Oxidative Stress-Responsive MicroRNAs in Heart Injury
Abstract
:1. Introduction
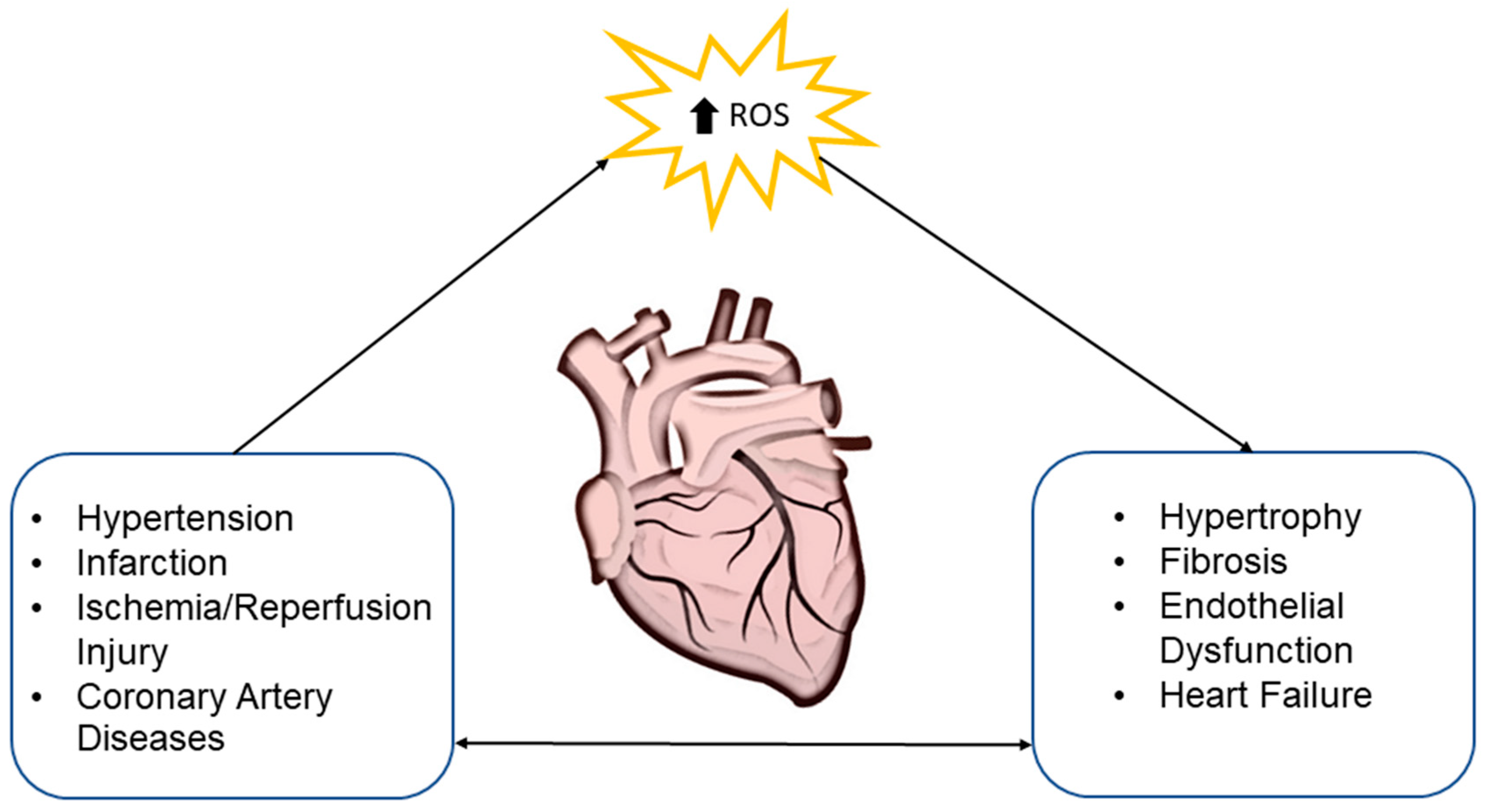
2. Oxidative Stress and Cardiovascular System
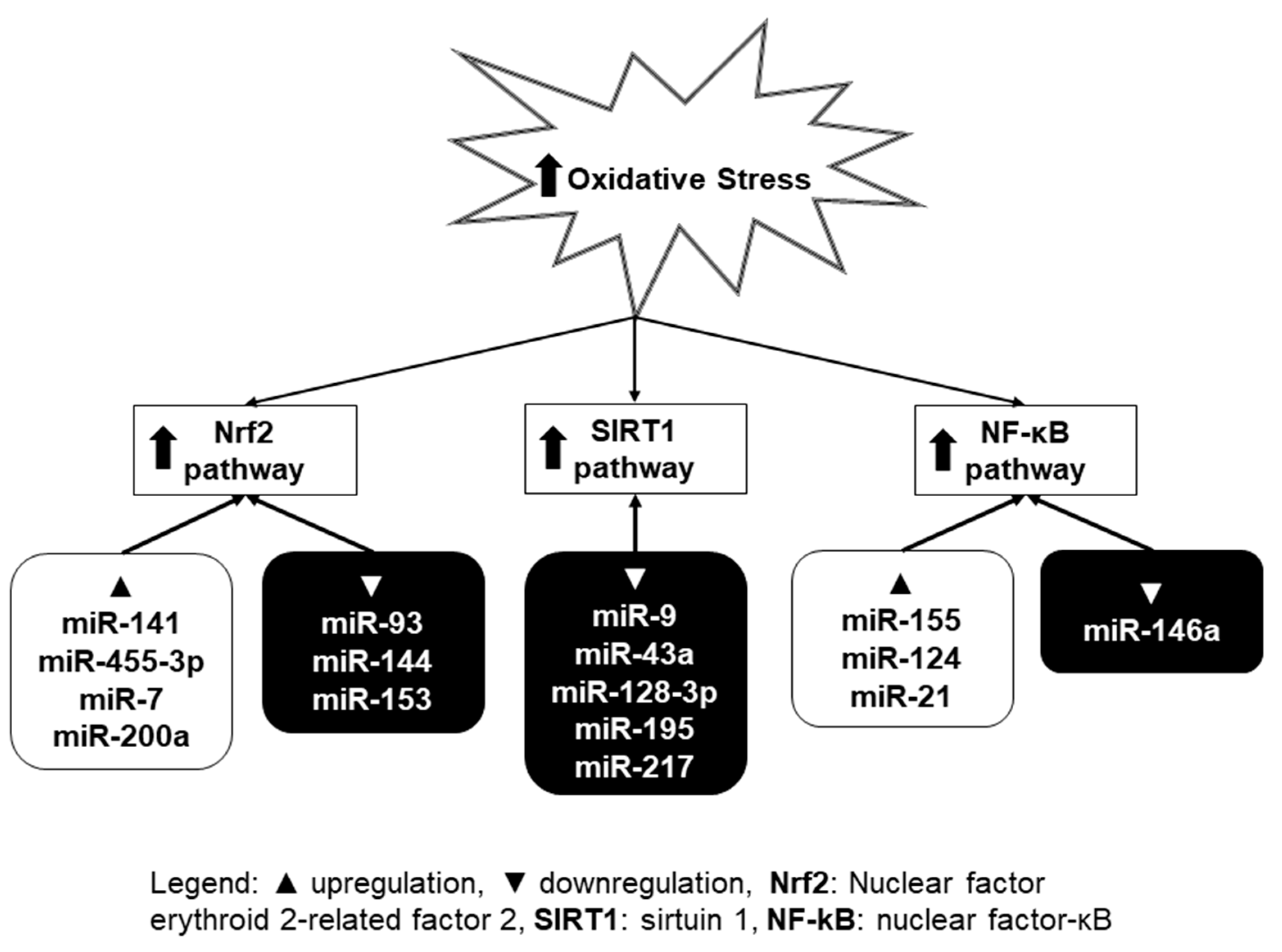
3. Oxidative Stress and MiRNA
3.1. Nrf2 Pathway
3.2. SIRT1 Pathway
3.3. NF-κB Pathway
4. MiRNA in Oxidative-Stress-Induced Heart Diseases
4.1. Cardiac Hypertrophy
4.2. Ischemia/Reperfusion Injury
4.3. Coronary Artery Diseases (CAD)
4.4. Heart Failure
5. Future Perspectives of Using MiRNA in Disease Diagnosis and Treatment
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- WHO. About Cardiovascular Diseases. Available online: https://www.who.int/cardiovascular_diseases/about_cvd/en/ (accessed on 30 October 2019).
- Li, M.; Duan, L.; Li, Y.; Liu, B. Long noncoding RNA/circular noncoding RNA–miRNA–mRNA axes in cardiovascular diseases. Life Sci. 2019, 233, 116440. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pract. 2019, 38, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugamura, K.; Keaney, J.F. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, D.B.; Siwik, D.A.; Xiao, L.; Pimentel, D.R.; Singh, K.; Colucci, W.S. Role of oxidative stress in myocardial hypertrophy and failure. J. Mol. Cell. Cardiol. 2002, 34, 379–388. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Woo, C.C.; Giannakakis, A.; Lin, X.Y.; Cheow, E.S.H.; Lee, C.N.; Richards, M.; Sze, S.K.; Nookaew, I.; Kuznetsov, V.A.; et al. Transcriptome alterations of vascular smooth muscle cells in aortic wall of myocardial infarction patients. Data Br. 2018, 17, 1112–1135. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Woo, C.C.; Giannakakis, A.; Lin, X.Y.; Cheow, E.S.H.; Lee, C.N.; Richards, M.; Sze, S.K.; Nookaew, I.; Kuznetsov, V.A.; et al. Distinctive molecular signature and activated signaling pathways in aortic smooth muscle cells of patients with myocardial infarction. Atherosclerosis 2018, 271, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Derda, A.A.; Woo, C.C.; Wongsurawat, T.; Richards, M.; Lee, C.N.; Kofidis, T.; Kuznetsov, V.A.; Sorokin, V.A. Gene expression profile analysis of aortic vascular smooth muscle cells reveals upregulation of cadherin genes in myocardial infarction patients. Physiol. Genom. 2018, 50, 648–657. [Google Scholar] [CrossRef]
- Date, R.A. Bradyrhizobium effectiveness responses in Stylosanthes hamata and S. seabrana. Trop. Grassl. 2010, 44, 141–157. [Google Scholar]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef] [Green Version]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Duygu, B.; de Windt, L.J.; da Costa Martins, P.A. Targeting microRNAs in heart failure. Trends Cardiovasc. Med. 2016, 26, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, R.M.W.; Calore, M. MicroRNAs in Cardiac Diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef] [Green Version]
- Valášková, Z.; Mladosievičová, B.; Hulín, I.; Maruščáková, L. MicroRNA—information about myocardial damage or biomarker of heart failure? Cardiol. Lett. 2017, 26, 299–302. [Google Scholar]
- Kreutzer, F.P.; Fiedler, J.; Thum, T. Non-coding RNAs: Key players in cardiac disease. J. Physiol. 2019. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Deng, J.; Zhong, Q. Advanced research on the microRNA mechanism in heart failure. Int. J. Cardiol. 2016, 220, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Luo, J.Y.; Wang, L.; Huang, Y. MicroRNAs Regulating Reactive Oxygen Species in Cardiovascular Diseases. Antioxid. Redox Signal. 2018, 29, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature Part 2 of a 3-Part Series HHS Public Access PATHOPHYSIOLOGICAL ROLE OF OXIDATIVE STRESS IN HEART FAILURE. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Biophys. Acta 2017, 1861, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Palade, F.; Alexa, I.D.; Azoicai, D.; Panaghiu, L.; Ungureanu, G. Oxidative stress in atherosclerosis. Rev. Med. 2003, 107, 502–511. [Google Scholar]
- Halliwell, B. Antioxidants in Human Health and Disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid. Med. Cell. Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef] [Green Version]
- Syu, J.-P.; Chi, J.-T.; Kung, H.-N. Nrf2 Contributes to the Poor Prognosis and Chemoresistance. A Master Regul. Oxidative Stress 2016. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Mccord, J.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Horke, S.; Förstermann, U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013, 34, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Cheung, O.; Sanyal, A.J. MicroRNAs. Signal. Pathw. Liver Dis. 2010, 107, 493–499. [Google Scholar]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef] [Green Version]
- Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction: Therapeutic implications. Ann. Med. 2011, 43, 259–272. [Google Scholar] [CrossRef]
- Maack, C.; Kartes, T.; Kilter, H.; Schäfers, H.J.; Nickenig, G.; Böhm, M.; Laufs, U. Oxygen free radical, release in human failing myocardium is associated with increased activity of Rac1-GTPase and represents a target for statin treatment. Circulation 2003, 108, 1567–1574. [Google Scholar] [CrossRef] [Green Version]
- Mollnau, H.; Oelze, M.; August, M.; Wendt, M.; Daiber, A.; Schulz, E.; Baldus, S.; Kleschyov, A.L.; Materne, A.; Wenzel, P.; et al. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2554–2559. [Google Scholar] [CrossRef] [Green Version]
- Bendall, J.K.; Cave, A.C.; Heymes, C.; Gall, N.; Shah, A.M. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 2002, 105, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Nakagami, H.; Takemoto, M.; Liao, J.K. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2003, 35, 851–859. [Google Scholar] [CrossRef]
- Wenzel, P.; Knorr, M.; Kossmann, S.; Stratmann, J.; Hausding, M.; Schuhmacher, S.; Karbach, S.H.; Schwenk, M.; Yogev, N.; Schulz, E.; et al. Lysozyme M-positive monocytes mediate angiotensin ii-induced arterial hypertension and vascular dysfunction. Circulation 2011, 124, 1370–1381. [Google Scholar] [CrossRef] [Green Version]
- Ago, T.; Kuroda, J.; Pain, J.; Fu, C.; Li, H.; Sadoshima, J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010, 106, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.D.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Romestaing, C.; Han, X.; Li, Y.; Hao, X.; Wu, Y.; Sun, C.; Liu, X.; Jefferson, L.S.; Xiong, J.; et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010, 12, 154–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadicherla, A.K.; Stowe, D.F.; Antholine, W.E.; Yang, M.; Camara, A.K.S. Damage to mitochondrial complex i during cardiac ischemia reperfusion injury is reduced indirectly by anti-anginal drug ranolazine. Biochim. Biophys. Acta 2012, 1817, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Ruggiero, F.M. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 2002, 286, 135–141. [Google Scholar] [CrossRef]
- Baldus, S.; Müllerleile, K.; Chumley, P.; Steven, D.; Rudolph, V.; Lund, G.K.; Staude, H.J.; Stork, A.; Köster, R.; Kähler, J.; et al. Inhibition of xanthine oxidase improves myocardial contractility in patients with ischemic cardiomyopathy. Free Radic. Biol. Med. 2006, 41, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Touyz, R.M.; Chen, X.; Tabet, F.; Yao, G.; He, G.; Quinn, M.T.; Pagano, P.J.; Schiffrin, E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: Regulation by angiotensin II. Circ. Res. 2002, 90, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Bitar, M.S.; Wahid, S.; Mustafa, S.; Al-Saleh, E.; Dhaunsi, G.S.; Al-Mulla, F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur. J. Pharmacol. 2005, 511, 53–64. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; Covas, M.I.; Martín, S.; Rubiés-Prat, J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J. Hum. Hypertens. 2000, 14, 343–345. [Google Scholar] [CrossRef] [Green Version]
- Münzel, T.; Gori, T.; Bruno, R.M.; Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2749. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Xue, F. Current antioxidant treatments in organ transplantation. Oxid. Med. Cell. Longev. 2016, 2016, 8678510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vela, C.; Thomsen, M.; Delbosc, S.; Calise, D.; Cristol, J.P.; Mourad, G. Lipid and Oxidative Stress Disorders in a Rat Model of Chronic Rejection. Transplant. Proc. 2007, 39, 2617–2619. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Webb, G.; Kennington, S.; Kelleher, N.; Sheppard, J.; Kuo, J.; Unsworth-White, J. Mannitol in cardioplegia as an oxygen free radical scavenger measured by malondialdehyde. Perfusion 2002, 17, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, E.R. Substrate enhancement of cardioplegic solution: Experimental studies and clinical evaluation. Ann. Thorac. Surg. 1995, 60, 797–800. [Google Scholar] [CrossRef]
