Stereocilia Rootlets: Actin-Based Structures That Are Essential for Structural Stability of the Hair Bundle
Abstract
:1. Introduction
2. Hair Cells Mediate Hearing and Balance
3. Compartments of Actin in Sensory Hair Cells
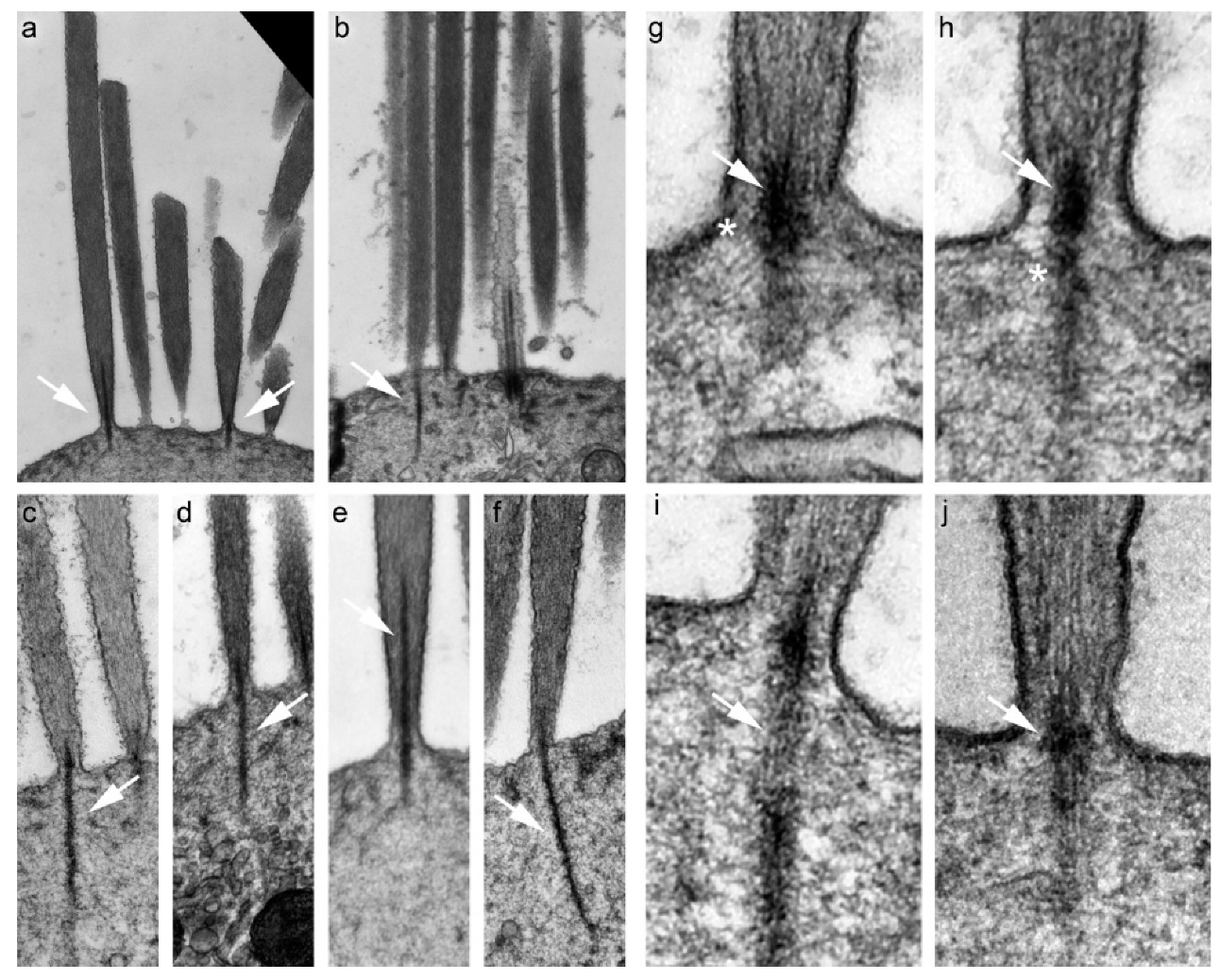
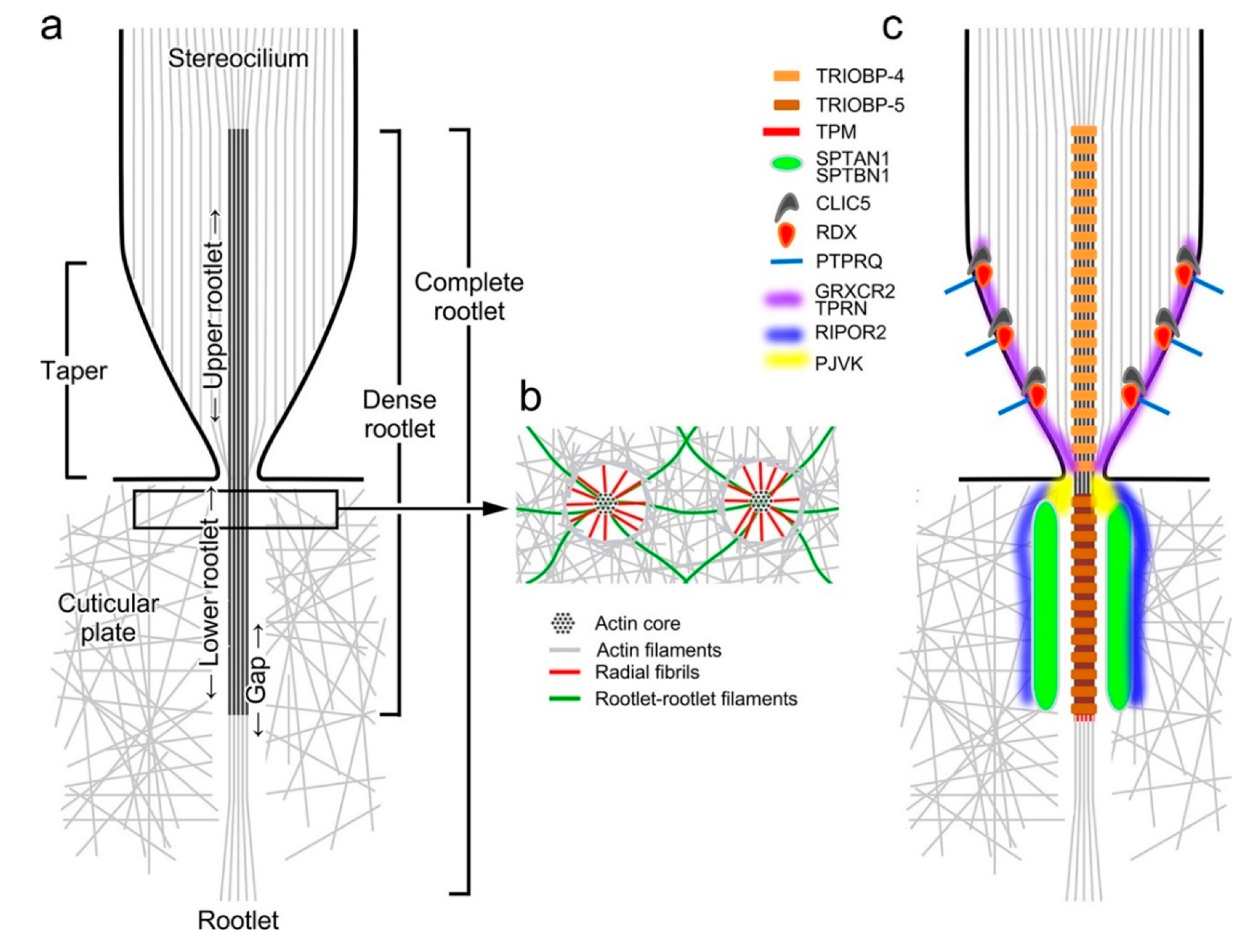
4. Morphology of the Rootlet
5. Rootlet Protein Composition
6. Function of the Rootlet
7. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TEM | Transmission electron microscopy |
| MET | Mechanoelectrical transduction |
References
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef] [Green Version]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhabra, E.S.; Higgs, H.N. The many faces of actin: Matching assembly factors with cellular structures. Nat. Cell Biol. 2007, 9, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Michelot, A.; Drubin, D.G. Building distinct actin filament networks in a common cytoplasm. Curr. Biol. 2011, 21, R560–R569. [Google Scholar] [CrossRef] [Green Version]
- Tilney, L.G.; DeRosier, D.J.; Mulroy, M.J. The organization of actin filaments in the stereocilia of cochlear hair cells. J. Cell Biol. 1980, 86, 244–259. [Google Scholar] [CrossRef]
- Tilney, L.G.; DeRosier, D.J. Actin filaments, stereocilia, and hair cells of the bird cochlea. IV. How the actin filaments become organized in developing stereocilia and in the cuticular plate. Dev. Biol. 1986, 116, 119–129. [Google Scholar] [CrossRef]
- DeRosier, D.J.; Tilney, L.G. The structure of the cuticular plate, an in vivo actin gel. J. Cell Biol. 1989, 109, 2853–2867. [Google Scholar] [CrossRef] [Green Version]
- Cotanche, D.A. Development of hair cell stereocilia in the avian cochlea. Hear. Res. 1987, 28, 35–44. [Google Scholar] [CrossRef]
- Hirokawa, N.; Tilney, L.G. Interactions between actin filaments and between actin filaments and membranes in quick-frozen and deeply etched hair cells of the chick ear. J. Cell Biol. 1982, 95, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Duckert, L.G.; Rubel, E.W. Ultrastructural observations on regenerating hair cells in the chick basilar papilla. Hear. Res. 1990, 48, 161–182. [Google Scholar] [CrossRef]
- Blest, A.D.; De Couet, H.G.; Sigmund, C. The cytoskeleton of microvilli of leech photoreceptors. A stable bundle of actin microfilaments. Cell Tissue Res. 1983, 234, 9–16. [Google Scholar] [CrossRef]
- Hirokawa, N.; Tilney, L.G.; Fujiwara, K.; Heuser, J.E. Organization of actin, myosin, and intermediate filaments in the brush border of intestinal epithelial cells. J. Cell Biol. 1982, 94, 425–443. [Google Scholar] [CrossRef] [Green Version]
