NTRK Fusions, from the Diagnostic Algorithm to Innovative Treatment in the Era of Precision Medicine
Abstract
:1. Introduction
2. NTRK Genes: Structure, Function, and Oncogenic Potential
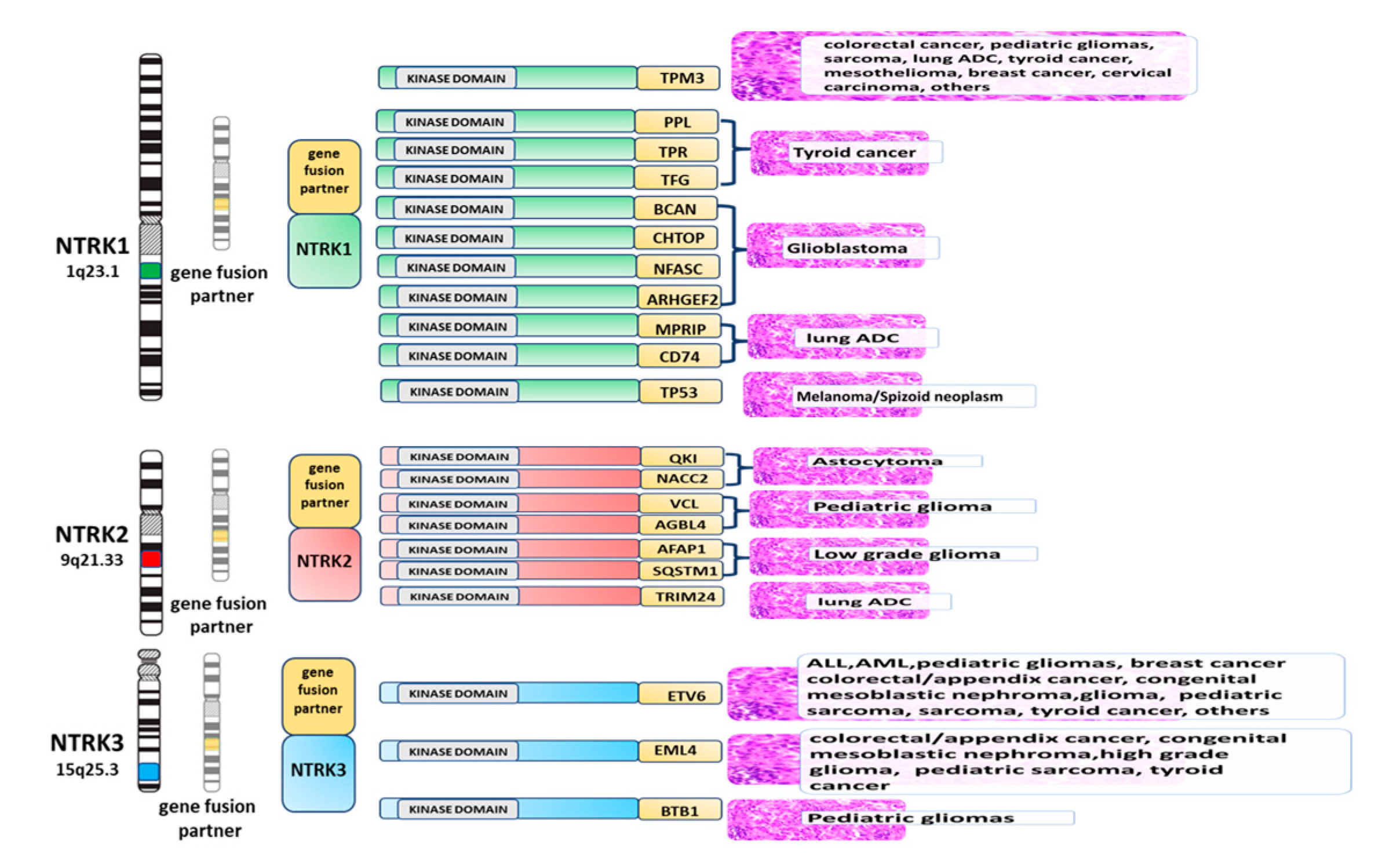
3. Tumors Harboring NTRK Gene Aberrations
4. The Choice of Biomaterials to Detect NTRK Fusions
5. Methods for the Detection of NTRK Fusions
5.1. Immunohistochemistry (IHC)
5.2. Fluorescence in Situ Hybridization (FISH)
5.3. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
5.4. Next-Generation Sequencing
5.5. DNA-Based Next-Generation Sequencing
5.6. RNA-Based Next-Generation Sequencing
6. Diagnostic Algorithm to Detect NTRK Gene Fusions
7. Expert Opinion
Funding
Conflicts of Interest
References
- Salto-Tellez, M.; James, J.A.; Hamilton, P.W. Molecular pathology—The value of an integrative approach. Mol. Oncol. 2014, 8, 1163–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheder, E.S.; Hong, D.S. Emerging Targeted Therapy for Tumors with NTRK Fusion Proteins. Clin. Cancer Res. 2018, 24, 5807–5814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schatch, A. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar]
- Hsiao, S.J.; Zehir, A.; Sireci, A.N.; Aisner, D.L. Detection of Tumor NTRK Gene Fusions to Identify Patients Who May Benefit from Tyrosine Kinase (TRK) Inhibitor Therapy. J. Mol. Diagn. 2019, 21, 553–571. [Google Scholar] [CrossRef] [Green Version]
- Lannon, C.L.; Sorensen, P.H. ETV6-NTRK3: A chimeric protein tyrosine kinase with transformation activity in multiple cell lineages. Semin. Cancer Biol. 2005, 15, 215–223. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophin receptors: A window into neuronal differentiation. Neuron 1992, 9, 583–593. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Stephens, R.M.; Loeb, D.M.; Copeland, T.D.; Pawson, T.; Greene, L.A.; Kaplan, D.R. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron 1994, 12, 691–705. [Google Scholar] [CrossRef]
- Martin-Zanca, D.; Hughes, S.H.; Barbacid, M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 1986, 319, 743–748. [Google Scholar] [CrossRef]
- Bongarzone, I.; Pierotti, M.A.; Monzini, N.; Mondellini, P.; Manenti, G.; Donghi, R.; Pilotti, S.; Grieco, M.; Santoro, M.; Fusco, A.; et al. High frequency of activation of tyrosine kinase oncogenes in human papillary thyroid carcinoma. Oncogene 1989, 4, 1457–1462. [Google Scholar]
- Vaishnavi, A.; Le, A.T.; Doebele, R.C. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015, 5, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 1998, 18, 184–187. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.G.; Laetsch, T.W.; Federman, N.; Turpin, B.K.; Albert, C.M.; Nagasubramanian, R.; Anderson, M.E.; Davis, J.L.; Qamoos, H.E.; Reynolds, M.E.; et al. The use of neoadjuvant larotrectinib in the management of children with locally advanced TRK fusion sarcomas. Cancer 2018, 124, 4241–4247. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Roberts, S.S.; Baki, M.O.; Mushtaq, Q.; Goss, P.E.; Park, B.H.; Gundem, G.; Tian, K.; Geiger, H.; Redfield, K.; et al. Successful Targeted Therapy of Refractory Pediatric ETV6-NTRK3 Fusion-Positive Secretory Breast Carcinoma. JCO Precis. Oncol. 