- Fudulu, D.; Angelini, G. Oxidative Stress after Surgery on the Immature Heart. Oxid. Med. Cell. Longev. 2016, 2016, 1971452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, J.B.; Hicks, M.; Cropper, J.R.; Nicholson, A.; Kesteven, S.H.; Wilson, M.K.; Feneley, M.P.; Macdonald, P.S. Lazaroid (U74389G)-supplemented cardioplegia: Results of a double-blind, randomized, controlled trial in a porcine model of orthotopic heart transplantation. J. Hear. Lung Transplant. 2003, 22, 347–356. [Google Scholar] [CrossRef]
- Watson, A.J.; Gao, L.; Sun, L.; Tsun, J.; Jabbour, A.; Ru Qiu, M.; Jansz, P.C.; Hicks, M.; MacDonald, P.S. Enhanced preservation of the rat heart after prolonged hypothermic ischemia with erythropoietin-supplemented Celsior solution. J. Hear. Lung Transplant. 2013, 32, 633–640. [Google Scholar] [CrossRef]
- Villanueva, J.E.; Gao, L.; Chew, H.C.; Hicks, M.; Doyle, A.; Qui, M.R.; Dhital, K.K.; MacDonald, P.S.; Jabbour, A. Functional recovery after dantrolenesupplementation of cold stored hearts using an ex vivo isolated working rat heart model. PLoS ONE 2018, 13, e0205850. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Engedal, N.; Žerovnik, E.; Rudov, A.; Galli, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Monsurrò, V.; Betti, M.; Albertini, M.C. From oxidative stress damage to pathways, networks, and autophagy via microRNAs. Oxid. Med. Cell. Longev. 2018, 2018, 4968321. [Google Scholar] [CrossRef]
- Wan, Y.; Cui, R.; Gu, J.; Zhang, X.; Xiang, X.; Liu, C.; Qu, K.; Lin, T. Identification of Four Oxidative Stress-Responsive MicroRNAs, miR-34a-5p, miR-1915-3p, miR-638, and miR-150-3p, in Hepatocellular Carcinoma. Oxid. Med. Cell. Longev. 2017, 2017, 5189138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, J.; Khanna, S.; Bhattacharya, A. MicroRNA Regulation of Oxidative Stress. Oxid. Med. Cell. Longev. 2017, 2017, 2872156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Zhu, G.Y.; Su, X.H.; Wang, R.; Liu, J.; Liao, K.; Ren, R.; Li, T.; Liu, L. 7-deacetylgedunin suppresses inflammatory responses through activation of Keap1/Nrf2/HO-1 signaling. Oncotarget 2017, 8, 55051–55063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Wang, S.; Qi, W.; Xu, X.; Liang, Y. Overexpression of miR-153 promotes oxidative stress in MPP+-induced PD model by negatively regulating the Nrf2/HO-1 signaling pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 4179–4187. [Google Scholar]
- Sangokoya, C.; Telen, M.J.; Chi, J.T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood 2010, 116, 4338–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Zhao, X.; Yang, Z.; Min, X. Downregulation of miR-93 elevates Nrf2 expression and alleviates reactive oxygen species and cell apoptosis in diabetic retinopathy. Int. J. Clin. Exp. Med. 2019, 12, 10235–10243. [Google Scholar]
- Cheng, L.B.; Li, K.; Yi, N.; Li, X.M.; Wang, F.; Xue, B.; Pan, Y.; Yao, J.; Jiang, Q.; Wu, Z.F. miRNA-141 attenuates UV-induced oxidative stress via activating Keap1-Nrf2 signaling in human retinal pigment epithelium cells and retinal ganglion cells. Oncotarget 2017, 8, 13186–13194. [Google Scholar] [CrossRef]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Jain, M.R.; Li, H.; Junn, E. MicroRNA-7 activates Nrf2 pathway by targeting Keap1 expression. Free Radic. Biol. Med. 2015, 89, 548–556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wu, W.; Jiao, G.; Li, C.; Liu, H. MiR-455-3p activates Nrf2/ARE signaling via HDAC2 and protects osteoblasts from oxidative stress. Int. J. Biol. Macromol. 2018, 107, 2094–2101. [Google Scholar] [CrossRef]
- Yang, J.J.; Tao, H.; Hu, W.; Liu, L.P.; Shi, K.H.; Deng, Z.Y.; Li, J. MicroRNA-200a controls Nrf2 activation by target Keap1 in hepatic stellate cell proliferation and fibrosis. Cell. Signal. 2014, 26, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, S.; Kim, Y.N.; Lee, I.H. Deacetylation of CHK2 by SIRT1 protects cells from oxidative stress-dependent DNA damage response. Exp. Mol. Med. 2019, 51, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, A.; Molkentin, J.D.; Paik, J.H.; DePinho, R.A.; Yutzey, K.E. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J. Biol. Chem. 2011, 286, 7468–7478. [Google Scholar] [CrossRef] [Green Version]
- Badi, I.; Burba, I.; Ruggeri, C.; Zeni, F.; Bertolotti, M.; Scopece, A.; Pompilio, G.; Raucci, A. MicroRNA-34a Induces Vascular Smooth Muscle Cells Senescence by SIRT1 Downregulation and Promotes the Expression of Age-Associated Pro-inflammatory Secretory Factors. J. Gerontol. 2015, 70, 1304–1311. [Google Scholar] [CrossRef]
