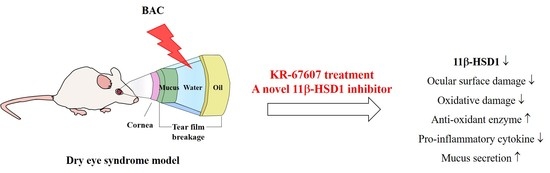
A Novel Selective 11β-HSD1 Inhibitor, (E)-4-(2-(6-(2,6-Dichloro-4-(Trifluoromethyl)Phenyl)-4-Methyl-1,1-Dioxido-1,2,6-Thiadiazinan-2-yl)Acetamido)Adamantan-1-Carboxamide (KR-67607), Prevents BAC-Induced Dry Eye Syndrome
Abstract
:1. Introduction
2. Results
2.1. Effects of 11β-HSD1 Inhibitors on Ocular Surface Damage in BAC-Treated SD Rat Eyes
2.2. Effects of 11β-HSD1 Inhibitors on Corneal Epithelial and Basement Membrane Thickness in BAC-Treated SD Rat Eyes
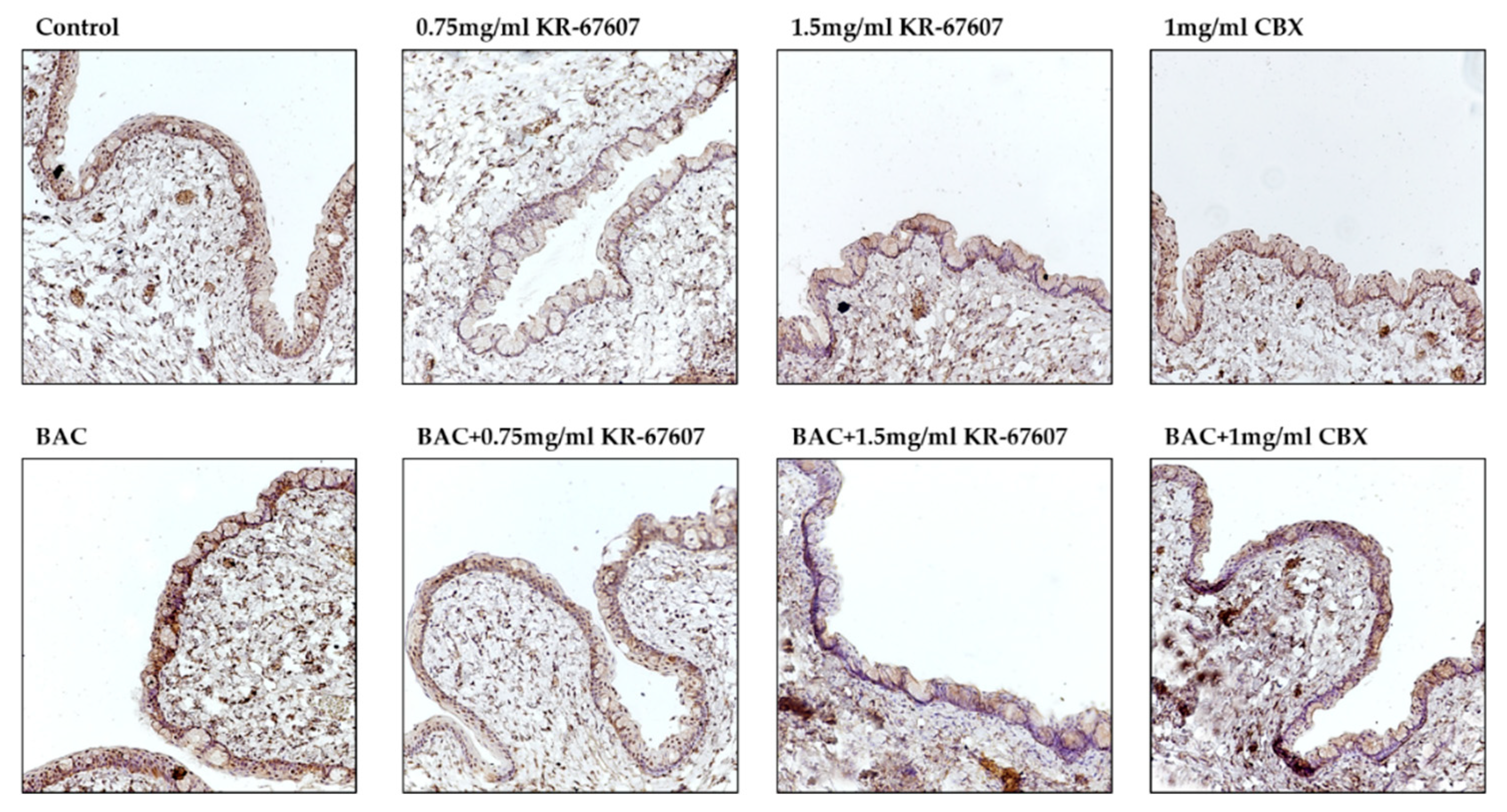
2.3. Effects of 11β-HSD1 Inhibitors on Conjunctival ROS Level in BAC-Treated SD Rat Eyes
2.4. Effects of 11β-HSD1 Inhibitors on Conjunctival Pro-Inflammatory Marker Expression in BAC-Treated SD Rat Eyes
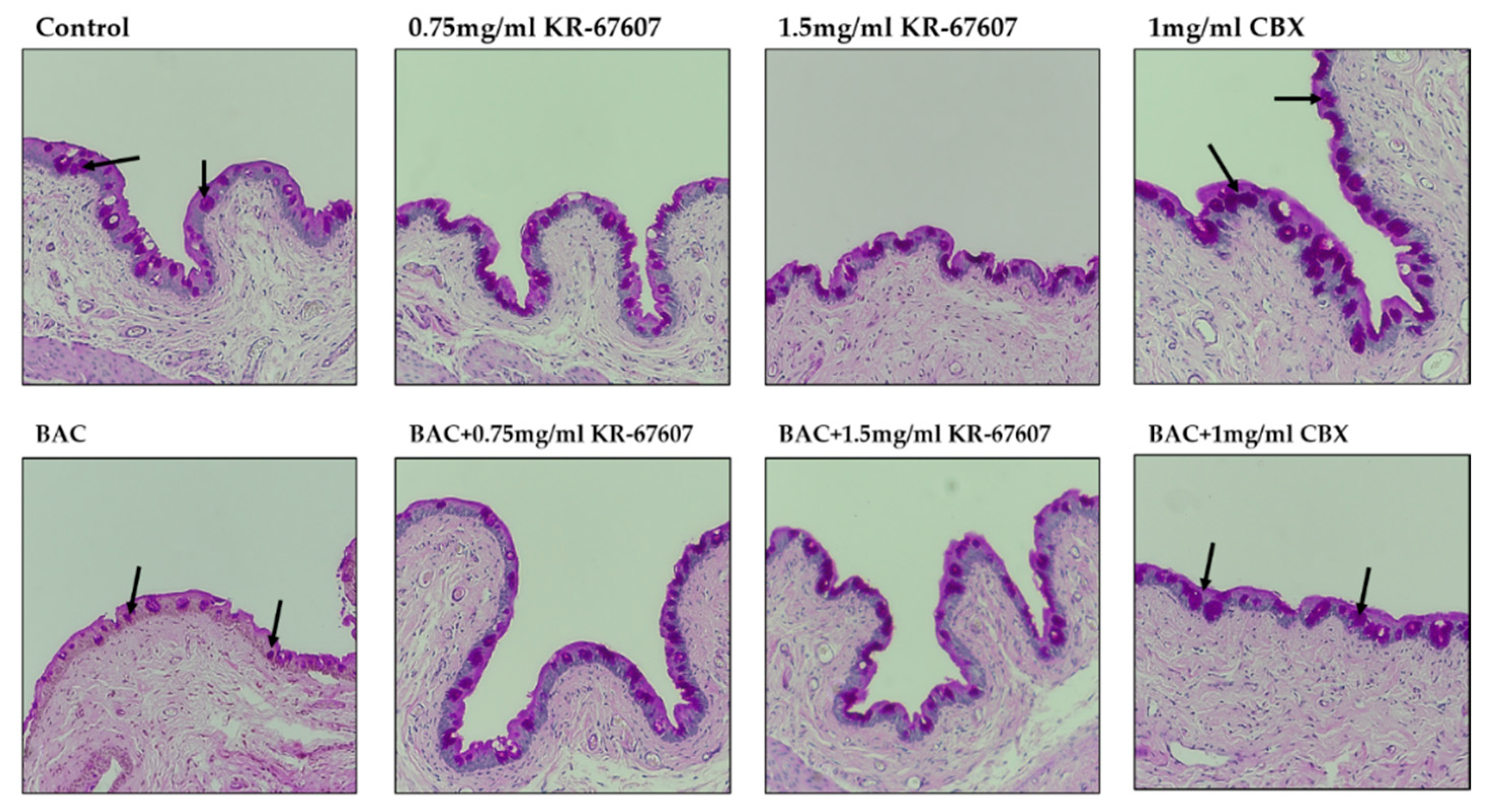
2.5. Effects of 11β-HSD1 Inhibitors on Mucus Secretion in Conjunctival Epithelium of BAC-Treated SD Rat Eyes
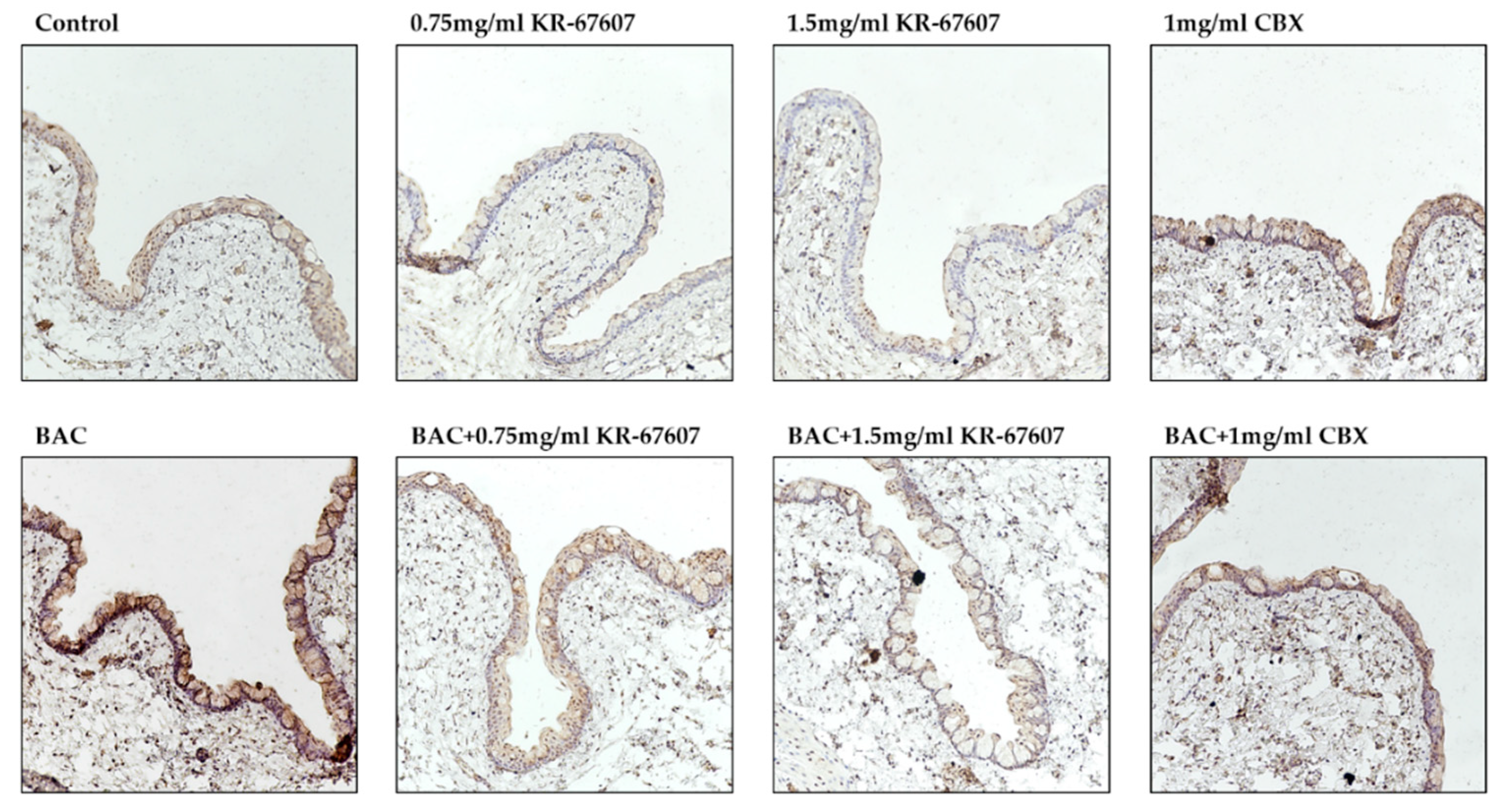
2.6. Effects of 11β-HSD1 Inhibitors on 11β-HSD1 Expression in BAC-Treated SD Rat Eyes
3. Discussion
4. Materials and Methods
4.1. Animals and Drug Administration
4.2. Rose Bengal Staining and Scoring
4.3. Histology
4.4. Immunohistochemistry (IHC) Staining
4.5. Periodic Acid and Schiff’s (PAS) Staining
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DES | Dry Eye Syndrome |
| 11β-HSD1 | 11beta-HydroxySteroid Dehydrogenase |
| CBX | Carbenoxolone |
| BAC | Benzalkonium Chloride |
| TNF-α | Tumor Necrosis Factor α |
| 4-HNE | 4-Hydroxynonenal |
| SOD1 | Superoxide Dismutase 1 |
References
- Aggarwal, S.; Galor, A. What’s new in dry eye disease diagnosis? Current advances and challenges. F1000Research 2018, 7, 1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messmer, E. The Pathophysiology, Diagnosis, and treatment of dry eye disease. Dtsch. Aerzteblatt Online 2015, 112, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcmonnies, C.W. Dry eye disease immune responses and topical therapy. Eye Vis. 2019, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Guia, R.; Rose, A.J.; Herzig, S. Glucocorticoid hormones and energy homeostasis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.-C.; Wang, J.-C. Regulation of glucose homeostasis by glucocorticoids. Single Mol. Single Cell Seq. 2015, 872, 99–126. [Google Scholar]