2017, 2017. [Google Scholar] [CrossRef]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, M.; Chibon, F.; Arnould, L.; Croce, S.; Ribeiro, A.; Perot, G.; Hostein, I.; Geha, S.; Bozon, C.; Garnier, A.; et al. Secretory Breast Carcinoma: A Histopathologic and Genomic Spectrum Characterized by a Joint Specific ETV6-NTRK3 Gene Fusion. Am. J. Surg. Pathol. 2015, 39, 1458–1467. [Google Scholar] [CrossRef]
- Church, A.J.; Calicchio, M.L.; Nardi, V.; Skalova, A.; Pinto, A.; Dillon, D.A.; Gomez-Fernandez, C.R.; Manoj, N.; Haimes, J.D.; Stahl, J.A.; et al. Recurrent EML4-NTRK3 fusions in infantile fibrosarcoma and congenital mesoblastic nephroma suggest a revised testing strategy. Mod. Pathol. 2018, 31, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.; Pavlick, D.; Brennan, T.; Yelensky, R.; Crawford, J.; Ross, J.S.; Miller, V.A.; Malicki, D.; Stephens, P.J.; Ali, S.M.; et al. Evaluation of a Congenital Infantile Fibrosarcoma by Comprehensive Genomic Profiling Reveals an LMNA-NTRK1 Gene Fusion Responsive to Crizotinib. J. Natl. Cancer Inst. 2015, 108, djv307. [Google Scholar] [CrossRef] [Green Version]
- Tannenbaum-Dvir, S.; Glade Bender, J.L.; Church, A.J.; Janeway, K.A.; Harris, M.H.; Mansukhani, M.M.; Nagy, P.L.; Andrews, S.J.; Murty, V.V.; Kadenhe-Chiweshe, A.; et al. Characterization of a novel fusion gene EML4-NTRK3 in a case of recurrent congenital fibrosarcoma. Cold Springer Harb. Mol. Case Stud. 2015, 1, a000471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skálová, A.; Vanecek, T.; Simpson, R.H.; Laco, J.; Majewska, H.; Baneckova, M.; Steiner, P.; Michal, M. Mammary Analogue Secretory Carcinoma of Salivary Glands: Molecular Analysis of 25 ETV6 Gene Rearranged Tumors With Lack of Detection of Classical ETV6-NTRK3 Fusion Transcript by Standard RT-PCR: Report of 4 Cases Harboring ETV6-X Gene Fusion. Am. J. Surg. Pathol. 2016, 40, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ishibashi, K.; Masaki, A.; Fujii, K.; Fujiyoshi, Y.; Hattori, H.; Kawakita, D.; Matsumoto, M.; Miyabe, S.; Shimozato, K.; et al. Mammary analogue secretory carcinoma of salivary glands: A clinicopathologic and molecular study including 2 cases harboring ETV6-X fusion. Am. J. Surg. Pathol. 2015, 39, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Neill, R.J.; Kelly, L.M.; Liu, P.; Brenner, A.V.; Little, M.P.; Bogdanova, T.; Evdokimova, V.N.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 2014, 120, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Ricarte-Filho, J.C.; Li, S.; Garcia-Rendueles, M.E.; Montero-Conde, C.; Voza, F.; Knauf, J.A.; Heguy, A.; Viale, A.; Bogdanova, T.; Thomas, G.A.; et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J. Clin. Investig. 2013, 123, 4935–4944. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.L.; Vyas, M.; Horne, M.J.; Virk, R.K.; Morotti, R.; Liu, Z.; Tallini, G.; Nikiforova, M.N.; Christison-Lagay, E.R.; Udelsman, R.; et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer 2016, 122, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Seethala, R.R.; Chiosea, S.I.; Liu, C.Z.; Nikiforova, M.; Nikiforov, Y.E. Clinical and Morphologic Features of ETV6-NTRK3 Translocated Papillary Thyroid Carcinoma in an Adult Population Without Radiation Exposure. Am. J. Surg. Pathol. 2017, 41, 446–457. [Google Scholar] [CrossRef] [Green Version]