- Menghini, R.; Casagrande, V.; Cardellini, M.; Martelli, E.; Terrinoni, A.; Amati, F.; Vasa-Nicotera, M.; Ippoliti, A.; Novelli, G.; Melino, G.; et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 2009, 120, 1524–1532. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Jin, Y.; Li, L.; Xu, L.; Tang, Z.; Qi, Y.; Yin, L.; Peng, J. MicroRNA-128-3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting Sirtuin-1. Pharmacol. Res. 2019, 146, 104276. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Wang, Y.; Li, J.; Schiller, P.W.; Peng, T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc. Res. 2011, 92, 75–84. [Google Scholar] [CrossRef] [Green Version]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Gaspar-Pereira, S.; Fullard, N.; Townsend, P.A.; Banks, P.S.; Ellis, E.L.; Fox, C.; Maxwell, A.G.; Murphy, L.B.; Kirk, A.; Bauer, R.; et al. The NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am. J. Pathol. 2012, 180, 929–939. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-κB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tong, L.; Wu, S. MicroRNA and NF-kappa B. Adv. Exp. Med. Biol. 2015, 887, 157–170. [Google Scholar] [PubMed]
- Gu, X.L. MicroRNA-124 Prevents H 2 O 2 -Induced Apoptosis and Oxidative Stress in Human Lens Epithelial Cells via Inhibition of the NF-κB Signaling Pathway. Pharmacology 2018, 102, 213–222. [Google Scholar] [CrossRef]
- Wei, C.; Li, L.; Kim, I.K.; Sun, P.; Gupta, S. NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free Radic. Res. 2014, 48, 282–291. [Google Scholar] [CrossRef]
- Xie, Y.; Chu, A.; Feng, Y.; Chen, L.; Shao, Y.; Luo, Q.; Deng, X.; Wu, M.; Shi, X.; Chen, Y. MicroRNA-146a: A comprehensive indicator of inflammation and oxidative stress status induced in the brain of chronic T2DM rats. Front. Pharmacol. 2018, 9, 478. [Google Scholar] [CrossRef]
- Farías, J.G.; Molina, V.M.; Carrasco, R.A.; Zepeda, A.B.; Figueroa, E.; Letelier, P.; Castillo, R.L. Antioxidant therapeutic strategies for cardiovascular conditions associated with oxidative stress. Nutrients 2017, 9, 966. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [Green Version]
- Chekulaeva, M.; Filipowicz, W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr. Opin. Cell Biol. 2009, 21, 452–460. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, W.; Liu, C.; Liu, P.; Li, P.; Wang, K. Reactive oxygen species related noncoding RNAs as regulators of cardiovascular diseases. Int. J. Biol. Sci. 2019, 15, 680–687. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ransom, J.F.; Li, A.; Vedantham, V.; von Drehle, M.; Muth, A.N.; Tsuchihashi, T.; McManus, M.T.; Schwartz, R.J.; Srivastava, D. Dysregulation of Cardiogenesis, Cardiac Conduction, and Cell Cycle in Mice Lacking miRNA-1-2. Cell 2007, 129, 303–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 18255–18260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, S.; He, A.; Kong, S.W.; Lu, J.; Bejar, R.; Bodyak, N.; Lee, K.-H.; Ma, Q.; Kang, P.M.; Golub, T.R.; et al. MicroRNA-1 Negatively Regulates Expression of the Hypertrophy-Associated Calmodulin and Mef2a Genes. Mol. Cell. Biol. 2009, 29, 2193–2204. [Google Scholar] [CrossRef] [Green Version]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Braniewska, A.; Kozar-Kamińska, K. MicroRNA in cardiovascular biology and disease. Adv. Clin. Exp. Med. 2017, 26, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef] [Green Version]
- Rawal, S.; Nagesh, P.T.; Coffey, S.; Van Hout, I.; Galvin, I.F.; Bunton, R.W.; Davis, P.; Williams, M.J.A.; Katare, R. Early dysregulation of cardiac-specific microRNA-208a is linked to maladaptive cardiac remodelling in diabetic myocardium. Cardiovasc. Diabetol. 2019, 18, 13. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Chen, Y.; Li, F. Attenuation of MicroRNA-495 Derepressed PTEN to Effectively Protect Rat Cardiomyocytes from Hypertrophy. Cardiology 2018, 139, 245–254. [Google Scholar] [CrossRef]
