Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications
Abstract
:1. Introduction
2. Discussion
2.1. Myokines
2.2. Sarcopenia
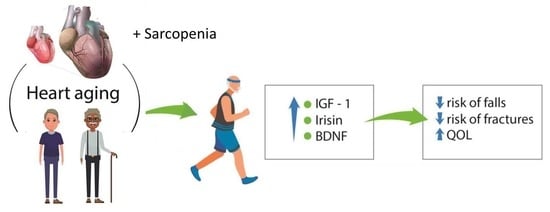
2.3. Myokines, Sarcopenia, and Cardiovascular Diseases
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2019, e13367. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1alpha, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, L. Circulating MicroRNAs as Diagnostic Biomarkers for Motor Neuron Disease. Front. Neurosci. 2020, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Shibasaki, I.; Sawaguchi, T.; Haruyama, A.; Kaneda, H.; Nakajima, T.; Hasegawa, T.; Arikawa, T.; Obi, S.; Sakuma, M.; et al. Growth differentiation factor-15 (GDF-15) is a biomarker of muscle wasting and renal dysfunction in preoperative cardiovascular surgery patients. J. Clin. Med. 2019, 8, 1576. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I. Evolution of sarcopenia research. Appl. Physiol. Nutr. Metab. 2010, 35, 707–712. [Google Scholar] [CrossRef]
- Linge, J.; Heymsfield, S.B.; Leinhard, O.D. On the definition of sarcopenia in the presence of aging and obesity-initial results from UK biobank. J. Gerontol. A 2019, 229. [Google Scholar] [CrossRef] [Green Version]
- Tinsley, G.M.; Paoli, A. Time-restricted eating and age-related muscle loss. Aging 2019, 11, 8741–8742. [Google Scholar] [CrossRef]
- Yin, J.; Lu, X.; Qian, Z.; Xu, W.; Zhou, X. New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure. Theranostics 2019, 9, 4019–4029. [Google Scholar] [CrossRef]
- Lai, S.; Muscaritoli, M.; Andreozzi, P.; Sgreccia, A.; de Leo, S.; Mazzaferro, S.; Mitterhofer, A.P.; Pasquali, M.; Protopapa, P.; Spagnoli, A.; et al. Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutrition 2019, 62, 108–114. [Google Scholar] [CrossRef]
- McLeod, J.C.; Stokes, T.; Phillips, S.M. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front. Physiol. 2019, 10, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.S.; Choi, K.M. Organokines in disease. Adv. Clin. Chem. 2020, 94, 261–321. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A. An Overview of the Beneficial Effects of Exercise on Health and Performance. Adv. Exp. Med. Biol. 2020, 1228, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.H.; Yi, H.K. Gradual downhill running improves age-related skeletal muscle and bone weakness: Implication of autophagy and bone morphogenetic proteins. Exp. Physiol. 2016, 101, 1528–1540. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-Induced Myokines with Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Trayhurn, P.; Drevon, C.A.; Eckel, J. Secreted proteins from adipose tissue and skeletal muscle—Adipokines, myokines and adipose/muscle cross-talk. Arch. Physiol. Biochem. 2011, 117, 47–56. [Google Scholar] [CrossRef]
- Tuena, C.; Pedroli, E.; Trimarchi, P.D.; Gallucci, A.; Chiappini, M.; Goulene, K.; Gaggioli, A.; Riva, G.; Lattanzio, F.; Giunco, F.; et al. Usability Issues of Clinical and Research Applications of Virtual Reality in Older People: A Systematic Review. Front. Hum. Neurosci. 2020, 14, 93. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.I.; Kakuma, T.; Sasaki, M.; Ishizaki, Y.; Fukami, A.; Enomoto, M.; Adachi, H.; Matsuse, H.; Shiba, N.; Ueno, T.; et al. The prevalence of sarcopenia and subtypes in cardiovascular diseases, and a new diagnostic approach. J. Cardiol. 2020. [Google Scholar] [CrossRef]
- Pedersen, B.K. The diseasome of physical inactivity—And the role of myokines in muscle—Fat cross talk. J. Physiol. 2009, 587, 5559–5568. [Google Scholar] [CrossRef]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garneau, L.; Aguer, C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019, 45, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Ohashi, K.; Shibata, R.; Murohara, T. Protective roles of adipocytokines and myokines in cardiovascular disease. Circ. J. 2016, 80, 2073–2080. [Google Scholar] [CrossRef] [Green Version]