- Bongarzone, I.; Vigneri, P.; Mariani, L.; Collini, P.; Pilotti, S.; Pierotti, M.A. RET/NTRK1 rearrangements in thyroid gland tumors of the papillary carcinoma family: Correlation with clinicopathological features. Clin. Cancer Res. 1998, 4, 223–228. [Google Scholar]
- Greco, A.; Miranda, C.; Pierotti, M.A. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Mol. Cell. Endocrinol. 2010, 321, 44–49. [Google Scholar] [CrossRef]
- Ronchi, A.; Montella, M.; Cozzolino, I.; Argenziano, G.; Moscarella, E.; Piccolo, V.; Iovino, F.; Troiani, T.; Alfano, R.; Errico, M.E.; et al. The potential diagnostic and predictive role of anaplastic lymphoma kinase (ALK) gene alterations in melanocytic tumors. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3829–3838. [Google Scholar]
- Lezcano, C.; Shoushtari, A.N.; Ariyan, C.; Hollmann, T.J.; Busam, K.J. Primary and Metastatic Melanoma with NTRK Fusions. Am. J. Surg. Pathol. 2018, 42, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Créancier, L.; Vandenberghe, I.; Gomes, B.; Dejean, C.; Blanchet, J.C.; Meilleroux, J.; Guimbaud, R.; Selves, J.; Kruczynski, A. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma. Cancer Lett. 2015, 365, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Ardini, E.; Bosotti, R.; Amatu, A.; Valtorta, E.; Somaschini, A.; Raddrizzani, L.; Palmeri, L.; Banfi, P.; Bonazzina, E.; et al. Sensitivity to Entrectinib Associated with a Novel LMNA-NTRK1 Gene Fusion in Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2015, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, A.; Capelletti, M.; Le, A.T.; Kako, S.; Butaney, M.; Ercan, D.; Mahale, S.; Davies, K.D.; Aisner, D.L.; Pilling, A.B.; et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat. Med. 2013, 19, 1469–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stransky, N.; Cerami, E.; Schalm, S.; Kim, J.L.; Lengauer, C. The landscape of kinase fusions in cancer. Nat. Commun. 2014, 5, 4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farago, A.F.; Taylor, M.S.; Doebele, R.C.; Zhu, V.W.; Kummar, S.; Spira, A.I.; Boyle, T.A.; Haura, E.B.; Arcila, M.E.; Benayed, R.; et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis. Oncol. 2018, 2018. [Google Scholar] [CrossRef]
- Farago, A.F.; Le, L.P.; Zheng, Z.; Muzikansky, A.; Drilon, A.; Patel, M.; Bauer, T.M.; Liu, S.V.; Ou, S.H.; Jackman, D.; et al. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1670–1674. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, Z.; Chen, X.; Ren, X.; Wei, J.; Zhou, S.; Yang, X.; Zeng, S.; Qian, L.; Wu, G.; et al. A tropomyosin receptor kinase family protein, NTRK2 is a potential predictive biomarker for lung adenocarcinoma. PeerJ 2019, 7, e7125. [Google Scholar] [CrossRef]
