Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells
Abstract
:1. Introduction
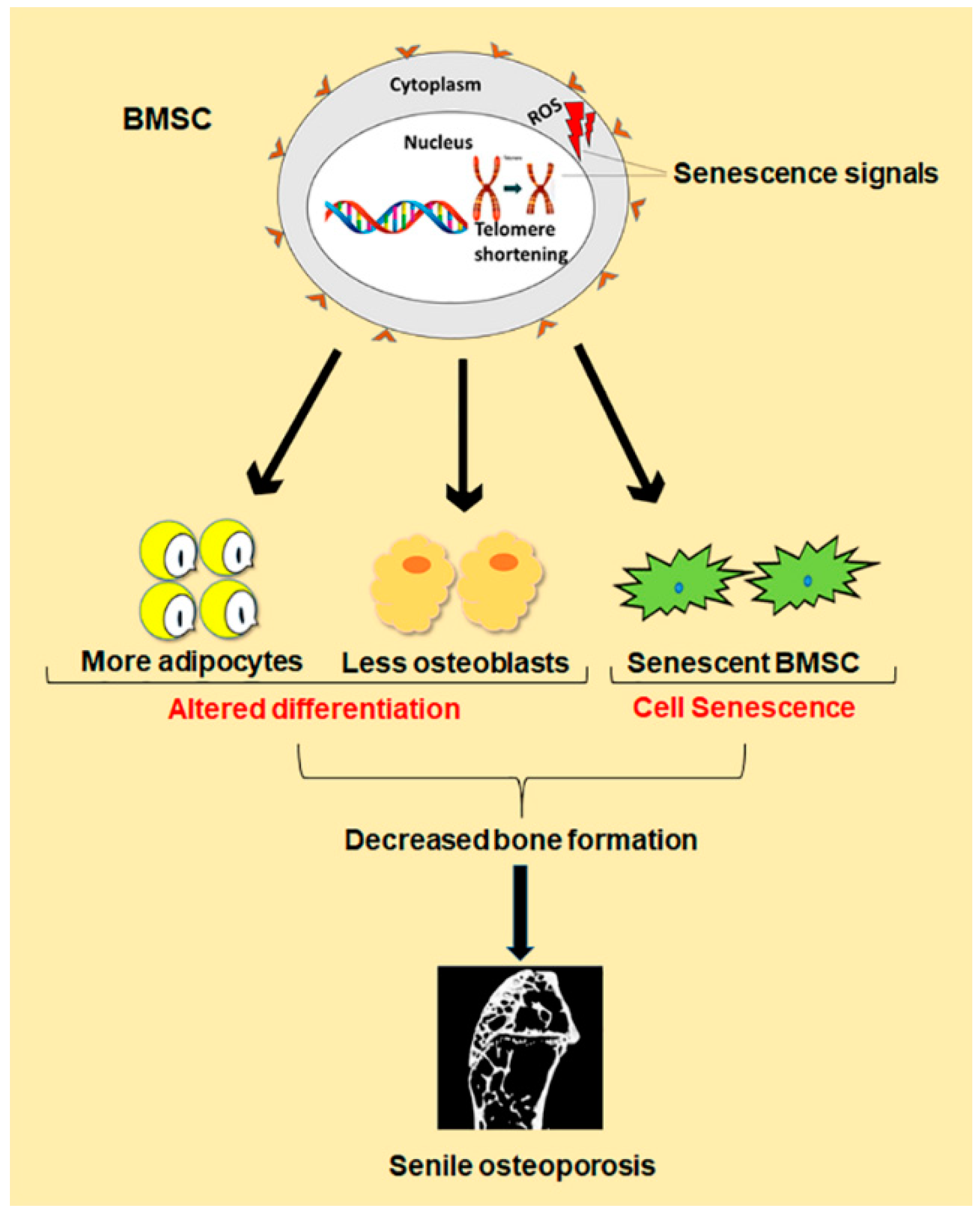
2. Bone Marrow Stromal Cells (BMSCs) and Function Alterations of BMSCs in Senile Osteoporosis
2.1. Differentiation of BMSCs and Senile Osteoporosis
2.2. Senescence of BMSCs and Senile Osteoporosis
3. Molecular Mechanisms Regulating Differentiation and Senescence of BMSCs during Senile Osteoporosis
3.1. Transcription Factors
3.1.1. Transcription Factors Involved in Osteogenic Differentiation of BMSCs
3.1.2. Transcription Factors Involved in Adipogenic Differentiation of BMSCs
3.1.3. Transcription Factors Involved in Senescence of BMSCs
3.2. Signaling Pathways
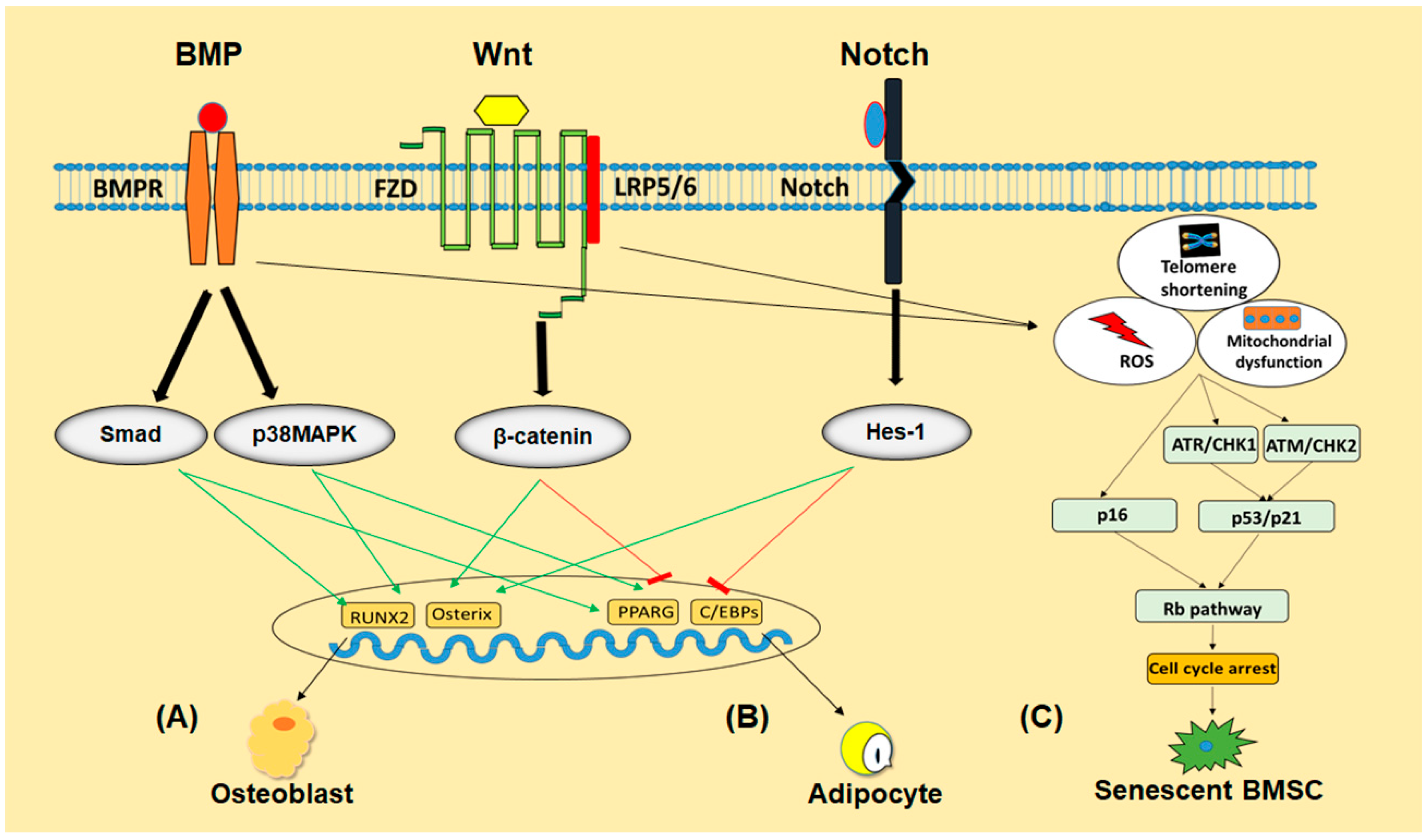
3.2.1. Signaling Pathways Involved in Differentiation of BMSCs
BMP Signaling
Wnt Signaling
Notch Signaling
Other Signaling Pathways
3.2.2. Signaling Pathways Involved in Senescence of BMSCs
p53/p21 and p16/Rb
Other Signaling Pathways
3.3. Epigenetic Regulations
3.3.1. Epigenetic Factors Involved in Osteogenic Differentiation of BMSCs
3.3.2. Epigenetic Factors Involved in Adipogenic Differentiation of BMSCs
3.3.3. Epigenetic Factors Involved in Senescence of BMSCs
3.4. Other Factors
3.4.1. Chemical Factors
3.4.2. Physical Factors
3.4.3. Biological Factors
4. Treatment of Senile Osteoporosis by Aiming at BMSCs
5. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | ascorbic acid |
| Ash1l | absent, small, or homeotic disc1 like |
| Ash1l | alkaline phosphatase |
| AMPK | adenosine monophosphate-activated protein kinase |
| βGP | β-glycerophosphate |
| BMD | bone mineral density |
| BMP | bone morphogenic protein |
| BMSCs | bone marrow stromal cells |
| CEBPB | CCAAT/enhancer-binding protein beta |
| C/EBPα | core binding factor α1 |
| DLX5 | distal-less Homeobox 5 |
| DDR | DNA damage response |
| DNMT | DNA methyltransferase |
| EBF-1 | early B cell factor |
| EZH2 | enhancer of zeste homology 2 |
| Ezh2-H3k27me3 | enhancer of zeste homolog2-tri-methylation of histone H3 at Lys 27 |
| FGFs | fibroblast growth factors Foxa1 |
| FOX | forkhead transcription factor |
| FZD | 7-transmembrane domain-spanning Frizzled receptor |
| GATA2 | GATA-binding factor 2 |
| HDACs | histone deacetylases |
| HOXA-AS2 | HOXA Cluster Antisense RNA 2 |
| HIF1 | Hypoxia-Inducible Factor 1 |
| IBMX | isobutylmethylxanthine |
| IGF-I | insulin-like growth factor-I |
| JAK | janus kinase |
| LRP5/6 | lipoprotein receptors-related protein 5/6 |
| MAPK | mitogen-activated protein kinase |
| miRNA | microRNA |
| Nampt | nicotinamide phosphoribosyltransferase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NELL-1 | neural epidermal growth factor-like (NEL)-like protein 1 |
| NRF2 | nuclear factor Erythroid 2-related factor 2 |
| ObI-1 | osteoblast inducer 1 |
| OC | osteocalcin |
| OG | orcinol glucoside |
| PcG | Polycomb group |
| PPARγ | peroxisome proliferator-activated receptor-gamma |
| Rb | retinoblastoma |
| ROS | reactive oxygen species |
| Runx2 | runt-related transcription factor 2 |
| SAMP6 | senescence accelerated mouse prone 6 |
| SASP | senescence-associated secretory phenotype |
| SIRT1 | silent information regulator 1 |
| Sox2 | sex determining region Y-box 2 |
| TGF-β | transforming growth factor-β TGF-β |
| TMP | tetramethylpyrazine |
| TNF-α | tumor necrosis factor-α |
| Twist | twist-related protein |
| Wnt | wingless-type MMTV integration site |
| Xist | X-inactive specific transcript |
| YAP | yes-associated protein 1 |
References
- Ge, D.W.; Wang, W.W.; Chen, H.T.; Yang, L.; Cao, X.J. Functions of microRNAs in osteoporosis. Eur. Rev. Med. Pharm. Sci. 2017, 21, 4784–4789. [Google Scholar]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vondracek, S.F.; Linnebur, S.A. Diagn116osis and management of osteoporosis in the older senior. Clin. Interv. Aging 2009, 4, 121–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R.; National Osteoporosis, F. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [Green Version]
- Briot, K.; Roux, C.; Thomas, T.; Blain, H.; Buchon, D.; Chapurlat, R.; Debiais, F.; Feron, J.M.; Gauvain, J.B.; Guggenbuhl, P.; et al. 2018 update of French recommendations on the management of postmenopausal osteoporosis. Jt. Bone Spine 2018, 85, 519–530. [Google Scholar] [CrossRef]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, J.; Davies, J.E.; Stanford, W.L. Concise Review: Musculoskeletal Stem Cells to Treat Age-Related Osteoporosis. Stem Cells Transl. Med. 2017, 6, 1930–1939. [Google Scholar] [CrossRef]