- Ebert, T.; Kralisch, S. Newly discovered myokines in chronic kidney disease. Pol. Arch. Med. Wewn. 2016, 126, 457–458. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Luo, A.L. Myokines: A promising therapeutic target for hepatic encephalopathy. J. Hepatol. 2017, 66, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
- Ojima, K.; Oe, M.; Nakajima, I.; Shibata, M.; Muroya, S.; Chikuni, K.; Hattori, A.; Nishimura, T. The importance of subfragment 2 and C-terminus of myosin heavy chain for thick filament assembly in skeletal muscle cells. Anim. Sci. J. 2015, 86, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Eckardt, K.; Holven, K.B.; Jensen, J.; Eckel, J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS ONE 2013, 8, e62008. [Google Scholar] [CrossRef] [Green Version]
- Jia, D.; Cai, M.; Xi, Y.; Du, S.; Tian, Z. Interval exercise training increases LIF expression and prevents myocardial infarction-induced skeletal muscle atrophy in rats. Life Sci. 2018, 193, 77–86. [Google Scholar] [CrossRef]
- Ishiuchi, Y.; Sato, H.; Komatsu, N.; Kawaguchi, H.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M.; Nedachi, T. Identification of CCL5/RANTES as a novel contraction-reducible myokine in mouse skeletal muscle. Cytokine 2018, 108, 17–23. [Google Scholar] [CrossRef]
- Rutti, S.; Dusaulcy, R.; Hansen, J.S.; Howald, C.; Dermitzakis, E.T.; Pedersen, B.K.; Pinget, M.; Plomgaard, P.; Bouzakri, K. Angiogenin and osteoprotegerin are type II muscle specific myokines protecting pancreatic beta-cells against proinflammatory cytokines. Sci. Rep. 2018, 8, 10072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho-Junior, H.J.; Picca, A.; Calvani, R.; Uchida, M.C.; Marzetti, E. If my muscle could talk: Myokines as a biomarker of frailty. Exp. Gerontol. 2019, 127, 110715. [Google Scholar] [CrossRef]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Iemura, S.; Kawao, N.; Okumoto, K.; Akagi, M.; Kaji, H. Role of irisin in androgen-deficient muscle wasting and osteopenia in mice. J. Bone Min. Metab. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, T.D.S.; Duarte, Y.A.O.; Santos, J.L.F.; Lebrão, M.L. Prevalence and associated factors of sarcopenia, dynapenia, and sarcodynapenia in community-dwelling elderly in São Paulo—SABE Study. Rev. Bras. Epidemiol. 2019, 21 (Suppl. 02), e180009. [Google Scholar] [CrossRef] [Green Version]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef] [Green Version]
- Durieux, A.C.; Amirouche, A.; Banzet, S.; Koulmann, N.; Bonnefoy, R.; Pasdeloup, M.; Mouret, C.; Bigard, X.; Peinnequin, A.; Freyssenet, D. Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology 2007, 148, 3140–3147. [Google Scholar] [CrossRef]
- Rentier, C.; Takayama, K.; Saitoh, M.; Nakamura, A.; Ikeyama, H.; Taguchi, A.; Taniguchi, A.; Hayashi, Y. Design and synthesis of potent myostatin inhibitory cyclic peptides. Bioorganic Med. Chem. 2019, 27, 1437–1443. [Google Scholar] [CrossRef]
- Yakabe, M.; Hosoi, T.; Akishita, M.; Ogawa, S. Updated concept of sarcopenia based on muscle-bone relationship. J. Bone Min. Metab. 2019, 38, 7–13. [Google Scholar] [CrossRef]
- Willis, S.A.; Sargeant, J.A.; Thackray, A.E.; Yates, T.; Stensel, D.J.; Aithal, G.P.; King, J.A. Effect of exercise intensity on circulating hepatokine concentrations in healthy men. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2019, 44, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- El Shafey, N.; Guesnon, M.; Simon, F.; Deprez, E.; Cosette, J.; Stockholm, D.; Scherman, D.; Bigey, P.; Kichler, A. Inhibition of the myostatin/Smad signaling pathway by short decorin-derived peptides. Exp. Cell Res. 2016, 341, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kanzleiter, T.; Rath, M.; Gorgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schurmann, A.; et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, S.; van Wittenberghe, L.; Georger, C.; Scherman, D.; Kichler, A. Identification of decorin derived peptides with a zinc dependent anti-myostatin activity. Neuromuscul. Disord. 