- Horridge, G.A. Statocysts of medusae and evolution of stereocilia. Tissue Cell 1969, 1, 341–353. [Google Scholar] [CrossRef]
- Salisbury, J.L.; Floyd, G.L. Calcium-induced contraction of the rhizoplast of a quadriflagellate green alga. Science 1978, 202, 975–977. [Google Scholar] [CrossRef]
- Salisbury, J.L.; Baron, A.; Surek, B.; Melkonian, M. Striated flagellar roots: Isolation and partial characterization of a calcium-modulated contractile organelle. J. Cell Biol. 1984, 99, 962–970. [Google Scholar] [CrossRef]
- Wolfrum, U. Cytoskeletal elements in arthropod sensilla and mammalian photoreceptors. Biol. Cell 1992, 76, 373–381. [Google Scholar] [CrossRef]
- Chen, J.V.; Kao, L.R.; Jana, S.C.; Sivan-Loukianova, E.; Mendonça, S.; Cabrera, O.A.; Singh, P.; Cabernard, C.; Eberl, D.F.; Bettencourt-Dias, M.; et al. Rootletin organizes the ciliary rootlet to achieve neuron sensory function in Drosophila. J. Cell Biol. 2015, 211, 435–453. [Google Scholar] [CrossRef] [Green Version]
- Worley, L.G.; Fischbeinn, E.; Shapiro, J.E. The structure of ciliated epithelial cells as revealed by the electron microscope and in phase contrast. J. Morphol. 1953, 92, 545–577. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Yue, G.; Adamian, M.; Bulgakov, O.; Li, T. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J. Cell Biol. 2002, 159, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gao, J.; Adamian, M.; Wen, X.H.; Pawlyk, B.; Zhang, L.; Sanderson, M.J.; Zuo, J.; Makino, C.L.; Li, T. The ciliary rootlet maintains long-term stability of sensory cilia. Mol. Cell Biol. 2005, 25, 4129–4137. [Google Scholar] [CrossRef] [Green Version]
- Hudspeth, A.J. How the ear’s works work. Nature 1989, 341, 397–404. [Google Scholar] [CrossRef]
- Roberts, W.M.; Howard, J.; Hudspeth, A.J. Hair cells: Transduction, tuning, and transmission in the inner ear. Annu. Rev. Cell. Biol. 1988, 4, 63–92. [Google Scholar] [CrossRef]
- Pickles, J.O.; Comis, S.D.; Osborne, M.P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 1984, 15, 103–112. [Google Scholar] [CrossRef]
- Fettiplace, R.; Kim, K.X. The physiology of mechanoelectrical transduction channels in hearing. Physiol. Rev. 2014, 94, 951–986. [Google Scholar] [CrossRef] [PubMed]