- Infante, A.; Rodriguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [Green Version]
- Nelson, G.; Wordsworth, J.; Wang, C.; Jurk, D.; Lawless, C.; Martin-Ruiz, C.; von Zglinicki, T. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y.; Van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, S.; Farr, J.N.; Kirkland, J.L. Inhibiting cellular senescence: A new therapeutic paradigm for age-related osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriano, C.A.; Issack, P.S.; Shindle, L.; Werner, C.M.; Helfet, D.L.; Lane, J.M. Recent advances toward the clinical application of PTH (1-34) in fracture healing. HSS J. 2009, 5, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Hilgenbrink, A.R.; Low, P.S. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Dinavahi, R.V.; Lazaretti-Castro, M.; Ebeling, P.R.; Adachi, J.D.; Miyauchi, A.; Gielen, E.; Milmont, C.E.; Libanati, C.; Grauer, A. One Year of Romosozumab Followed by Two Years of Denosumab Maintains Fracture Risk Reductions: Results of the FRAME Extension Study. J. Bone Miner. Res. 2019, 34, 419–428. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat. Rev. Genet. 2011, 12, 341–355. [Google Scholar] [CrossRef]
- Rehman, Z.; Zuhorn, I.S.; Hoekstra, D. How cationic lipids transfer nucleic acids into cells and across cellular membranes: Recent advances. J. Control. Release 2013, 166, 46–56. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [Green Version]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tsai, Y.-T.; DiMarco, N.M.; Long, M.A.; Sun, X.; Tang, L. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci. Rep. 2011, 1, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Meng, H.; Wang, X.; Zhao, C.; Peng, J.; Wang, Y. Differentiation of Bone Marrow Mesenchymal Stem Cells in Osteoblasts and Adipocytes and its Role in Treatment of Osteoporosis. Med. Sci. Monit. 2016, 22, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Justesen, J.; Stenderup, K.; Ebbesen, E.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef]
- Verma, S.; Rajaratnam, J.; Denton, J.; Hoyland, J.; Byers, R. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 2002, 55, 693–698. [Google Scholar] [CrossRef]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.; Boyette, L.B.; Tuan, R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 2015, 70, 37–47. [Google Scholar] [CrossRef]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef] [Green Version]
- Mattiucci, D.; Maurizi, G.; Leoni, P.; Poloni, A. Aging-and Senescence-associated Changes of Mesenchymal Stromal Cells in Myelodysplastic Syndromes. Cell Transplant. 2018, 27, 754–764. [Google Scholar] [CrossRef] [Green Version]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef]
- Fathi, E.; Charoudeh, H.N.; Sanaat, Z.; Farahzadi, R. Telomere shortening as a hallmark of stem cell senescence. Stem Cell Investig. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, U.; Helmbold, H.; Squillaro, T.; Alessio, N.; Komm, N.; Khadang, B.; Cipollaro, M.; Bohn, W.; Giordano, A. In vitro senescence of rat mesenchymal stem cells is accompanied by downregulation of stemness-related and DNA damage repair genes. Stem Cells Dev. 2009, 18, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Vono, R.; Jover Garcia, E.; Spinetti, G.; Madeddu, P. Oxidative stress in mesenchymal stem cell senescence: Regulation by coding and noncoding RNAs. Antioxid. Redox Signal. 2018, 29, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Roninson, I.B. Oncogenic functions of tumour suppressor p21Waf1/Cip1/Sdi1: Association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 2002, 179, 1–14. [Google Scholar] [CrossRef]
- Kang, C.; Xu, Q.; Martin, T.D.; Li, M.Z.; Demaria, M.; Aron, L.; Lu, T.; Yankner, B.A.; Campisi, J.; Elledge, S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015, 349, aaa5612. [Google Scholar] [CrossRef] [Green Version]
- Acosta, J.C.; O’Loghlen, A.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; Da Costa, M.; Brown, C.; Popov, N. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef] [Green Version]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, C.; Choi, Y.S.; Kim, M.; Park, C.; Suh, Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: Implication to age-associated bone diseases and defects. Mech. Ageing Dev. 2012, 133, 215–225. [Google Scholar] [CrossRef]