- Girod, J.P.; Brotman, D.J. Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiovasc. Res. 2004, 64, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Vaidya, A. Cortisol dysregulation in obesity-related metabolic disorders. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Bellaire, S.; Walzer, M.; Wang, T.; Krauwinkel, W.; Yuan, N.; Marek, G.J. Safety, pharmacokinetics, and pharmacodynamics of ASP3662, a novel 11β-hydroxysteroid dehydrogenase type 1 inhibitor, in healthy young and elderly subjects. Clin. Transl. Sci. 2019, 12, 291–301. [Google Scholar] [CrossRef]
- Zou, C.; Li, W.; Pan, Y.; Khan, Z.A.; Li, J.; Wu, X.; Wang, Y.; Deng, L.; Liang, G.; Zhao, Y. 11β-HSD1 inhibition ameliorates diabetes-induced cardiomyocyte hypertrophy and cardiac fibrosis through modulation of EGFR activity. Oncotarget 2017, 8, 96263–96275. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.S.; Rabbitt, E.H.; Goddard, P.E.; Bartlett, W.A.; Hewison, M.; Stewart, P.M. Osteoblastic 11β-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J. Bone Miner. Res. 2002, 17, 979–986. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Gray, M.; Brownstein, D.G.; Salter, D.M.; Sawatzky, D.A.; Clay, S.; Gilmour, J.S.; Seckl, J.R.; Savill, J.S.; Chapman, K.E. 11β-Hydroxysteroid dehydrogenase type 1, but not type 2, deficiency worsens acute inflammation and experimental arthritis in mice. Endocrinology 2012, 153, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, A.; Maeda, N.; Nakatsuji, H.; Hiuge-Shimizu, A.; Okada, T.; Funahashi, T.; Shimomura, I. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem. Biophys. Res. Commun. 2012, 419, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Rauz, S.; Cheung, C.M.; Wood, P.J.; Coca-Prados, M.; Walker, E.A.; Murray, P.I.; Stewart, P.M. Inhibition of 11β-hydroxysteroid dehydrogenase type 1 lowers intraocular pressure in patients with ocular hypertension. QJM 2003, 96, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, D.; Sturm, C.; Portron, A.; Fuerst-Recktenwald, S.; Hainzl, D.; Jordan, P.; Stewart, W.C.; Tepedino, M.E.; DuBiner, H. Oral administration of the 11β-hydroxysteroid-dehydrogenase type 1 inhibitor RO5093151 to patients with glaucoma: An adaptive, randomised, placebo-controlled clinical study. BMJ Open Ophthalmol. 