- Flock, A.; Cheung, H.C. Actin filaments in sensory hairs of inner ear receptor cells. J. Cell Biol. 1977, 75, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Flock, A.; Cheung, H.C.; Flock, B.; Utter, G. Three sets of actin filaments in sensory cells of the inner ear. Identification and functional orientation determined by gel electrophoresis, immunofluorescence, and electron microscopy. J. Neurocytol. 1981, 10, 133–147. [Google Scholar] [CrossRef]
- Flock, A.; Flock, B.; Murray, E. Studies on the sensory hairs of receptor cells in the inner ear. Acta Otolaryngol. 1977, 83, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Pollock, L.M.; McDermott, B.M. The cuticular plate: A riddle, wrapped in a mystery, inside a hair cell. Birth Defects Res. C Embryo Today 2015, 105, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Slepecky, N.B.; Ulfendahl, M. Actin-binding and microtubule-associated proteins in the organ of Corti. Hear. Res. 1992, 57, 201–215. [Google Scholar] [CrossRef]
- Du, T.T.; Dewey, J.B.; Wagner, E.L.; Cui, R.; Heo, J.; Park, J.J.; Francis, S.P.; Perez-Reyes, E.; Guillot, S.J.; Sherman, N.E.; et al. LMO7 deficiency reveals the significance of the cuticular plate for hearing function. Nat. Commun. 2019, 10, 1117. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.P.; Krey, J.F.; Krystofiak, E.S.; Cui, R.; Nanda, S.; Xu, W.; Kachar, B.; Barr-Gillespie, P.G.; Shin, J.B. A short splice form of Xin-actin binding repeat containing 2 (XIRP2) lacking the Xin repeats is required for maintenance of stereocilia morphology and hearing function. J. Neurosci. 2015, 35, 1999–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, D.I.; Zhang, D.S.; Shen, J.; Indzhykulian, A.; Karavitaki, K.D.; Xu, Y.J.; Wang, Q.; Lin, J.J.; Chen, Z.Y.; Corey, D.P. XIRP2, an actin-binding protein essential for inner ear hair-cell stereocilia. Cell Rep. 2015, 10, 1811–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flock, A.; Duvall, A.J. The ultrastructure of the kinocilium of the senosry cells in the inner ear and lateral line organs. J. Cell Biol. 1965, 25, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, M.; Nakashima, T. Structure of the hair rootlets on cochlear sensory cells by tannic acid fixation. Acta Otolaryngol. 1980, 90, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M. Preservation and visualization of actin-containing filaments in the apical zone of cochlear sensory cells. Hear. Res. 1982, 6, 277–289. [Google Scholar] [CrossRef]
- Slepecky, N.; Chamberlain, S.C. Immunoelectron microscopic and immunofluorescent localization of cytoskeletal and muscle-like contractile proteins in inner ear sensory hair cells. Hear. Res. 1985, 20, 245–260. [Google Scholar] [CrossRef]