- Sepúlveda, J.C.; Tomé, M.; Fernández, M.E.; Delgado, M.; Campisi, J.; Bernad, A.; González, M.A. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cells 2014, 32, 1865–1877. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, X.; Wang, L.; Liu, G.; Li, Y.; Wu, X.; Jing, Y.; Li, H.; Wang, G. Senescent mesenchymal stem cells promote colorectal cancer cells growth via galectin-3 expression. Cell Biosci. 2015, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Skolekova, S.; Matuskova, M.; Bohac, M.; Toro, L.; Durinikova, E.; Tyciakova, S.; Demkova, L.; Gursky, J.; Kucerova, L. Cisplatin-induced mesenchymal stromal cells-mediated mechanism contributing to decreased antitumor effect in breast cancer cells. Cell Commun. Signal. 2016, 14, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L. Identification of senescent cells in the bone microenvironment. J. Bone Miner. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Geyh, S.; Öz, S.; Cadeddu, R.; Fröbel, J.; Brückner, B.; Kündgen, A.; Fenk, R.; Bruns, I.; Zilkens, C.; Hermsen, D. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia 2013, 27, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Jang, D.-J. Protein Kinase CK2 Regulates Cytoskeletal Reorganization during Ionizing Radiation–Induced Senescence of Human Mesenchymal Stem Cells. Cancer Res. 2009, 69, 8200–8207. [Google Scholar] [CrossRef] [Green Version]
- Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995, 55, 2284–2292. [Google Scholar]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Varela, N.; Aranguiz, A.; Lizama, C.; Sepulveda, H.; Antonelli, M.; Thaler, R.; Moreno, R.D.; Montecino, M.; Stein, G.S.; Van Wijnen, A.J. Mitotic inheritance of mRNA facilitates translational activation of the osteogenic-Lineage commitment factor runx2 in progeny of osteoblastic cells. J. Cell. Physiol. 2016, 231, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.C.; Yang, M.H.; Tsai, C.-C.; Huang, T.F.; Chen, Y.H.; Hung, S.C. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS ONE 2011, 6, e23965. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Mishima, H.; Sakai, S.; Liu, Y.K.; Ohyabu, Y.; Uemura, T. Gene expression analysis of major lineage-defining factors in human bone marrow cells: Effect of aging, gender, and age-related disorders. J. Orthop. Res. 2008, 26, 910–917. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Querques, F.; D’Agostino, A.; Cozzolino, C.; Cozzuto, L.; Lombardo, B.; Leggiero, E.; Ruosi, C.; Pastore, L. Identification of a Novel Transcription Factor Required for Osteogenic Differentiation of Mesenchymal Stem Cells. Stem Cells Dev. 2019, 28, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.E.; Moldes, M.; Farmer, S.R. The forkhead transcription factor FoxC2 inhibits white adipocyte differentiation. J. Biol. Chem. 2004, 279, 42453–42461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okitsu, Y.; Takahashi, S.; Minegishi, N.; Kameoka, J.; Kaku, M.; Yamamoto, M.; Sasaki, T.; Harigae, H. Regulation of adipocyte differentiation of bone marrow stromal cells by transcription factor GATA-2. Biochem. Biophys. Res. Commun. 2007, 364, 383–387. [Google Scholar] [CrossRef]
- Seifert, A.; Werheid, D.F.; Knapp, S.M.; Tobiasch, E. Role of Hox genes in stem cell differentiation. World J. Stem Cells 2015, 7, 583–595. [Google Scholar] [CrossRef]
- La Cour Poulsen, L.; Siersbæk, M.; Mandrup, S. PPARs: Fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 2012, 23, 631–639. [Google Scholar] [CrossRef]
- Bionaz, M.; Monaco, E.; Wheeler, M.B. Transcription adaptation during in vitro adipogenesis and osteogenesis of porcine mesenchymal stem cells: Dynamics of pathways, biological processes, up-stream regulators, and gene networks. PLoS ONE 2015, 10, e0137644. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Qi, C.; Korenberg, J.R.; Chen, X.-N.; Noya, D.; Rao, M.S.; Reddy, J.K. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: Alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc. Natl. Acad. Sci. USA 1995, 92, 7921–7925. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.H.; Li, F.G.; Chen, X.Y.; Li, J.T.; Wu, Y.H.; Huang, L.H.; Wang, Z.; Li, P.; Wang, T.; Lahn, B.T. PPARγ suppression inhibits adipogenesis but does not promote osteogenesis of human mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 377–384. [Google Scholar] [CrossRef]