- Da Costa Martins, P.A.; Salic, K.; Gladka, M.M.; Armand, A.S.; Leptidis, S.; El Azzouzi, H.; Hansen, A.; Coenen-De Roo, C.J.; Bierhuizen, M.F.; Van Der Nagel, R.; et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 2010, 12, 1220–1227. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; Liu, L.; Hou, N.; An, X.; Zhan, D.; Li, Y.; Zhou, L.; Li, P.; Yu, L.; et al. MiR-199a impairs autophagy and induces cardiac hypertrophy through mTOR activation. Cell Death Differ. 2017, 24, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Parikh, M.; Slezak, J.; Pierce, G.N. The influence of diet on microRNAs that impact cardiovascular disease. Molecules 2019, 24, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, D.; Yang, B. Role of microRNAs in cardiac hypertrophy, myocardial fibrosis and heart failure. Acta Pharm. Sin. B 2011, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Cai, J. The role of microRNAs in heart failure. Biochim. Biophys. Acta 2017, 1863, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Van Empel, V.P.M.; De Windt, L.J.; Da Costa Martins, P.A. Circulating miRNAs: Reflecting or affecting cardiovascular disease. Curr. Hypertens. Rep. 2012, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.D.; Esfandyari, D.; Papadopoulou, A.S.; De Strooper, B.; et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Piper, H.M.; Meuter, K.; Schäfer, C. Cellular mechanisms of ischemia-reperfusion injury. Ann. Thorac. Surg. 2003, 75, S644–S648. [Google Scholar] [CrossRef]
- Xiao, X.; Lu, Z.; Lin, V.; May, A.; Shaw, D.H.; Wang, Z.; Che, B.; Tran, K.; Du, H.; Shaw, P.X. MicroRNA miR-24-3p reduces apoptosis and regulates Keap1-Nrf2 pathway in mouse cardiomyocytes responding to ischemia/reperfusion injury. Oxid. Med. Cell. Longev. 2018, 2018, 7042105. [Google Scholar] [CrossRef]
- Jiang, H.; Lu, Z. MicroRNA-144 attenuates cardiac ischemia/reperfusion injury by targeting FOXO1. Exp. Ther. Med. 2019, 17, 2152–2160. [Google Scholar]
- Puthanveetil, P.; Zhang, D.; Wang, Y.; Wang, F.; Wan, A.; Abrahani, A.; Rodrigues, B. Diabetes triggers a PARP1 mediated death pathway in the heart through participation of FoxO1. J. Mol. Cell. Cardiol. 2012, 53, 677–686. [Google Scholar] [CrossRef]
- Chen, C.J.; Yu, W.; Fu, Y.C.; Wang, X.; Li, J.L.; Wang, W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem. Biophys. Res. Commun. 2009, 378, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.C.; Yeh, C.H. Inhibition of MIR-302 Suppresses Hypoxia-Reoxygenation-Induced H9c2 Cardiomyocyte Death by Regulating Mcl-1 Expression. Oxid. Med. Cell. Longev. 2017, 2017, 7968905. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Wongsurawat, T.; Lin, X.Y.; Sorokin, V. The miRNA 30B-5P targeting mRNA MBNL1 leads to pro-myogenic VSMC phenotype modulation in myocardial infarction patients. Atherosclerosis 2018, 275, e46. [Google Scholar] [CrossRef]
- Woo, C.C.; Wongsurawat, T.; Soong, R.; Lee, C.N.; Richards, M.; Kuznetsov, V.; Sorokin, V. Distinctive pattern of LET-7B and MIR-30B in human aortic smooth muscle cells of myocardial infarction patients. Atherosclerosis 2017, 263, e63. [Google Scholar] [CrossRef]
- Sorokin, V.; Woo, C.C.; Lin, X.Y.; Kofidis, T.; Lee, C.N. Role of Micro RNA in Arterial Wall Remodeling in Patients with Advanced Coronary Artery Disease Undergoing Bypass. In Proceedings of the 22nd Annual Meeting of the Asian Society for Cardiovascular and Thoracic Surgery (ASCVTS’14), Istanbul, Turkey, 18–22 August 2014. [Google Scholar]
- Long, B.; Gan, T.Y.; Zhang, R.C.; Zhang, Y.H. miR-23a regulates cardiomyocyte apoptosis by targeting manganese superoxide dismutase. Mol. Cells 2017, 40, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, Y.; Li, J.; Ren, H.; Cai, Q.; Chen, X.; Liang, H.; Shan, H.; Fu, Z.D.; Gao, X.; et al. MicroRNA-1 aggravates cardiac oxidative stress by post-transcriptional modification of the antioxidant network. Cell Stress Chaperones 2015, 20, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Du, Y.; Cao, J.; Gao, Q.; Li, H.; Chen, Y.; Lu, N. MiR-130a inhibition protects rat cardiac myocytes from hypoxia-triggered apoptosis by targeting Smad4. Kardiol. Pol. 2018, 76, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Liu, H.; Guo, J.; Yu, Y.; Yang, D.; He, F.; Du, Z. MicroRNA-98 negatively regulates myocardial infarction-induced apoptosis by down-regulating Fas and caspase-3. Sci. Rep. 2017, 7, 7460. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Sun, Y.; Yu, B. Microrna-208a correlates apoptosis and oxidative stress induced by h2o2 through protein tyrosine kinase/phosphatase balance in cardiomyocytes. Int. Heart J. 2018, 59, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Zang, G.; Zhang, G.; Wang, H.; Zhang, X.; Johnston, N.; Min, W.; Luke, P.; Jevnikar, A.; Haig, A.; et al. MicroRNA and mRNA signatures in ischemia reperfusion injury in heart transplantation. PLoS ONE 2013, 8, e79805. [Google Scholar] [CrossRef]
