties of Colloidal Chitosans by Sonication. Food Hydrocoll. 2016, 56, 29–40. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G.W. Nanocomposites in food packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef] [PubMed]
- Himeoka, Y.; Kaneko, K. Theory for transitions between exponential and stationary phases: Universal laws for lag time. Phys. Rev. X 2017, 7, 021049. [Google Scholar] [CrossRef] [Green Version]
- Yahyaei, M.; Mehrnejad, F.; Naderi-Manesh, H.; Rezayan, A.H. Protein adsorption onto polysaccharides: Comparison of chitosan and chitin polymers. Carbohydr. Polym. 2018, 191, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Proofreading in Trans by an Aminoacyl-tRNA Synthetases Model for Single Site Editing by Isoleucyl-tRNA Synthetase. Nucleic Acids Res. 1996, 24, 2505–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.-Y.; Song, J.-A.; Ahn, K.-Y.; Park, J.-S.; Seo, H.-S.; Lee, J. Enhanced solubility of heterologous proteins by fusion expression using stress-induced Escherichia coli protein, Tsf. FEMS Microbiol. Lett. 2007, 274, 132–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, P.; Liu, S.; Zhao, Y.; Wang, Z.; Chen, T. glyA gene knock-out in Escherichia coli enhances L-serine production without glycine addition. Biotechnol. Bioprocess. Eng. 2017, 22, 390–396. [Google Scholar] [CrossRef]
- Rodina, E.; Vorobieva, N.; Kurilova, S.; Mikulovich, J.; Vainonen, J.; Aro, E.M.; Nazarova, T. Identification of new protein complexes of Escherichia coli inorganic pyrophosphatase using pull-down assay. Biochimie 2011, 93, 1576–1583. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, F.; Li, Y.; Zhang, X.; Li, J.; Yang, P.; Qi, Q. Comparison of individual component deletions in a glucose-specific phosphotransferase system revealed their different applications. Sci. Rep. 2015, 5, 13200. [Google Scholar] [CrossRef] [Green Version]
- Applebee, M.K.; Joyce, A.R.; Conrad, T.M.; Pettigrew, D.W.; Palsson, B.Ø. Functional and metabolic effects of adaptive glycerol kinase (GLPK) mutants in Escherichia coli. J. Biol. Chem. 2011, 286, 23150–23159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, A.F.V.; Schultz, S.; Bomke, J.; Pils, T.; Lehmann, W.D.; Knappe, J. YfiD of Escherichia coli and Y06I of Bacteriophage T4 as Autonomous Glycyl Radical Cofactors Reconstituting the Catalytic Center of Oxygen-Fragmented Pyruvate Formate-Lyase. Biochem. Biophys. Res. Commun. 2001, 285, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.; Zeth, K.; Linke, D. Structure and function of colicin S4, a colicin with a duplicated receptor-binding domain. J. Biol. Chem. 2009, 284, 6403–6413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Lai, Y.; Sun, J.; Chen, G.; Yan, A. Transcriptional regulation of the outer membrane porin gene ompW reveals its physiological role during the transition from the aerobic to the anaerobic lifestyle of Escherichia coli. Front. Microbiol. 2016, 7, 799. [Google Scholar] [CrossRef] [Green Version]
- Johansen, J.; Rasmussen, A.A.; Overgaard, M.; Valentin-Hansen, P. Conserved small non-coding RNAs that belong to the σE regulon: Role in down-regulation of outer membrane proteins. J. Mol. Biol. 2006, 364, 1–8. [Google Scholar] [CrossRef]
- Reimer, A.; Maffenbeier, V.; Dubey, M.; Sentchilo, V.; Tavares, D.; Gil, M.H.; Beggah, S.; van der Meer, J.R. Complete alanine scanning of the Escherichia coli RbsB ribose binding protein reveals residues important for chemoreceptor signaling and periplasmic abundance. Sci. Rep. 2017, 7, 8245. [Google Scholar] [CrossRef] [Green Version]
- Aseev, L.V.; Koledinskaya, L.S.; Boni, I.V. Regulation of ribosomal protein operons rplM-rpsI, rpmB-rpmG, and rplU-rpmA at the transcriptional and translational levels. J. Bacteriol. 2016, 198, 2494–2502. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Ruckenstein, E. Cross-linked macroporous chitosan anion-exchange membranes for protein separations. J. Membr. Sci. 1998, 148, 195–205. [Google Scholar] [CrossRef]
- Chiou, M.-S.; Ho, P.-Y.; Li, H.-Y. Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dye. Pigment. 2004, 60, 69–84. [Google Scholar] [CrossRef]
- Clifton, L.A.; Skoda, M.W.; Daulton, E.L.; Hughes, A.V.; Le Brun, A.P.; Lakey, J.H.; Holt, S.I.A. Asymmetric phospholipid: Lipopolysaccharide bilayers; a Gram-negative bacterial outer membrane mimic. J. R. Soc. Interface 2013, 10, 20130810. [Google Scholar] [CrossRef]
- De Cock, H.; Brandenburg, K.; Wiese, A.; Holst, O.; Seydel, U. Non-lamellar structure and negative charges of lipopolysaccharides required for efficient folding of outer membrane protein PhoE of Escherichia coli. J. Biol. Chem. 1999, 274, 5114–5119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonntag, I.; Schwarz, H.; Hirota, Y.; Henning, U. Cell envelope and shape of Escherichia coli: Multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J. Bacteriol. 1978, 136, 280–285. [Google Scholar] [PubMed]