- Ozono, K.; Ohishi, Y.; Onishi, H.; Nakamura, K.; Motoshita, J.; Kato, M.; Nakanishi, R.; Nakamura, M.; Oda, Y. Brain-derived neurotrophic factor/tropomyosin-related kinase B signaling pathway contributes to the aggressive behavior of lung squamous cell carcinoma. Lab. Investig. 2017, 97, 1332–1342. [Google Scholar] [CrossRef]
- Rolfo, C.; Raez, L. New targets bring hope in squamous cell lung cancer: Neurotrophic tyrosine kinase gene fusions. Lab. Investig. 2017, 97, 1268–1270. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.; Yip, S.; Sorensen, P.H. Methods for Identifying Patients with Tropomyosin Receptor Kinase (TRK) Fusion Cancer. Pathol. Oncol. Res. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbareschi, M.; Barberis, M.; Buttitta, F.; Doglioni, C.; Fiorentino, M.; Fontanini, G.; Franco, R.; Marchetti, A.; Rossi, G.; Troncone, G. Predictive markers in lung cancer: A few hints for the practicing pathologist. Pathologica 2018, 110, 29–38. [Google Scholar]
- Negri, T.; Tamborini, E.; Dagrada, G.P.; Greco, A.; Staurengo, S.; Guzzo, M.; Locati, L.D.; Carbone, A.; Pierotti, M.A.; Licitra, L.; et al. TRK-A, HER-2/neu, and KIT expression/activation profiles in salivary gland carcinoma. Transl. Oncol. 2008, 1, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Liu, L.; Cai, B.; He, Y.; Wan, X. Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Sci. 2008, 99, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Nag, T.C.; Jindal, A.; Kushwaha, R.; Mahapatra, A.K.; Sarkar, C. Expression of the neurotrophin receptors Trk A and Trk B in adult human astrocytoma and glioblastoma. J. Biosci. 2003, 28, 181–188. [Google Scholar] [CrossRef]
- Chiang, S.; Cotzia, P.; Hyman, D.M.; Drilon, A.; Tap, W.D.; Zhang, L.; Hechtman, J.F.; Frosina, D.; Jungbluth, A.A.; Murali, R.; et al. NTRK Fusions Define a Novel Uterine Sarcoma Subtype with Features of Fibrosarcoma. Am. J. Surg. Pathol. 2018, 42, 791–798. [Google Scholar] [CrossRef]
- Rudzinski, E.R.; Lockwood, C.M.; Stohr, B.A.; Vargas, S.O.; Sheridan, R.; Black, J.O.; Rajaram, V.; Laetsch, T.W.; Davis, J.L. Pan-Trk Immunohistochemistry Identifies NTRK rearrangements in Pediatric Mesenchymal Tumors. Am. J. Surg. Pathol. 2018, 42, 927–935. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef]
- Gatalica, Z.; Xiu, J.; Swensen, J.; Vranic, S. Molecular characterization of cancers with NTRK gene fusions. Mod. Pathol. 2019, 32, 147–153. [Google Scholar] [CrossRef]
- Hung, Y.P.; Fletcher, C.D.M.; Hornick, J.L. Evaluation of pan-TRK immunohistochemistry in infantile fibrosarcoma, lipofibromatosis-like neural tumour and histological mimics. Histopathology 2018, 73, 634–644. [Google Scholar] [CrossRef]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK fusion detection across multiple assays and 33,997 cases: Diagnostic implications and pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef]
- Solomon, J.P.; Benayed, R.; Hechtman, J.F.; Ladanyi, M. Identifying patients with NTRK fusion cancer. Ann. Oncol. 2019, 30 (Suppl. 8), viii16–viii22. [Google Scholar] [CrossRef] [Green Version]