- Shah, P.; Bristow, M.R.; Port, J.D. MicroRNAs in Heart Failure, Cardiac Transplantation, and Myocardial Recovery: Biomarkers with Therapeutic Potential. Curr. Heart Fail. Rep. 2017, 14, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Hamdorf, M.; Kawakita, S.; Everly, M. The Potential of MicroRNAs as Novel Biomarkers for Transplant Rejection. J. Immunol. Res. 2017, 2017, 4072364. [Google Scholar] [CrossRef] [PubMed]
- Van Huyen, J.P.D.; Tible, M.; Gay, A.; Guillemain, R.; Aubert, O.; Varnous, S.; Iserin, F.; Rouvier, P.; François, A.; Vernerey, D.; et al. MicroRNAs as non-invasive biomarkers of heart transplant rejection. Eur. Heart J. 2014, 35, 3194–3202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Shi, C.; Manduchi, E.; Civelek, M.; Davies, P.F. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 13450–13455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, Y.; Wang, C.; Manes, T.D.; Pober, J.S. Cutting Edge: TNF-Induced MicroRNAs Regulate TNF-Induced Expression of E-Selectin and Intercellular Adhesion Molecule-1 on Human Endothelial Cells: Feedback Control of Inflammation. J. Immunol. 2010, 184, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhou, M.; Wang, Y.; Huang, W.; Qin, G.; Weintraub, N.L.; Tang, Y. MiR-92a inhibits vascular smooth muscle cell apoptosis: Role of the MKK4-JNK pathway. Apoptosis 2014, 19, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Wang, M.; Qu, X.; Mah, A.; Xiong, X.; Harris, A.G.C.; Phillips, L.K.; Martinez, O.M.; Krams, S.M. Differential expression of microRNAs during allograft rejection. Am. J. Transplant. 2012, 12, 1113–1123. [Google Scholar] [CrossRef]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef]
- Li, T.; Song, X.; Zhang, J.; Zhao, L.; Shi, Y.; Li, Z.; Liu, J.; Liu, N.; Yan, Y.; Xiao, Y.; et al. Protection of Human Umbilical Vein Endothelial Cells against Oxidative Stress by MicroRNA-210. Oxid. Med. Cell. Longev. 2017, 2017, 3565613. [Google Scholar] [CrossRef]
- Fu, X.M.; Zhou, Y.Z.; Cheng, Z.; Liao, X.B.; Zhou, X.M. MicroRNAs: Novel players in aortic aneurysm. Biomed. Res. Int. 2015, 2015, 831641. [Google Scholar] [CrossRef] [Green Version]
- Marcil, V.; Delvin, E.; Sané, A.T.; Tremblay, A.; Levy, E. Oxidative stress influences cholesterol efflux in THP-1 macrophages: Role of ATP-binding cassette A1 and nuclear factors. Cardiovasc. Res. 2006, 72, 473–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, M.; Wang, D.-Z. Non-Coding RNAs Including miRNAs and lncRNAs in Cardiovascular Biology and Disease. Cells 2014, 3, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Du, Y.; Shu, Y.; Gao, M.; Sun, F.; Luo, S.; Yang, T.; Zhan, L.; Yuan, Y.; Chu, W.; et al. MIAT is a Pro-fibrotic Long Non-coding RNA Governing Cardiac Fibrosis in Post-infarct Myocardium. Sci. Rep. 2017, 7, 42657. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Liu, G.; Qi, X.; Cao, X. MicroRNA-24 inhibits the proliferation and migration of endothelial cells in patients with atherosclerosis by targeting importin-α3 and regulating inflammatory responses. Exp. Ther. Med. 2018, 15, 338–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Cai, W.; Fan, Z.; Yang, C.; Wang, W.; Xiong, M.; Ma, C.; Yang, J. MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis 2019, 290, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Zhao, L.; Song, W.; Wang, L.; Liu, J.; Zhang, H.; Lau, C.W.; Yao, X.; Tian, X.Y.; Wong, W.T.; et al. Inhibition of miR-92a Suppresses Oxidative Stress and Improves Endothelial Function by Upregulating Heme Oxygenase-1 in db/db Mice. Antioxid. Redox Signal. 2018, 28, 358–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamac, A.H.; Huyut, M.A.; Yilmaz, E.; Celikkale, I.; Bacaksiz, A.; Demir, Y.; Demir, A.R.; Erturk, M.; Bakhshaliyev, N.; Ozdemir, R.; et al. MicroRNA 199a is downregulated in patients after coronary artery bypass graft surgery and is associated with increased levels of sirtuin 1 (SIRT 1) protein and major adverse cardiovascular events at 3-year follow-up. Med. Sci. Monit. 2018, 24, 6245–6254. [Google Scholar] [CrossRef]
- Guo, M.; Mao, X.; Ji, Q.; Lang, M.; Li, S.; Peng, Y.; Zhou, W.; Xiong, B.; Zeng, Q. MiR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol. Cell Biol. 2010, 88, 555–564. [Google Scholar] [CrossRef]