- Tilney, L.G.; Tilney, M.S.; DeRosier, D.J. Actin filaments, stereocilia, and hair cells: How cells count and measure. Ann. Rev. Cell Biol. 1992, 8, 257–274. [Google Scholar] [CrossRef]
- Furness, D.N.; Mahendrasingam, S.; Ohashi, M.; Fettiplace, R.; Hackney, C.M. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: Comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J. Neurosci. 2008, 28, 6342–6353. [Google Scholar] [CrossRef]
- Anniko, M. Cytodifferentiation of cochlear hair cells. Am. J. Otolaryngol. 1983, 4, 375–388. [Google Scholar] [CrossRef]
- Kitajiri, S.; Sakamoto, T.; Belyantseva, I.A.; Goodyear, R.J.; Stepanyan, R.; Fujiwara, I.; Bird, J.E.; Riazuddin, S.; Riazuddin, S.; Ahmed, Z.M.; et al. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell 2010, 141, 786–798. [Google Scholar] [CrossRef] [Green Version]
- Slepecky, N.; Chamberlain, S.C. Distribution and polarity of actin in the sensory hair cells of the chinchilla cochlea. Cell Tissue Res. 1982, 224, 15–24. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandekerckhove, J.; Weber, K. At least six different actins are expressed in a higher mammal: An analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J. Mol. Biol. 1978, 126, 783–802. [Google Scholar] [CrossRef]
- Furness, D.N.; Katori, Y.; Mahendrasingam, S.; Hackney, C.M. Differential distribution of beta- and gamma-actin in guinea-pig cochlear sensory and supporting cells. Hear. Res. 2005, 207, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Arima, T.; Uemura, T.; Yamamoto, T. Three-dimensional visualizations of the inner ear hair cell of the guinea pig. A rapid-freeze, deep-etch study of filamentous and membranous organelles. Hear. Res. 1987, 25, 61–68. [Google Scholar] [CrossRef]
- Vranceanu, F.; Perkins, G.A.; Terada, M.; Chidavaenzi, R.L.; Ellisman, M.H.; Lysakowski, A. Striated organelle, a cytoskeletal structure positioned to modulate hair-cell transduction. Proc. Natl. Acad. Sci. USA 2012, 109, 4473–4478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoendlin, H. The organization of the cochlear receptor. Fortschr. Hals Nasen Ohrenheilkd. 1966, 13, 1–227. [Google Scholar] [PubMed]
- Slepecky, N.; Hamernik, R.; Henderson, D. The consistent occurrence of a striated organelle (Friedmann body) in the inner hair cells of the normal chinchilla. Acta Otolaryngol. 1981, 91, 189–198. [Google Scholar] [CrossRef]
- Ross, M.D.; Bourne, C. Interrelated striated elements in vestibular hair cells of the rat. Science 1983, 220, 622–624. [Google Scholar] [CrossRef]