2012, 22, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Marquez, J.; Le, L.T.; Nguyen, A.T.T.; Kim, H.K.; Han, J. The role of decorin in cardiovascular diseases: More than just a decoration. Free Radic. Res. 2018, 52, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Manole, E.; Ceafalan, L.C.; Popescu, B.O.; Dumitru, C.; Bastian, A.E. Myokines as Possible Therapeutic Targets in Cancer Cachexia. J. Immunol. Res. 2018, 2018, 8260742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re Cecconi, A.D.; Forti, M.; Chiappa, M.; Zhu, Z.; Zingman, L.V.; Cervo, L.; Beltrame, L.; Marchini, S.; Piccirillo, R. Musclin, a myokine induced by aerobic exercise, retards muscle atrophy during cancer cachexia in mice. Cancers 2019, 11, E1541. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, J.; Bian, H.; Bo, J.; Lv, S.; Pan, W.; Lv, X. Apelin promotes hepatic fibrosis through ERK signaling in LX-2 cells. Mol. Cell Biochem. 2019, 460, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradere, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Vinel, C.; Schanstra, J.P.; Boizard, F.; Pereira, O.; Auriau, J.; Dortignac, A.; Breuil, B.; Feuillet, G.; Nkuipou-Kenfack, E.; Zurbig, P.; et al. Apelin affects the mouse aging urinary peptidome with minimal effects on kidney. Sci. Rep. 2019, 9, 10647. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Calvani, R.; Leeuwenburgh, C.; Coelho-Junior, H.J.; Bernabei, R.; Landi, F.; Marzetti, E. Targeting mitochondrial quality control for treating sarcopenia: Lessons from physical exercise. Expert Opin. Ther. Targets 2019, 23, 153–160. [Google Scholar] [CrossRef]
- Otaka, N.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kambara, T.; Enomoto, T.; Ogawa, H.; Ito, M.; Kawanishi, H.; Maruyama, S.; et al. Myonectin Is an Exercise-Induced Myokine That Protects the Heart From Ischemia-Reperfusion Injury. Circ. Res. 2018, 123, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.M.; Peschard-Franco, M.; Castillo, E.C.; Gutierrez-DelBosque, G.; Trevino, V.; Silva-Platas, C.; Perez-Villarreal, L.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Myokine-adipokine cross-talk: Potential mechanisms for the association between plasma irisin and adipokines and cardiometabolic risk factors in Mexican children with obesity and the metabolic syndrome. Diabetol. Metab. Syndr. 2019, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drey, M.; Sieber, C.C.; Bertsch, T.; Bauer, J.M.; Schmidmaier, R. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin. Exp. Res. 2016, 28, 895–899. [Google Scholar] [CrossRef]
- Planella-Farrugia, C.; Comas, F.; Sabater-Masdeu, M.; Moreno, M.; Moreno-Navarrete, J.M.; Rovira, O.; Ricart, W.; Fernandez-Real, J.M. Circulating Irisin and Myostatin as Markers of Muscle Strength and Physical Condition in Elderly Subjects. Front. Physiol. 2019, 10, 871. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of white fat: Novel insight into factors, mechanisms, and therapeutics. J. Cell Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Lattanzi, B.; Aprile, F. Sarcopenic obesity in fatty liver. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: A role for inflammation. Oxid. Med. Cell Longev. 2017, 2017, 3292087. [Google Scholar] [CrossRef]
- Halon-Golabek, M.; Borkowska, A.; Herman-Antosiewicz, A.; Antosiewicz, J. Iron Metabolism of the Skeletal Muscle and Neurodegeneration. Front. Neurosci. 2019, 13, 165. [Google Scholar] [CrossRef] [Green Version]
- Drey, M.; Krieger, B.; Sieber, C.C.; Bauer, J.M.; Hettwer, S.; Bertsch, T. Motoneuron loss is associated with sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 435–439. [Google Scholar] [CrossRef]
- Park, S.J.; Ryu, S.Y.; Park, J.; Choi, S.W. Association of sarcopenia with metabolic syndrome in Korean population using 2009–2010 Korea national health and nutrition examination survey. Metab. Syndr. Relat. Disord. 2019, 17, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef]