- Hesslein, D.G.; Fretz, J.A.; Xi, Y.; Nelson, T.; Zhou, S.; Lorenzo, J.A.; Schatz, D.G.; Horowitz, M.C. Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone 2009, 44, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Ambrosi, G.; Gammoh, O.; Blandini, F.; Cereda, C. SOD1 and DJ-1 converge at Nrf2 pathway: A clue for antioxidant therapeutic potential in neurodegeneration. Oxidative Med. Cell. Longev. 2013, 2013, 836760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liu, P.; Xu, S.; Li, Y.; Dekker, J.D.; Li, B.; Fan, Y.; Zhang, Z.; Hong, Y.; Yang, G. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J. Clin. Investig. 2017, 127, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Freire, M.O.; You, H.-K.; Kook, J.-K.; Choi, J.-H.; Zadeh, H.H. Antibody-mediated osseous regeneration: A novel strategy for bioengineering bone by immobilized anti–bone morphogenetic protein-2 antibodies. Tissue Eng. Part. A 2011, 17, 2911–2918. [Google Scholar] [CrossRef]
- Kang, Q.; Song, W.-X.; Luo, Q.; Tang, N.; Luo, J.; Luo, X.; Chen, J.; Bi, Y.; He, B.C.; Park, J.K. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2008, 18, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef] [Green Version]
- Zur Nieden, N.I.; Kempka, G.; Rancourt, D.E.; Ahr, H.-J. Induction of chondro-, osteo-and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: Effect of cofactors on differentiating lineages. BMC Dev. Biol. 2005, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.L.; Sharff, K.A.; Tang, N.; Song, W.X.; Luo, J.; Luo, X.; Chen, J.; Bennett, E.; Reid, R.; Manning, D. Regulation of osteogenic differentiation during skeletal development. Front. Biosci. 2008, 13, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Miyazono, K.; Maeda, S.; Imamura, T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005, 16, 251–263. [Google Scholar] [CrossRef]
- Li, X.; Cao, X. BMP signaling and skeletogenesis. Ann. N. Y. Acad. Sci. 2006, 1068, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- White, B.D.; Chien, A.J.; Dawson, D.W. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H.; Loh, K.M.; Nusse, R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Roman, A.; Sinal, C. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.; Hwang, J.; Kim, A.; Kim, K.; Hwang, E.; Yaffe, M.; Hong, J.-H. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ. 2014, 21, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, C.N.; Ouyang, H.; Ma, Y.L.; Zeng, Q.; Gerin, I.; Sousa, K.M.; Lane, T.F.; Krishnan, V.; Hankenson, K.D.; MacDougald, O.A. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J. Bone Miner. Res. 2007, 22, 1924–1932. [Google Scholar] [CrossRef]
- Stevens, J.R.; Miranda-Carboni, G.A.; Singer, M.A.; Brugger, S.M.; Lyons, K.M.; Lane, T.F. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J. Bone Miner. Res. 2010, 25, 2138–2147. [Google Scholar] [CrossRef] [Green Version]
- Arango, N.A.; Szotek, P.P.; Manganaro, T.F.; Oliva, E.; Donahoe, P.K.; Teixeira, J. Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev. Biol. 2005, 288, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Tanaka, T.; Iso, T.; Matsui, H.; Ooyama, Y.; Kawai-Kowase, K.; Arai, M.; Kurabayashi, M. Notch signaling pathway enhances bone morphogenetic protein 2 (BMP2) responsiveness of Msx2 gene to induce osteogenic differentiation and mineralization of vascular smooth muscle cells. J. Biol. Chem. 2011, 286, 19138–19148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinella-Jaegle, S.; Rawadi, G.; Kawai, S.; Gallea, S.; Faucheu, C.; Mollat, P.; Courtois, B.; Bergaud, B.; Ramez, V.; Blanchet, A.M. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J. Cell Sci. 2001, 114, 2085–2094. [Google Scholar] [PubMed]
- James, A.W.; Pan, A.; Chiang, M.; Zara, J.N.; Zhang, X.; Ting, K.; Soo, C. A new function of Nell-1 protein in repressing adipogenic differentiation. Biochem. Biophys. Res. Commun. 2011, 411, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zara, J.; Siu, R.; Ting, K.; Soo, C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J. Dent. Res. 2010, 89, 865–878. [Google Scholar] [CrossRef]
- Neubauer, M.; Fischbach, C.; Bauer-Kreisel, P.; Lieb, E.; Hacker, M.; Tessmar, J.; Schulz, M.B.; Goepferich, A.; Blunk, T. Basic fibroblast growth factor enhances PPARγ ligand-induced adipogenesis of mesenchymal stem cells. FEBS Lett. 2004, 577, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Woei Ng, K.; Speicher, T.; Dombrowski, C.; Helledie, T.; Haupt, L.M.; Nurcombe, V.; Cool, S.M. Osteogenic differentiation of murine embryonic stem cells is mediated by fibroblast growth factor receptors. Stem Cells Dev. 2007, 16, 305–318. [Google Scholar] [CrossRef]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008, 29, 535–559. [Google Scholar] [CrossRef] [Green Version]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Di Fagagna, F.d.A. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Senescence and immortalization: Role of telomeres and telomerase. Carcinogenesis 2004, 26, 867–874. [Google Scholar] [CrossRef]