- Karavitaki, K.D.; Corey, D.P. Sliding adhesion confers coherent motion to hair cell stereocilia and parallel gating to transduction channels. J. Neurosci. 2010, 30, 9051–9063. [Google Scholar] [CrossRef] [Green Version]
- Hudspeth, A.J. The hair cells of the inner ear. Sci. Am. 1983, 248, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A.J. Hair-bundle mechanics and a model for mechanoelectrical transduction by hair cells. Soc. Gen. Physiol. Ser. 1992, 47, 357–370. [Google Scholar] [PubMed]
- Gunning, P.W.; Hardeman, E.C.; Lappalainen, P.; Mulvihill, D.P. Tropomyosin—Master regulator of actin filament function in the cytoskeleton. J. Cell Sci. 2015, 128, 2965–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seipel, K.; O’Brien, S.P.; Iannotti, E.; Medley, Q.G.; Streuli, M. Tara, a novel F-actin binding protein, associates with the Trio guanine nucleotide exchange factor and regulates actin cytoskeletal organization. J. Cell Sci. 2001, 114, 389–399. [Google Scholar]
- Shahin, H.; Walsh, T.; Sobe, T.; Abu Sa’ed, J.; Abu Rayan, A.; Lynch, E.D.; Lee, M.K.; Avraham, K.B.; King, M.C.; Kanaan, M. Mutations in a novel isoform of TRIOBP that encodes a filamentous-actin binding protein are responsible for DFNB28 recessive nonsyndromic hearing loss. Am. J. Hum. Genet. 2006, 78, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Riazuddin, S.; Khan, S.N.; Ahmed, Z.M.; Ghosh, M.; Caution, K.; Nazli, S.; Kabra, M.; Zafar, A.U.; Chen, K.; Naz, S.; et al. Mutations in TRIOBP, which encodes a putative cytoskeletal-organizing protein, are associated with nonsyndromic recessive deafness. Am. J. Hum. Genet. 2006, 78, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.; Bielski, E.; Bachhawat, A.; Taha, D.; Gunther, L.K.; Thirumurugan, K.; Kitajiri, S.; Sakamoto, T. R1 motif is the major actin-binding domain of TRIOBP-4. Biochemistry 2013, 52, 5256–5264. [Google Scholar] [CrossRef]
- Katsuno, T.; Belyantseva, I.A.; Cartagena-Rivera, A.X.; Ohta, K.; Crump, S.M.; Petralia, R.S.; Ono, K.; Tona, R.; Imtiaz, A.; Rehman, A.; et al. TRIOBP-5 sculpts stereocilia rootlets and stiffens supporting cells enabling hearing. JCI Insight 2019, 4, 128561. [Google Scholar] [CrossRef]
- Kazmierczak, M.; Kazmierczak, P.; Peng, A.W.; Harris, S.L.; Shah, P.; Puel, J.L.; Lenoir, M.; Franco, S.J.; Schwander, M. Pejvakin, a candidate stereociliary rootlet protein, regulates hair cell function in a cell-autonomous manner. J. Neurosci. 2017, 37, 3447–3464. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.U.; Morell, R.J.; Belyantseva, I.A.; Khan, S.Y.; Boger, E.T.; Shahzad, M.; Ahmed, Z.M.; Riazuddin, S.; Khan, S.N.; Riazuddin, S.; et al. Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am. J. Hum. Genet. 2010, 86, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Men, Y.; Li, X.; Tu, H.; Zhang, A.; Fu, X.; Wang, Z.; Jin, Y.; Hou, C.; Zhang, T.; Zhang, S.; et al. Tprn is essential for the integrity of stereociliary rootlet in cochlear hair cells in mice. Front. Med. 2018, 13, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wu, Z.; Müller, U. Murine Fam65b forms ring-like structures at the base of stereocilia critical for mechanosensory hair cell function. Elife 2016, 5, e14222. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, N.; Tung, C.Y.; Perrin, B.J.; Zhao, B. GRXCR2 regulates taperin localization critical for stereocilia morphology and hearing. Cell Rep. 2018, 25, 1268–1280.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, D.I.; Shen, J.; Corey, D.P.; Chen, Z.Y. Gene expression by mouse inner ear hair cells during development. J. Neurosci. 2015, 35, 6366–6380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Qi, J.; Chen, X.; Tang, M.; Chu, C.; Zhu, W.; Li, H.; Tian, C.; Yang, G.; Zhong, C.; et al. Critical role of spectrin in hearing development and deafness. Sci. Adv. 2019, 5, eaav7803. [Google Scholar] [CrossRef] [Green Version]
- Salles, F.T.; Andrade, L.R.; Tanda, S.; Grati, M.; Plona, K.L.; Gagnon, L.H.; Johnson, K.R.; Kachar, B.; Berryman, M.A. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton (Hoboken) 2014, 71, 61–78. [Google Scholar] [CrossRef]
- Hasson, T.; Gillespie, P.G.; Garcia, J.A.; MacDonald, R.B.; Zhao, Y.; Yee, A.G.; Mooseker, M.S.; Corey, D.P. Unconventional myosins in inner-ear sensory epithelia. J. Cell Biol. 1997, 137, 1287–1307. [Google Scholar] [CrossRef] [Green Version]
- Furness, D.N.; Karkanevatos, A.; West, B.; Hackney, C.M. An immunogold investigation of the distribution of calmodulin in the apex of cochlear hair cells. Hear. Res. 2002, 173, 10–20. [Google Scholar] [CrossRef]
- Liberman, M.C. Chronic ultrastructural changes in acoustic trauma: Serial-section reconstruction of stereocilia and cuticular plates. Hear. Res. 1987, 26, 65–88. [Google Scholar] [CrossRef]
- Howard, J.; Hudspeth, A.J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron 1988, 1, 189–199. [Google Scholar] [CrossRef]
- Pickles, J.O. A model for the mechanics of the stereociliar bundle on acousticolateral hair cells. Hear. Res. 1993, 68, 159–172. [Google Scholar] [CrossRef]