- Tibana, R.A.; Nascimento, D.C.; de Souza, N.M.F.; de Souza, V.C.; Neto, I.V.S.; Voltarelli, F.A.; Pereira, G.B.; Navalta, J.W.; Prestes, J. Irisin levels are not associated to resistance training-induced alterations in body mass composition in older untrained women with and without obesity. J. Nutr. Health Aging 2017, 21, 241–246. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Z.; Qu, C.; Dong, Y. Effects of 12 weeks resistance training on serum irisin in older male adults. Front. Physiol. 2017, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Diggins, N.H.; Gunderson, Z.J.; Fehrenbacher, J.C.; White, F.A.; Kacena, M.A. No pain, no gain? The effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone 2019, 131, 115109. [Google Scholar] [CrossRef] [PubMed]
- Sustar, A.; Perkovic, M.N.; Erjavec, G.N.; Strac, D.S.; Pivac, N. Association between reduced brain-derived neurotrophic factor concentration & coronary heart disease. Indian J. Med. Res. 2019, 150, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.B.; Ahuja, P.; Ye, K. Developing Insulin and BDNF Mimetics for Diabetes Therapy. Curr. Top. Med. Chem. 2019, 19, 2188–2204. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Bataille, S.; Quinonez, K.; Buckinx, F.; Warling, X.; Krzesinski, J.M.; Pottel, H.; Burtey, S.; Bruyere, O.; Cavalier, E. Myostatin and insulin-like growth factor 1 are biomarkers of muscle strength, muscle mass, and mortality in patients on hemodialysis. J. Ren. Nutr. 2019, 29, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Kinugawa, S.; Fukushima, A.; Takada, S.; Homma, T.; Masaki, Y.; Abe, T.; Yokota, T.; Oba, K.; Okita, K.; et al. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int. J. Cardiol. 2016, 220, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.R.; Chen, R.C. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir. Med. 2012, 106, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Itoh, N. FGF21 as a hepatokine, adipokine, and myokine in metabolism and diseases. Front. Endocrinol. 2014, 5, 107. [Google Scholar] [CrossRef] [Green Version]
- Seto, D.N.; Kandarian, S.C.; Jackman, R.W. A key role for leukemia inhibitory factor in C26 cancer cachexia. J. Biol. Chem. 2015, 290, 19976–19986. [Google Scholar] [CrossRef] [Green Version]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Wolden-Hanson, T. Serum and muscle interleukin-15 levels decrease in aging mice: Correlation with declines in soluble interleukin-15 receptor alpha expression. Exp. Gerontol. 2010, 45, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Bloch, S.A.; Lee, J.Y.; Wort, S.J.; Polkey, M.I.; Kemp, P.R.; Griffiths, M.J. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit. Care Med. 2013, 41, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Chae, S.A.; Testroet, E.D.; Du, M.; Jun, H.P. Exercise-induced myokines: A brief review of controversial issues of this decade. Expert Rev. Endocrinol. Metab. 2018, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Shin, D.W.; Han, K.; Jung, J.H.; Kim, B.; Jung, H.W.; Son, K.Y.; Lee, S.P.; Lee, S.C. The timed up and go test and the ageing heart: Findings from a national health screening of 1,084,875 community-dwelling older adults. Eur. J. Prev. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.C. Sarcopenia, frailty, and diabetes in older adults. Diabetes Metab. J. 2016, 40, 182–189. [Google Scholar] [CrossRef]
- Dillon, L.M.; Rebelo, A.P.; Moraes, C.T. The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life 2012, 64, 231–241. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A time for action. An SCWD position paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Hida, T.; Imagama, S.; Ando, K.; Kobayashi, K.; Muramoto, A.; Ito, K.; Ishikawa, Y.; Tsushima, M.; Nishida, Y.; Ishiguro, N.; et al. Sarcopenia and physical function are associated with inflammation and arteriosclerosis in community-dwelling people: The Yakumo study. Mod. Rheumatol. 2018, 28, 345–350. [Google Scholar] [CrossRef]
- Nozoe, M.; Kanai, M.; Kubo, H.; Yamamoto, M.; Shimada, S.; Mase, K. Prestroke sarcopenia and stroke severity in elderly patients with acute stroke. J. Stroke Cereb. Dis 2019, 28, 2228–2231. [Google Scholar] [CrossRef]