- Marchiò, C.; Scaltriti, M.; Ladanyi, M.; Iafrate, A.J.; Bibeau, F.; Dietel, M.; Hechtman, J.F.; Troiani, T.; López-Rios, F.; Douillard, J.Y.; et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann. Oncol. 2019, 30, 1417–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, K.M.; López-Ríos, F. Precision medicine in NSCLC and pathology: How does ALK fit in the pathway? Ann. Oncol. 2016, 27, iii16–iii24. [Google Scholar] [CrossRef] [PubMed]
- Penault-Llorca, F.; Rudzinski, E.R.; Sepulveda, A.R. Testing algorithm for identification of patients with TRK fusion cancer. J. Clin. Pathol. 2019, 72, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Adem, C.; Gisselsson, D.; Dal Cin, P.; Nascimento, A.G. ETV6 rearrangements in patients with infantile fibrosarcomas and congenital mesoblastic nephromas by fluorescence in situ hybridization. Mod. Pathol. 2001, 14, 1246–1251. [Google Scholar] [CrossRef]
- Makretsov, N.; He, M.; Hayes, M.; Chia, S.; Horsman, D.E.; Sorensen, P.H.; Huntsman, D.G. A fluorescence in situ hybridization study of ETV6-NTRK3 fusion gene in secretory breast carcinoma. Genes Chromosomes Cancer 2004, 40, 152–157. [Google Scholar] [CrossRef]
- Beadling, C.; Wald, A.I.; Warrick, A.; Neff, T.L.; Zhong, S.; Nikiforov, Y.E.; Corless, C.L.; Nikiforova, M.N. A multiplexed amplicon approach for detecting gene fusions by next-generation sequencing. J. Mol. Diagn. 2016, 18, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, J.M.; Knezevich, S.R.; Mathers, J.A.; Sorensen, P.H. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am. J. Surg. Pathol. 2000, 24, 937–946. [Google Scholar] [CrossRef]
- Russell, J.P.; Powell, D.J.; Cunnane, M.; Greco, A.; Portella, G.; Santoro, M.; Fusco, A.; Rothstein, J.L. The TRK-T1 fusion protein induces neoplastic transformation of thyroid epithelium. Oncogene 2000, 19, 5729–5735. [Google Scholar] [CrossRef] [Green Version]
- Frattini, V.; Trifonov, V.; Chan, J.M.; Castano, A.; Lia, M.; Abate, F.; Keir, S.T.; Ji, A.X.; Zoppoli, P.; Niola, F.; et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat. Genet. 2013, 45, 1141–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skálová, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.; Passador-Santos, F. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Diallo, R.; Tognon, C.; Knezevich, S.R.; Sorensen, P.; Poremba, C. Secretory carcinoma of the breast: A genetically defined carcinoma entity. Verh. Dtsch. Ges. Pathol. 2003, 87, 193–203. [Google Scholar] [PubMed]
- Knezevich, S.R.; Garnett, M.J.; Pysher, T.J.; Beckwith, J.B.; Grundy, P.E.; Sorensen, P.H. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998, 58, 5046–5048. [Google Scholar]
- Sheng, W.Q.; Hisaoka, M.; Okamoto, S.; Tanaka, A.; Meis-Kindblom., J.M.; Kindblom, L.G.; Ishida, T.; Nojima, T.; Hashimoto, H. CongenitalCongenitalinfantile fibrosarcoma: A clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin-embedded tissues. Am. J. Clin. Pathol. 2001, 115, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.D.; Le, A.T.; Sheren, J.; Nijmeh, H.; Gowan, K.; Jones, K.L.; Varella-Garcia, M.; Aisner, D.L.; Doebele, R.C. Comparison of molecular testing modalities for detection of ROS1 rearrangements in a cohort of positive patient samples. J. Thorac. Oncol. 