2017, 1, e000063. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.; Shanker Dubey, R.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Babizhayev, M.A.; Yegorov, Y.E. Reactive Oxygen Species and the aging eye: Specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation-induced maturity onset cataract—A novel platform of mitochondria-targeted antioxidants with broad therapeutic potential for redox regulation and detoxification of oxidants in eye diseases. Am. J. Ther. 2016, 23, e98–e117. [Google Scholar]
- Pinazo-Duran, M.D.; Gallego-Pinazo, R.; Garcia-Medina, J.J.; Zanon-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martinez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramirez, C.; Lopez-Galvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef] [Green Version]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef] [Green Version]
- McCormick, K.L.; Wang, X.; Mick, G.J. Modification of microsomal 11β-HSD1 activity by cytosolic compounds: Glutathione and hexose phosphoesters. J. Steroid Biochem. Mol. Biol. 2008, 111, 18–23. [Google Scholar] [CrossRef]
- Kim, J.; Jung, E.J.; Moon, S.S.; Seo, M. Protective effect of carbenoxolone on ER stress-induced cell death in hypothalamic neurons. Biochem. Biophys. Res. Commun. 2015, 468, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.J.; Choi, K.J.; Park, S.B.; Sung, H.R.; Jung, W.H.; Kim, H.Y.; Rhee, S.D.; Kim, K.Y. Protective effects of carbenoxolone, an 11β-HSD1 inhibitor, against chemical induced dry eye syndrome. Apoptosis 2017, 22, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Na, Y.J.; Jung, W.H.; Park, S.B.; Kang, S.; Nam, H.J.; Ahn, J.H.; Kim, K.Y. Protective effect of a novel selective 11β-HSD1 inhibitor on eye ischemia-reperfusion induced glaucoma. Biochem. Pharmacol. 2019, 169, 113632. [Google Scholar] [CrossRef] [PubMed]