- Moussavi-Harami, F.; Duwayri, Y.; Martin, J.A.; Moussavi-Harami, F.; Buckwalter, J.A. Oxygen effects on senescence in chondrocytes and mesenchymal stem cells: Consequences for tissue engineering. Iowa Orthop. J. 2004, 24, 15. [Google Scholar]
- Wu, J.; Niu, J.; Li, X.; Wang, X.; Guo, Z.; Zhang, F. TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev. Biol. 2014, 14, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Tan, W.; Feng, G.; Meng, Y.; Shen, B.; Liu, H.; Cheng, C. Wnt/β-catenin signaling mediates the senescence of bone marrow-mesenchymal stem cells from systemic lupus erythematosus patients through the p53/p21 pathway. Mol. Cell. Biochem. 2014, 387, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Yan, Z.; Pei, M. A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration. Cell Biosci. 2019, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Westendorf, J.J. Transcriptional co-repressors of Runx2. J. Cell. Biochem. 2006, 98, 54–64. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B.; van Wijnen, A.J.; Stein, J.L. The osteocalcin gene: A model for multiple parameters of skeletal-specific transcriptional control. Mol. Biol. Rep. 1997, 24, 185–196. [Google Scholar] [CrossRef]
- Shen, J.; Hovhannisyan, H.; Lian, J.B.; Montecino, M.A.; Stein, G.S.; Stein, J.L.; Van Wijnen, A.J. Transcriptional induction of the osteocalcin gene during osteoblast differentiation involves acetylation of histones h3 and h4. Mol. Endocrinol. 2003, 17, 743–756. [Google Scholar] [CrossRef]
- Villar-Garea, A.; Esteller, M. Histone deacetylase inhibitors: Understanding a new wave of anticancer agents. Int. J. Cancer 2004, 112, 171–178. [Google Scholar] [CrossRef]
- Villagra, A.; Gutiérrez, J.; Paredes, R.; Sierra, J.; Puchi, M.; Imschenetzky, M.; Van Wijnen, A.; Lian, J.; Stein, G.; Stein, J. Reduced CpG methylation is associated with transcriptional activation of the bone-specific rat osteocalcin gene in osteoblasts. J. Cell. Biochem. 2002, 85, 112–122. [Google Scholar] [CrossRef]
- Arnsdorf, E.J.; Tummala, P.; Castillo, A.B.; Zhang, F.; Jacobs, C.R. The epigenetic mechanism of mechanically induced osteogenic differentiation. J. Biomech. 2010, 43, 2881–2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, M.; Huang, P.; Islam, S.; Heruth, D.P.; Li, X.; Zhang, L.Q.; Li, D.-Y.; Hu, Z.; Ye, S.Q. Epigenetic regulation of Runx2 transcription and osteoblast differentiation by nicotinamide phosphoribosyltransferase. Cell Biosci. 2017, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominaga, H.; Maeda, S.; Hayashi, M.; Takeda, S.; Akira, S.; Komiya, S.; Nakamura, T.; Akiyama, H.; Imamura, T. CCAAT/enhancer-binding protein β promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol. Biol. Cell 2008, 19, 5373–5386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, B.; Yu, F.; Wang, C.; Li, B.; Liu, M.; Ye, L. Epigenetic Control of Mesenchymal Stem Cell Fate Decision via Histone Methyltransferase Ash1l. Stem Cells 2019, 37, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baglìo, S.R.; Devescovi, V.; Granchi, D.; Baldini, N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene 2013, 527, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, T.; Taipaleenmäki, H.; Stenvang, J.; Abdallah, B.M.; Ditzel, N.; Nossent, A.Y.; Bak, M.; Kauppinen, S.; Kassem, M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 6139–6144. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhao, L.; Xing, L.; Chen, D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 2010, 28, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fu, W.M.; He, M.L.; Wang, H.; Wang, W.M.; Yu, S.C.; Bian, X.W.; Zhou, J.; Lin, M.C.; Lu, G. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol. Biol. Cell 2011, 22, 3955–3961. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Guo, Y.; Wang, Y.; Li, Y.; Wang, J. MicroRNA profiles of BMSCs induced into osteoblasts with osteoinductive medium. Exp. Ther. Med. 2018, 15, 2589–2596. [Google Scholar] [CrossRef] [Green Version]