2018, 13, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Russo, M.; Misale, S.; Wei, G.; Siravegna, G.; Crisafulli, G.; Lazzari, L.; Corti, G.; Rospo, G.; Novara, L.; Mussolin, B.; et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discov. 2016, 6, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Sheikine, Y.; Kuo, F.C.; Lindeman, N.I. Clinical and technical aspects of genomic diagnostics for precision oncology. J. Clin. Oncol. 2017, 35, 929–933. [Google Scholar] [CrossRef]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. Wiley Interdiscip. Rev. RNA 2017, 8, e1364. [Google Scholar] [CrossRef] [Green Version]
- Yohe, S.; Thyagarajan, B. Review of Clinical Next-Generation Sequencing. Arch. Pathol. Lab. Med. 2017, 141, 1544–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, P.; Touzani, R.; Perrier, L.; Rouleau, E.; Kossi, D.S.; Zhaomin, Z.; Charrier, N.; Goardon, N.; Preudhomme, C.; Durand-Zaleski, I.; et al. Cost of cancer diagnosis using next-generation sequencing targeted gene panels in routine practice: A nationwide French study. Eur. J. Hum. Genet. 2018, 26, 1396–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. NCCN Guidelines Version 5—Non-Small Cell Lung Cancer. In NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesVR); NCCN Evidence Blocks™: Washington, DC, USA, 2019. [Google Scholar]


| Adult Tumors | Pediatric Tumors | |
|---|---|---|
| <5% | Lung, colorectal, pancreatic, appendiceal cancer, cholangiocarcinoma, melanoma, glioma and several sarcoma histotypes | Glioma and several sarcoma histotypes |
| 5–75% | Thyroid and Gastrointestinal Stromal Tumors (GIST) | Thyroid, Spitzoid tumors and congenital mesoblastic nephroma |
| >75% | Secretory breast carcinoma and secretory salivary gland tumors | Infantile fibrosarcoma and breast secretory carcinoma |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zito Marino, F.; Pagliuca, F.; Ronchi, A.; Cozzolino, I.; Montella, M.; Berretta, M.; Errico, M.E.; Donofrio, V.; Bianco, R.; Franco, R. NTRK Fusions, from the Diagnostic Algorithm to Innovative Treatment in the Era of Precision Medicine. Int. J. Mol. Sci. 2020, 21, 3718. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103718
Zito Marino F, Pagliuca F, Ronchi A, Cozzolino I, Montella M, Berretta M, Errico ME, Donofrio V, Bianco R, Franco R. NTRK Fusions, from the Diagnostic Algorithm to Innovative Treatment in the Era of Precision Medicine. International Journal of Molecular Sciences. 2020; 21(10):3718. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103718
Chicago/Turabian StyleZito Marino, Federica, Francesca Pagliuca, Andrea Ronchi, Immacolata Cozzolino, Marco Montella, Massimiliano Berretta, Maria Elena Errico, Vittoria Donofrio, Roberto Bianco, and Renato Franco. 2020. "NTRK Fusions, from the Diagnostic Algorithm to Innovative Treatment in the Era of Precision Medicine" International Journal of Molecular Sciences 21, no. 10: 3718. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103718