- Jaramillo, F.; Hudspeth, A.J. Displacement-clamp measurement of the forces exerted by gating springs in the hair bundle. Proc. Natl. Acad. Sci. USA 1993, 90, 1330–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobin, M.; Chaiyasitdhi, A.; Michel, V.; Michalski, N.; Martin, P. Stiffness and tension gradients of the hair cell’s tip-link complex in the mammalian cochlea. Elife 2019, 8, e43473. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Mehta, A.D.; Hudspeth, A.J. Negative hair-bundle stiffness betrays a mechanism for mechanical amplification by the hair cell. Proc. Natl. Acad. Sci. USA 2000, 97, 12026–12031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J. Unconventional mechanics of lipid membranes: A potential role for mechanotransduction of hair cell stereocilia. Biophys. J. 2015, 108, 610–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilney, L.G.; Saunders, J.C.; Egelman, E.; DeRosier, D.J. Changes in the organization of actin filaments in the stereocilia of noise-damaged lizard cochleae. Hear. Res. 1982, 7, 181–197. [Google Scholar] [CrossRef]
- Tilney, L.G.; Egelman, E.H.; DeRosier, D.J.; Saunders, J.C. Actin filaments, stereocilia, and hair cells of the bird cochlea. II. Packing of actin filaments in the stereocilia and in the cuticular plate and what happens to the organization when the stereocilia are bent. J. Cell. Biol. 1983, 96, 822–834. [Google Scholar] [CrossRef] [Green Version]
- Narayan, K.; Subramaniam, S. Focused ion beams in biology. Nat. Methods 2015, 12, 1021. [Google Scholar] [CrossRef]
- Pfeffer, S.; Mahamid, J. Unravelling molecular complexity in structural cell biology. Curr Opin. Struct. Biol. 2018, 52, 111. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacentine, I.; Chatterjee, P.; Barr-Gillespie, P.G. Stereocilia Rootlets: Actin-Based Structures That Are Essential for Structural Stability of the Hair Bundle. Int. J. Mol. Sci. 2020, 21, 324. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010324
Pacentine I, Chatterjee P, Barr-Gillespie PG. Stereocilia Rootlets: Actin-Based Structures That Are Essential for Structural Stability of the Hair Bundle. International Journal of Molecular Sciences. 2020; 21(1):324. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010324
Chicago/Turabian StylePacentine, Itallia, Paroma Chatterjee, and Peter G. Barr-Gillespie. 2020. "Stereocilia Rootlets: Actin-Based Structures That Are Essential for Structural Stability of the Hair Bundle" International Journal of Molecular Sciences 21, no. 1: 324. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010324