- Morrison, R.F.; Farmer, S.R. Insights into the transcriptional control of adipocyte differentiation. J. Cell. Biochem. 1999, 75, 59–67. [Google Scholar] [CrossRef]
- Noer, A.; Sørensen, A.L.; Boquest, A.C.; Collas, P. Stable CpG hypomethylation of adipogenic promoters in freshly isolated, cultured, and differentiated mesenchymal stem cells from adipose tissue. Mol. Biol. Cell 2006, 17, 3543–3556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowers, R.R.; Kim, J.W.; Otto, T.C.; Lane, M.D. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: Role of the BMP-4 gene. Proc. Natl. Acad. Sci. USA 2006, 103, 13022–13027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musri, M.M.; Corominola, H.; Casamitjana, R.; Gomis, R.; Párrizas, M. Histone H3 lysine 4 dimethylation signals the transcriptional competence of the adiponectin promoter in preadipocytes. J. Biol. Chem. 2006, 281, 17180–17188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajas, L.; Egler, V.; Reiter, R.; Hansen, J.; Kristiansen, K.; Debril, M.-B.; Miard, S.; Auwerx, J. The retinoblastoma-histone deacetylase 3 complex inhibits PPARγ and adipocyte differentiation. Dev. Cell 2002, 3, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Qadir, A.S.; Woo, K.M.; Ryoo, H.-M.; Baek, J.-H. Insulin suppresses distal-less homeobox 5 expression through the up-regulation of microRNA-124 in 3T3-L1 cells. Exp. Cell Res. 2013, 319, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Hamam, D.; Ali, D.; Vishnubalaji, R.; Hamam, R.; Al-Nbaheen, M.; Chen, L.; Kassem, M.; Aldahmash, A.; Alajez, N.M. microRNA-320/RUNX2 axis regulates adipocytic differentiation of human mesenchymal (skeletal) stem cells. Cell Death Amp. Dis. 2014, 5, e1499. Available online: https://0-www-nature-com.brum.beds.ac.uk/articles/cddis2014462#supplementary-information (accessed on 12 December 2019). [CrossRef] [PubMed] [Green Version]
- Zaragosi, L.-E.; Wdziekonski, B.; Brigand, K.L.; Villageois, P.; Mari, B.; Waldmann, R.; Dani, C.; Barbry, P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011, 12, R64. [Google Scholar] [CrossRef] [Green Version]
- Li, C.J.; Cheng, P.; Liang, M.K.; Chen, Y.S.; Lu, Q.; Wang, J.Y.; Xia, Z.Y.; Zhou, H.D.; Cao, X.; Xie, H. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015, 125, 1509–1522. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, O.; Mitchum, R.D., Jr.; Schrier, L.; Ferns, S.P.; Barnes, K.M.; Troendle, J.F.; Baron, J. Growth plate senescence is associated with loss of DNA methylation. J. Endocrinol. 2005, 186, 241–249. [Google Scholar] [CrossRef]
- So, A.Y.; Jung, J.W.; Lee, S.; Kim, H.S.; Kang, K.S. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS ONE 2011, 6, e19503. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.W.; Lee, S.; Seo, M.S.; Park, S.B.; Kurtz, A.; Kang, S.K.; Kang, K.S. Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cell Mol. Life Sci. 2010, 67, 1165–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, A.F.; Bayon, G.F.; Urdinguio, R.G.; Torano, E.G.; Garcia, M.G.; Carella, A.; Petrus-Reurer, S.; Ferrero, C.; Martinez-Camblor, P.; Cubillo, I.; et al. H3K4me1 marks DNA regions hypomethylated during aging in human stem and differentiated cells. Genome Res. 2015, 25, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.K.; Kim, J.; Choi, S.J.; Noh, H.M.; Kwon, Y.D.; Yoo, H.; Yi, H.S.; Chung, H.M.; Kim, J.K. Discovery and characterization of novel microRNAs during endothelial differentiation of human embryonic stem cells. Stem Cells Dev. 2012, 21, 2049–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhamed, M.; Herbig, U.; Ye, T.; Dejean, A.; Bischof, O. Senescence is an endogenous trigger for microRNA-directed transcriptional gene silencing in human cells. Nat. Cell Biol. 2012, 14, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Kim, H.H.; Abdelmohsen, K.; Kuwano, Y.; Pullmann, R., Jr.; Srikantan, S.; Subrahmanyam, R.; Martindale, J.L.; Yang, X.; Ahmed, F.; et al. p16(INK4a) translation suppressed by miR-24. PLoS ONE 2008, 3, e1864. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [Green Version]
- Yamakuchi, M.; Lowenstein, C.J. MiR-34, SIRT1 and p53: The feedback loop. Cell Cycle 2009, 8, 712–715. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Liu, D.; Zhao, J.; Xu, J.; Ren, J.; Hu, Y.; Wang, Z.; Hou, Y.; Zhao, G. MiR-495 Promotes Senescence of Mesenchymal Stem Cells by Targeting Bmi-1. Cell Physiol. Biochem. 2017, 42, 780–796. [Google Scholar] [CrossRef]