- Ortiz-Suarez, M.L.; Samsudin, F.; Piggot, T.J.; Bond, P.J.; Khalid, S. Full-Length OmpA: Structure, Function, and Membrane Interactions Predicted by Molecular Dynamics Simulations. Biophys. J. 2016, 111, 1692–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, K.; Chen, X.G.; Liu, C.S.; Cha, D.S.; Park, H.J. Oleoyl-chitosan nanoparticles inhibits Escherichia coli and Staphylococcus aureus by damaging the cell membrane and putative binding to extracellular or intracellular targets. Int. J. Food Microbiol. 2009, 132, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Burmann, B.M.; Wang, C.; Hiller, S. Conformation and dynamics of the periplasmic membrane-protein–chaperone complexes OmpX–Skp and tOmpA–Skp. Nat. Struct. Mol. Biol. 2013, 20, 1265. [Google Scholar] [CrossRef] [PubMed]
- Schulz, G.E. β-barrel membrane proteins. Curr. Opin. Struct. Biol. 2000, 10, 443–447. [Google Scholar] [CrossRef]
- Patel, G.J.; Behrens-Kneip, S.; Holst, O.; Kleinschmidt, J.r.H. The periplasmic chaperone Skp facilitates targeting, insertion, and folding of OmpA into lipid membranes with a negative membrane surface potential. Biochemistry 2009, 48, 10235–10245. [Google Scholar] [CrossRef]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [CrossRef] [Green Version]
- Swain, A.; Fagan, W.F. A mathematical model of the Warburg Effect: Effects of cell size, shape and substrate availability on growth and metabolism in bacteria. Math. Biosci. Eng. MBE 2018, 16, 168–186. [Google Scholar] [CrossRef]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p) ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298. [Google Scholar] [CrossRef] [Green Version]
- Myka, K.K.; Küsters, K.; Washburn, R.; Gottesman, M.E. DksA–RNA polymerase interactions support new origin formation and DNA repair in Escherichia coli. Mol. Microbiol. 2019, 111, 1382–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.-P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.-Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R. Mapping differential interactomes by affinity purification coupled with data-independent mass spectrometry acquisition. Nat. Methods 2013, 10, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, B.C.; Gillet, L.C.; Rosenberger, G.; Röst, H.L.; Vichalkovski, A.; Gstaiger, M.; Aebersold, R. Quantifying protein interaction dynamics by SWATH mass spectrometry: Application to the 14-3-3 system. Nat. Methods 2013, 10, 1246. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol update for large-scale genome and gene function analysis with the PANTHER classification system (v. 14.0). Nat. Prot. 2019, 14, 703. [Google Scholar] [CrossRef]





| Sample | L | LN | M | MN | ||||
|---|---|---|---|---|---|---|---|---|
| ζ potential (mV) | 50.2 ± 1.9 | 40.1 ± 3.6 | 40.1 ± 1.1 | 33.1 ± 2.8 | ||||
| Rh (nm) | 1219 | 4468 | 468 | 4784 | 840 | 5132 | 355 | 50 |
| Intensity (%) | 62 | 38 | 94 | 6 | 50 | 18 | 84 | 8 |
| PdI | 0.55 ± 0.02 | 0.47 ± 0.01 | 0.99 ± 0.01 | 0.78 ± 0.02 | ||||
| L | LN | M | MN | |
|---|---|---|---|---|
| Overexpressed | ||||
| STRING Parameters | ||||
| Edges | 420 | 675 | 280 | 598 |
| PPI | <1.0 × 10−16 | <1.0 × 10−16 | <1.0 × 10−16 | <1.0 × 10−16 |
| Purine metabolism | guaA, pnp, purB, purL, pykF, gnd, rpoA | guaA, pnp, prs, purB, purH, gnd, rpoA, rpoB | guaA, prs, pykF | prs, purB, purF, purH, gnd, rpoA |
| Amino acid biosynthesis | fbaA, glyA, pheA, pykF, kbl | eno, gapA, glyA, pheA, prs, serC | glyA, pheA, prs, pykF | eno, fbaA, glyA, pheA, prs |
| Outer membrane proteins | accC, dnaK, dksA, hslU, ompA, ompT, tig | accC, dksA, mdoG, ompA, ompT, skp | accC, hslU, infB, mdoG, skp, tig | accC, bamA, dksA, infB, ompA, ompT |
| Underexpressed | ||||
| String Parameters | ||||
| Edges | 3 | 8 | 0 | 1 |
| PPI | 0.0111 | 6.92 × 10−16 | − | 0.366 |
| TCA | sdhA, sdhB | acnB, fumA, sdhA, sdhB | − | pckA, sdhB |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.P.; Anjo, S.I.; Manadas, B.; Coelho, A.V.; Paschoalin, V.M.F. Proteomic Analyses Reveal New Insights on the Antimicrobial Mechanisms of Chitosan Biopolymers and Their Nanosized Particles against Escherichia coli. Int. J. Mol. Sci. 2020, 21, 225. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010225
Gomes LP, Anjo SI, Manadas B, Coelho AV, Paschoalin VMF. Proteomic Analyses Reveal New Insights on the Antimicrobial Mechanisms of Chitosan Biopolymers and Their Nanosized Particles against Escherichia coli. International Journal of Molecular Sciences. 2020; 21(1):225. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010225
Chicago/Turabian StyleGomes, Laidson P., Sandra I. Anjo, Bruno Manadas, Ana V. Coelho, and Vania M. F. Paschoalin. 2020. "Proteomic Analyses Reveal New Insights on the Antimicrobial Mechanisms of Chitosan Biopolymers and Their Nanosized Particles against Escherichia coli" International Journal of Molecular Sciences 21, no. 1: 225. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010225