- Pisella, P.J.; Fillacier, K.; Elena, P.P.; Debbasch, C.; Baudouin, C. Comparison of the effects of preserved and unpreserved formulations of timolol on the ocular surface of albino rabbits. Ophthalmic Res. 2000, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.S.; Duncan, A.J.; Jay, J.L. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br. J. Ophthalmol. 1975, 59, 667–669. [Google Scholar] [CrossRef] [Green Version]
- Kuppens, E.V.; de Jong, C.A.; Stolwijk, T.R.; de Keizer, R.J.; van Best, J.A. Effect of timolol with and without preservative on the basal tear turnover in glaucoma. Br. J. Ophthalmol. 1995, 79, 339–342. [Google Scholar] [CrossRef]
- Liu, Z.; Pflugfelder, S.C. Corneal thickness is reduced in dry eye. Cornea 1999, 18, 403–407. [Google Scholar] [CrossRef]
- Chen, L.; Zong, R.; Zhou, J.; Ge, L.; Zhou, T.; Ma, J.X.; Liu, Z.; Zhou, Y. The oxidant role of 4-hydroxynonenal in corneal epithelium. Sci. Rep. 2015, 5, 10630. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Sakai, O.; Uchida, T.; Imai, H.; Ueta, T.; Amano, S. Role of glutathione peroxidase 4 in conjunctival epithelial cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.D.; Wright, J.C. Conjunctival goblet cell densities in ocular surface disease. Arch. Ophthalmol. 1984, 102, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Tseng, S.C.; Yoshino, K.; Monroy, D.; Felix, C.; Reis, B.L. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology 1997, 104, 223–235. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; De Paiva, C.S.; Moore, Q.L.; Volpe, E.A.; Li, D.Q.; Gumus, K.; Zaheer, M.L.; Corrales, R.M. Aqueous Tear Deficiency Increases Conjunctival Interferon-gamma (IFN-gamma) Expression and Goblet Cell Loss. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7545–7550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.; Carreiro, S.; Quenzer, T.; Gale, D.; Xiang, C.; Gukasyan, H.; Lafontaine, J.; Cheng, H.; Krauss, A.; Prasanna, G. In vivo evaluation of 11β-hydroxysteroid dehydrogenase activity in the rabbit eye. J. Ocul. Pharmacol. Ther. 2009, 25, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sasano, H.; Kaneko, C.; Ogawa, S.; Darnel, A.D.; Krozowski, Z.S. Immunohistochemical distribution of 11β-hydroxysteroid dehydrogenase in human eye. Mol. Cell. Endocrinol. 2001, 173, 121–125. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Luo, B.; Li, X.; Lu, W.; Yang, J.; Hu, Y.; Huang, P.; Wen, S. Inhibition of cancer growth in vitro and in vivo by a novel ROS-modulating agent with ability to eliminate stem-like cancer cells. Cell Death Dis. 2017, 8, e2887. [Google Scholar] [CrossRef]