- Okada, M.; Kim, H.W.; Matsu-ura, K.; Wang, Y.G.; Xu, M.; Ashraf, M. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells 2016, 34, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.; Fernandes, M. Human bone cell cultures in biocompatibility testing. Part II: Effect of ascorbic acid, β-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials 2000, 21, 1095–1102. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brindle, P.K.; Montminy, M.R. The CREB family of transcription activators. Curr. Opin. Genet. Dev. 1992, 2, 199–204. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93, S52–S59. [Google Scholar] [CrossRef]
- Chang, T.-C.; Hsu, M.-F.; Wu, K.K. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS ONE 2015, 10, e0126537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochane, M.; Trichet, V.; Pecqueur, C.; Avril, P.; Oliver, L.; Denis, J.; Brion, R.; Amiaud, J.; Pineau, A.; Naveilhan, P. Low-Dose Pesticide Mixture Induces Senescence in Normal Mesenchymal Stem Cells (MSC) and Promotes Tumorigenic Phenotype in Premalignant MSC. Stem Cells 2017, 35, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Stemig, M.E.; Takahashi, Y.; Hui, S.K. Radiation response of mesenchymal stem cells derived from bone marrow and human pluripotent stem cells. J. Radiat Res. 2015, 56, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessio, N.; Del Gaudio, S.; Capasso, S.; Di Bernardo, G.; Cappabianca, S.; Cipollaro, M.; Peluso, G.; Galderisi, U. Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget 2014, 6, 8155–8166. [Google Scholar] [CrossRef] [Green Version]
- Menuki, K.; Mori, T.; Sakai, A.; Sakuma, M.; Okimoto, N.; Shimizu, Y.; Kunugita, N.; Nakamura, T. Climbing exercise enhances osteoblast differentiation and inhibits adipogenic differentiation with high expression of PTH/PTHrP receptor in bone marrow cells. Bone 2008, 43, 613–620. [Google Scholar] [CrossRef]
- Demiray, L.; Ozcivici, E. Bone Marrow Stem Cells Adapt to Low-Magnitude Vibrations by Altering Theircytoskeleton During Quiescence and. Turk. J. Biol. 2015, 39, 88–97. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Gathings, W.E.; McDonald, J.M. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 2004, 145, 2421–2432. [Google Scholar] [CrossRef] [Green Version]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, S.V.; Renovato-Martins, M.; Ribeiro-Pereira, C.; Citelli, M.; Barja-Fidalgo, C. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity 2016, 24, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. From cells to organisms: Can we learn about aging from cells in culture? Exp. Gerontol. 2001, 36, 607–618. [Google Scholar] [CrossRef]
- Phetfong, J.; Sanvoranart, T.; Nartprayut, K.; Nimsanor, N.; Seenprachawong, K.; Prachayasittikul, V.; Supokawej, A. Osteoporosis: The current status of mesenchymal stem cell-based therapy. Cell. Mol. Biol. Lett. 2016, 21, 12. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Zhang, P.; Xue, S.; Xu, Y.; Tan, J.; Liu, G. Adipose-derived stem cells alleviate osteoporosis by enchancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy 2014, 16, 1643–1655. [Google Scholar] [CrossRef]
- Ichioka, N.; Inaba, M.; Kushida, T.; Esumi, T.; Takahara, K.; Inaba, K.; Ogawa, R.; Iida, H.; Ikehara, S. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells 2002, 20, 542–551. [Google Scholar] [CrossRef]
- Takada, K.; Inaba, M.; Ichioka, N.; Ueda, Y.; Taira, M.; Baba, S.; Mizokami, T.; Wang, X.; Hisha, H.; Iida, H.; et al. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells 2006, 24, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Ocarino Nde, M.; Boeloni, J.N.; Jorgetti, V.; Gomes, D.A.; Goes, A.M.; Serakides, R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect. Tissue Res. 2010, 51, 426–433. [Google Scholar] [CrossRef]
- Kiernan, J.; Hu, S.; Grynpas, M.D.; Davies, J.E.; Stanford, W.L. Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis. Stem Cells Transl. Med. 2016, 5, 683–693. [Google Scholar] [CrossRef] [Green Version]
- An, Q.; Wu, D.; Ma, Y.; Zhou, B.; Liu, Q. Suppression of Evi1 promotes the osteogenic differentiation and inhibits the adipogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro. Int. J. Mol. Med. 2015, 36, 1615–1622. [Google Scholar] [CrossRef]