- O′Sullivan, J.F.; Neylon, A.; McGorrian, C.; Blake, G.J. miRNA-93-5p and other miRNAs as predictors of coronary artery disease and STEMI. Int. J. Cardiol. 2016, 224, 310–316. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart. Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [Green Version]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.V.; Ponikowski, P.; Poole-Wilson, P.A.; Strömberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur. J. Heart Fail. 2008, 10, 933–989. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed. J. Am. Coll. Cardiol. 2009, 53, e1–e90. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, S.; Kinugawa, S.; Tsuchihashi-Makaya, M.; Goto, K.; Goto, D.; Yokota, T.; Yamada, S.; Yokoshiki, H.; Takeshita, A.; Tsutsui, H. Spironolactone use at discharge was associated with improved survival in hospitalized patients with systolic heart failure. Am. Heart J. 2010, 160, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, S.; Tsuchihashi-Makaya, M.; Kinugawa, S.; Yokota, T.; Ide, T.; Takeshita, A.; Tsutsui, H. Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan—Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ. J. 2009, 73, 1442–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, H.; Tsuchihashi-Makaya, M.; Kinugawa, S.; Goto, D.; Takeshita, A. Clinical characteristics and outcome of hospitalized patients with heart failure in Japan—Rationale and design of Japanese Cardiac Registry Of Heart Failure In Cardiology (JCARE-CARD). Circ. J. 2006, 70, 1617–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchihashi-Makaya, M.; Hamaguchi, S.; Kinugawa, S.; Yokota, T.; Goto, D.; Yokoshiki, H.; Kato, N.; Takeshita, A.; Tsutsui, H. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction—A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ. J. 2009, 73, 1893–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieve, D.J.; Shah, A.M. Oxidative stress in heart failure. More than just damage. Eur. Heart J. 2003, 24, 2161–2163. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, D.B. Oxidative stress in heart failure: What are we missing? Am. J. Med. Sci. 2011, 342, 120–124. [Google Scholar] [CrossRef] [Green Version]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Shan, H.; Zhang, Y.; Lu, Y.; Zhang, Y.; Pan, Z.; Cai, B.; Wang, N.; Li, X.; Feng, T.; Hong, Y.; et al. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc. Res. 2009, 83, 465–472. [Google Scholar] [CrossRef]
- Ye, Y.; Perez-polo, J.R.; Qian, J.; Birnbaum, Y.; Ye, Y.; Perez-polo, J.R.; Qian, J.; Birnbaum, Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol. Genom. 2010, 43, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Price, N.L.; Fernández-Hernando, C. Non-coding RNAs in lipid metabolism. Vascul. Pharmacol. 2019, 114, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Karakas, M.; Zeller, T. MicroRNAs in cardiovascular disease—Clinical application. Clin. Chem. Lab. Med. 2017, 55, 687–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.S.; Kala, C.; Abid, M.; Ahmad, N.; Sharma, U.S.; Khan, N.A. Pathological microRNAs in acute cardiovascular diseases and microRNA therapeutics. J. Acute Dis. 2016, 5, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Sue, Y.M.; Chou, Y.; Cheng, C.F.; Chang, C.C.; Li, H.F.; Chen, C.C.; Juan, S.H. Activation of a nuclear factor of activated T-lymphocyte-3 (NFAT3) by oxidative stress in carboplatin-mediated renal apoptosis. Br. J. Pharmacol. 2010, 161, 1661–1676. [Google Scholar] [CrossRef] [Green Version]
- Kakita, T.; Hasegawa, K.; Iwai-Kanai, E.; Adachi, S.; Morimoto, T.; Wada, H.; Kawamura, T.; Yanazume, T.; Sasayama, S. Calcineurin pathway is required for endothelin-1-mediated protection against oxidant stress-induced apoptosis in cardiac myocytes. Circ. Res. 2001, 88, 1239–1246. [Google Scholar] [CrossRef]
- Wang, X.; Lian, Y.; Wen, X.; Guo, J.; Wang, Z.; Jiang, S.; Hu, Y. Expression of miR-126 and its potential function in coronary artery disease. Afr. Health Sci. 2017, 17, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Reddy, L.L.; Shah, S.A.V.; Ponde, C.K.; Rajani, R.M.; Ashavaid, T.F. Circulating miRNA-33: A potential biomarker in patients with coronary artery disease. Biomarkers 2019, 24, 36–42. [Google Scholar] [CrossRef]
- Schulte, C.; Zeller, T. microRNA-based diagnostics and therapy in cardiovascular disease-Summing up the facts. Cardiovasc. Diagn. Ther. 2015, 5, 17–36. [Google Scholar]
- Cakmak, H.A.; Barman, H.A.; Coskunpinar, E.; Oltulu, Y.M.; Ikitimur, B.; Can, G.; Ozcan, S.; Vural, V.A. The Diagnostic Importance of MicroRNAs in Congestive Heart Failure. J. Am. Coll. Cardiol. 2013, 62, C17–C18. [Google Scholar] [CrossRef] [Green Version]