- Yao, J.; Cheng, Y.; Zhou, M.; Zhao, S.; Lin, S.; Wang, X.; Wu, J.; Li, S.; Wei, H. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem. Sci. 2018, 9, 2927–2933. [Google Scholar] [CrossRef] [Green Version]
- Choy, C.K.; Benzie, I.F.; Cho, P. Ascorbic acid concentration and total antioxidant activity of human tear fluid measured using the FRASC assay. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3293–3298. [Google Scholar]
- Jee, D.; Park, S.H.; Kim, M.S.; Kim, E.C. Antioxidant and inflammatory cytokine in tears of patients with dry eye syndrome treated with preservative-free versus preserved eye drops. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5081–5089. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Chen, D.; Liu, J.; Liu, B.; Li, N.; Zhou, Y.; Liang, X.; Ma, P.; Ye, C.; Ge, J.; et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1850–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feenstra, R.P.G.; Tseng, S.C.G. What is actually stained by rose bengal? Arch. Ophthalmol. 1992, 110, 984–993. [Google Scholar] [CrossRef]
- Ehlers, N.; Heegaard, S.; Hjortdal, J.; Ivarsen, A.; Nielsen, K.; Prause, J.U. Morphological evaluation of normal human corneal epithelium. Acta Ophthalmol. 2010, 88, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The corneal epithelial basement membrane: Structure, function, and disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef] [PubMed]
- Haworth, K.M.; Chandler, H.L. Oxidative stress measures of lipid and dna damage in human tears. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO151–BIO157. [Google Scholar] [CrossRef] [Green Version]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, H.; Yang, J.; Ye, J. Sodium perbarate and benzalkonium chloride induce DNA damage in Chang conjunctival epithelial cells. Cutan. Ocul. Toxicol. 2017, 36, 336–342. [Google Scholar] [CrossRef]