- Jing, H.; Liao, L.; An, Y.; Su, X.; Liu, S.; Shuai, Y.; Zhang, X.; Jin, Y. Suppression of EZH2 prevents the shift of osteoporotic MSC fate to adipocyte and enhances bone formation during osteoporosis. Mol. Ther. 2016, 24, 217–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liu, Z.; Huang, B.; Yan, H.; Yang, C.; Li, Q.; Jin, D. Orcinol glucoside facilitates the shift of MSC fate to osteoblast and prevents adipogenesis via Wnt/β-catenin signaling pathway. Drug Des. Dev. Ther. 2019, 13, 2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Li, H.; Wang, S.; Li, T.; Fan, J.; Liang, X.; Li, J.; Han, Q.; Zhu, L.; Fan, L. let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014, 23, 1452–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Li, X.; Zou, D.; Lian, Y.; Tian, S.; Dou, Z. Expression of microRNA-21 in osteoporotic patients and its involvement in the regulation of osteogenic differentiation. Exp. Ther. Med. 2019, 17, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Xiao, Y.; Yang, M.; Su, T.; Sun, X.; Guo, Q.; Huang, Y.; Luo, X.-H. Long noncoding RNA Bmncr regulates mesenchymal stem cell fate during skeletal aging. J. Clin. Investig. 2018, 128, 5251–5266. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, L.; Ge, D.; Wang, W.; Yin, Z.; Yan, J.; Cao, X.; Jiang, C.; Zheng, S.; Liang, B. Long non‑coding RNA XIST promotes osteoporosis through inhibiting bone marrow mesenchymal stem cell differentiation. Exp. Ther. Med. 2019, 17, 803–811. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, J.; Du, J.; Zhong, G.; Qiao, L.; Lin, J. LncRNA HOXA-AS2 positively regulates osteogenesis of mesenchymal stem cells through inactivating NF-κB signalling. J. Cell. Mol. Med. 2019, 23, 1325–1332. [Google Scholar] [CrossRef]
- Kim, H.N.; Chang, J.; Shao, L.; Han, L.; Iyer, S.; Manolagas, S.C.; O’Brien, C.A.; Jilka, R.L.; Zhou, D.; Almeida, M. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell 2017, 16, 693–703. [Google Scholar] [CrossRef]
- Gao, B.; Lin, X.; Jing, H.; Fan, J.; Ji, C.; Jie, Q.; Zheng, C.; Wang, D.; Xu, X.; Hu, Y. Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice. Aging Cell 2018, 17, e12741. [Google Scholar] [CrossRef]
- Sun, J.; Ming, L.; Shang, F.; Shen, L.; Chen, J.; Jin, Y. Apocynin suppression of NADPH oxidase reverses the aging process in mesenchymal stem cells to promote osteogenesis and increase bone mass. Sci. Rep. 2015, 5, 18572. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Yan, Y.; Zhao, C.; Xu, Y.; Wang, Q.; Xu, N. Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Rep. 2019, 24, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Transcriptional Factors | Function | References |
|---|---|---|
| Runx2 | Promotes osteogenic differentiation, and inhibits adipogenic differentiation and senescence | [48,49,50] |
| Osterix | Promotes osteogenic differentiation | [51] |
| Obl-1 | Promotes osteogenic differentiation | [52] |
| PPARγ | Promotes adipogenic differentiation and senescence, and inhibits osteogenic differentiation | [50,56,57,58,59] |
| EBF-1 | Promotes adipogenic differentiation | [60] |
| NRF2 | Inhibits senescence | [61,62] |
| FOXP | Inhibits senescence | [63] |
| Signaling Pathways | Functions | References |
|---|---|---|
| TGF-β/BMP | Controls both osteogenesis and adipogenesis in a proper manner, and also induces senescence | [66,92] |
| Wnt | Induces osteogenesis and inhibits adipogenesis | [77,78] |
| Notch | Promotes osteogenesis and inhibits adipogenesis | [80] |
| Hedgehog | Promotes osteogenesis and suppresses adipogenesis | [82] |
| NELL-1 | Induces osteogenesis with antiadipogenic effects | [83] |
| FGFs | Control both osteogenesis and adipogenesis with equal effects | [85,86] |
| IGF-I | Promotes adipogenic differentiation | [87] |
| p53/p21 | Induces senescence | [88,89,90,91] |
| p16/Rb | Induces senescence | [88,89,90,91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadir, A.; Liang, S.; Wu, Z.; Chen, Z.; Hu, L.; Qian, A. Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells. Int. J. Mol. Sci. 2020, 21, 349. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010349
Qadir A, Liang S, Wu Z, Chen Z, Hu L, Qian A. Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells. International Journal of Molecular Sciences. 2020; 21(1):349. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010349
Chicago/Turabian StyleQadir, Abdul, Shujing Liang, Zixiang Wu, Zhihao Chen, Lifang Hu, and Airong Qian. 2020. "Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells" International Journal of Molecular Sciences 21, no. 1: 349. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010349