- Anaszewicz, M.; Banas, W.; Wawrzenczyk, A.; Budzynski, J. Body composition in patients with atrial fibrillation. Acta Cardiol. Sin. 2019, 35, 484–492. [Google Scholar] [CrossRef]
- Santana, N.M.; Mendes, R.M.L.; Silva, N.F.D.; Pinho, C.P.S. Sarcopenia and sarcopenic obesity as prognostic predictors in hospitalized elderly patients with acute myocardial infarction. Einstein 2019, 17, eAO4632. [Google Scholar] [CrossRef]
- Yamashita, M.; Kamiya, K.; Matsunaga, A.; Kitamura, T.; Hamazaki, N.; Matsuzawa, R.; Nozaki, K.; Tanaka, S.; Nakamura, T.; Maekawa, E.; et al. Prognostic value of sarcopenic obesity estimated by computed tomography in patients with cardiovascular disease and undergoing surgery. J. Cardiol. 2019, 74, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Frobert, O.; Frobert, A.M.; Kindberg, J.; Arnemo, J.M.; Overgaard, M.T. The brown bear as a translational model for sedentary lifestyle-related diseases. J. Intern. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.; Tran, K.D.; Bui, K.X.; Le, D.; Nguyen, T.N. A preliminary study to identify the likely risk for sarcopenia in older hospitalised patients with cardiovascular disease in Vietnam. Australas. J. Ageing 2020. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, B.; Wang, X. Lower irisin levels in coronary artery disease: A meta-analysis. Miner. Endocrinol. 2017. [Google Scholar] [CrossRef]
- Abe, K.; Yano, T.; Katano, S.; Ohori, K.; Ishigo, T.; Moniwa, N.; Miura, T. Utility of the sarcopenia index for assessment of muscle mass and nutritional status in patients with chronic heart failure: Comparison with anthropometric parameters. Geriatr. Gerontol. Int. 2020, 20, 388–389. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.H. Impact of Skeletal Muscle Mass on Metabolic Health. Endocrinol. Metab. 2020, 35, 1–6. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas 2020, 133, 60–67. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-Released Myokines in the Control of Energy Metabolism. Front. Physiol. 2020, 11, 91. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Gao, F. Exercise and Cardiovascular Protection. Adv. Exp. Med. Biol. 2020, 1228, 205–216. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciorkowska, M.; Musiałowska, D.; Małyszko, J. Adropin and irisin in arterial hypertension, diabetes mellitus and chronic kidney disease. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2019, 28, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Ogura, Y.; Ouchi, N.; Ohashi, K.; Shibata, R.; Kataoka, Y.; Kambara, T.; Kito, T.; Maruyama, S.; Yuasa, D.; Matsuo, K.; et al. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation 2012, 126, 1728–1738. [Google Scholar] [CrossRef]
- Nakano, I.; Kinugawa, S.; Hori, H.; Fukushima, A.; Yokota, T.; Takada, S.; Kakutani, N.; Obata, Y.; Yamanashi, K.; Anzai, T. Serum Brain-Derived Neurotrophic Factor Levels Are Associated with Skeletal Muscle Function but Not with Muscle Mass in Patients with Heart Failure. Int. Heart J. 2020, 61, 96–102. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.d.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103607
Barbalho SM, Flato UAP, Tofano RJ, Goulart RdA, Guiguer EL, Detregiachi CRP, Buchaim DV, Araújo AC, Buchaim RL, Reina FTR, et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. International Journal of Molecular Sciences. 2020; 21(10):3607. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103607
Chicago/Turabian StyleBarbalho, Sandra Maria, Uri Adrian Prync Flato, Ricardo José Tofano, Ricardo de Alvares Goulart, Elen Landgraf Guiguer, Cláudia Rucco P. Detregiachi, Daniela Vieira Buchaim, Adriano Cressoni Araújo, Rogério Leone Buchaim, Fábio Tadeu Rodrigues Reina, and et al. 2020. "Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications" International Journal of Molecular Sciences 21, no. 10: 3607. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103607