- Moore, J.E.; Vasey, G.T.; Dartt, D.A.; McGilligan, V.E.; Atkinson, S.D.; Grills, C.; Lamey, P.J.; Leccisotti, A.; Frazer, D.G.; Moore, T.C. Effect of tear hyperosmolarity and signs of clinical ocular surface pathology upon conjunctival goblet cell function in the human ocular surface. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6174–6180. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.L.; Liu, X.J.; Ma, J.X.; Zhu, J. Effects of matrine on airway inflammation and early airway remodeling in asthmatic mice. Chin. J. Tuberc. Respir. Dis. 2009, 32, 165–170. [Google Scholar]
- Shirai, K.; Saika, S. Ocular surface mucins and local inflammation—Studies in genetically modified mouse lines. BMC Ophthalmol. 2015, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Takamura, E.; Tsubota, K.; Watanabe, H.; Ohashi, Y. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br. J. Ophthalmol. 2012, 96, 1310–1315. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, Y.-J.; Choi, K.J.; Jung, W.H.; Park, S.B.; Kang, S.; Ahn, J.H.; Kim, K.Y. A Novel Selective 11β-HSD1 Inhibitor, (E)-4-(2-(6-(2,6-Dichloro-4-(Trifluoromethyl)Phenyl)-4-Methyl-1,1-Dioxido-1,2,6-Thiadiazinan-2-yl)Acetamido)Adamantan-1-Carboxamide (KR-67607), Prevents BAC-Induced Dry Eye Syndrome. Int. J. Mol. Sci. 2020, 21, 3729. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103729
Na Y-J, Choi KJ, Jung WH, Park SB, Kang S, Ahn JH, Kim KY. A Novel Selective 11β-HSD1 Inhibitor, (E)-4-(2-(6-(2,6-Dichloro-4-(Trifluoromethyl)Phenyl)-4-Methyl-1,1-Dioxido-1,2,6-Thiadiazinan-2-yl)Acetamido)Adamantan-1-Carboxamide (KR-67607), Prevents BAC-Induced Dry Eye Syndrome. International Journal of Molecular Sciences. 2020; 21(10):3729. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103729
Chicago/Turabian StyleNa, Yoon-Ju, Kyoung Jin Choi, Won Hoon Jung, Sung Bum Park, Sein Kang, Jin Hee Ahn, and Ki Young Kim. 2020. "A Novel Selective 11β-HSD1 Inhibitor, (E)-4-(2-(6-(2,6-Dichloro-4-(Trifluoromethyl)Phenyl)-4-Methyl-1,1-Dioxido-1,2,6-Thiadiazinan-2-yl)Acetamido)Adamantan-1-Carboxamide (KR-67607), Prevents BAC-Induced Dry Eye Syndrome" International Journal of Molecular Sciences 21, no. 10: 3729. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21103729