- Murach, K.A.; McCarthy, J.J. MicroRNAs, heart failure, and aging: Potential interactions with skeletal muscle. Heart Fail. Rev. 2017, 22, 209–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, E.F.; Ohashi, P.S. Mir-155, a central modulator of T-cell responses. Eur. J. Immunol. 2014, 44, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Jones Buie, J.N.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Fan, H. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis 2016, 254, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum microRNAs are promising novel biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebolts, U.; Vamholt, H.; Drebber, U.; Dienes, H.P.; Wickenhauser, C.; Odenthal, M. Tissues from routine pathology archives are suitable for microRNA analyses by quantitative PCR. J. Clin. Pathol. 2009, 62, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaarz, A.; Debey-Pascher, S.; Classen, S.; Eggle, D.; Gathof, B.; Chen, J.; Fan, J.B.; Voss, T.; Schultze, J.L.; Staratschek-Jox, A. Bead array-based microRNA expression profiling of peripheral blood and the impact of different RNA isolation approaches. J. Mol. Diagnostics 2010, 12, 335–344. [Google Scholar] [CrossRef]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.S.; Tam, W.L.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A Pattern-Based Method for the Identification of MicroRNA Binding Sites and Their Corresponding Heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef] [Green Version]
- Olson, E.N. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci. Transl. Med. 2014, 6, 239ps3. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.R.; Wu, M.; Yu, H.; Long, S.; Stevens, A.; Engers, D.W.; Sackin, H.; Daniels, J.S.; Dawson, E.S.; Hopkins, C.R.; et al. Selective inhibition of the K ir2 family of inward rectifier potassium channels by a small molecule probe: The discovery, SAR, and pharmacological characterization of ML133. ACS Chem. Biol. 2011, 6, 845–856. [Google Scholar] [CrossRef] [Green Version]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with antagomirs. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Oliveira-Carvalho, V.; Carvalho, V.O.; Silva, M.M.; Guimarães, G.V.; Bocchi, E.A. MicroRNAs: A new paradigm in the treatment and diagnosis of heart failure? Arq. Bras. Cardiol. 2012, 98, 362–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, M.S.; Neilson, J.R.; Sharp, P.A. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 2007, 4, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.S.; Sharp, P.A. MicroRNA sponges: Progress and possibilities. RNA 2010, 16, 2043–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broderick, J.A.; Zamore, P.D. MicroRNA therapeutics. Gene Ther. 2011, 18, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Cardillo, M.T.; Galea, R.; Biasucci, L.M. Potential therapeutic role of microRNAs in ischemic heart disease. J. Cardiol. 2013, 61, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Disease | miRNA | Expression | Target | References |
|---|---|---|---|---|
| Cardiac hypertrophy | miRNA-1 | Downregulated | Mef2a; Gata4 | [93,94,95] |
| miRNA-133 | Downregulated | GDP–GTP exchange protein; Cdc42 | [93,96,97] | |
| miRNA-208a | Downregulated | Myh7 | [18,98,99] | |
| Ischemia/reperfusion injury | miRNA-24-3p | Downregulated | Keap1/Nrf2 | [109] |
| miRNA-144 | Downregulated | FoxO1 | [110,111] | |
| miRNA-302 | Upregulated | Mcl-1 | [113] | |
| miRNA-23a | Upregulated | MnSOD | [117] | |
| Heart transplantation | miRNA-10a | Downregulated | NF-κB | [126] |
| miRNA-31 | Upregulated | TNF-α | [127] | |
| miRNA-92a | Upregulated | Integrin a5, S1P1, MKK4, eNOS | [128] | |
| miRNA-155 | Upregulated | T-cell receptor, IFN receptor | [164] | |
| Coronary artery diseases | miRNA-24 | Downregulated | Ogt, Keap1/Nrf2 | [132,134,135,136,137] |
| miRNA-92a | Upregulated | HO-1 | [138] | |
| miRNA-199a | Downregulated | SIRT1 | [139] | |
| Heart failure | miRNA-199b | Upregulated | calcineurin/NFAT | [101,157,158,159] |
| miRNA-21 | Upregulated | natriuretic peptide B | [156,163] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kura, B.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010358
Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. International Journal of Molecular Sciences. 2020; 21(1):358. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010358
Chicago/Turabian StyleKura, Branislav, Barbara Szeiffova Bacova, Barbora Kalocayova, Matus Sykora, and Jan Slezak. 2020. "Oxidative Stress-Responsive MicroRNAs in Heart Injury" International Journal of Molecular Sciences 21, no. 1: 358. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21010358





