Recovery of BDNF and CB1R in the Prefrontal Cortex Underlying Improvement of Working Memory in Prenatal DEHP-Exposed Male Rats after Aerobic Exercise
Abstract
:1. Introduction
2. Results
2.1. Delayed Non-Match-to-Sample Task
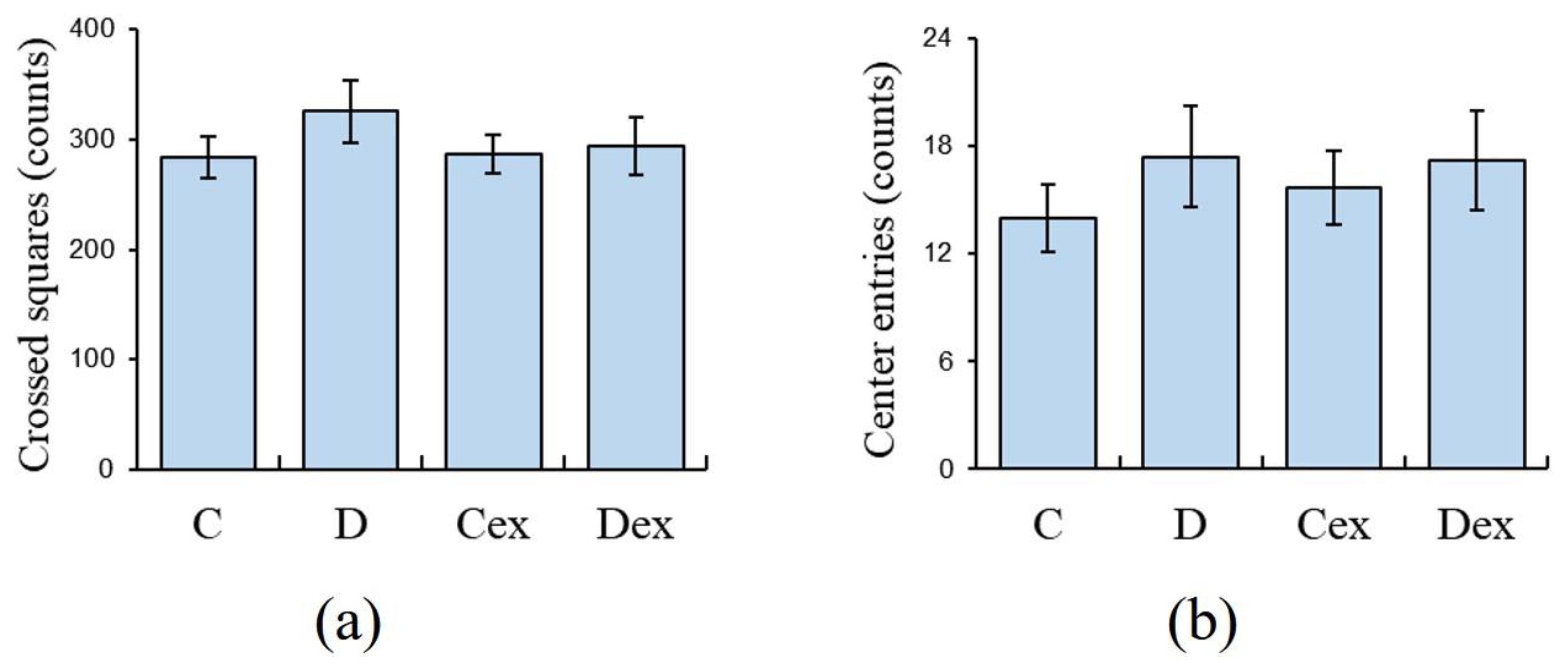
2.2. Open Field Test
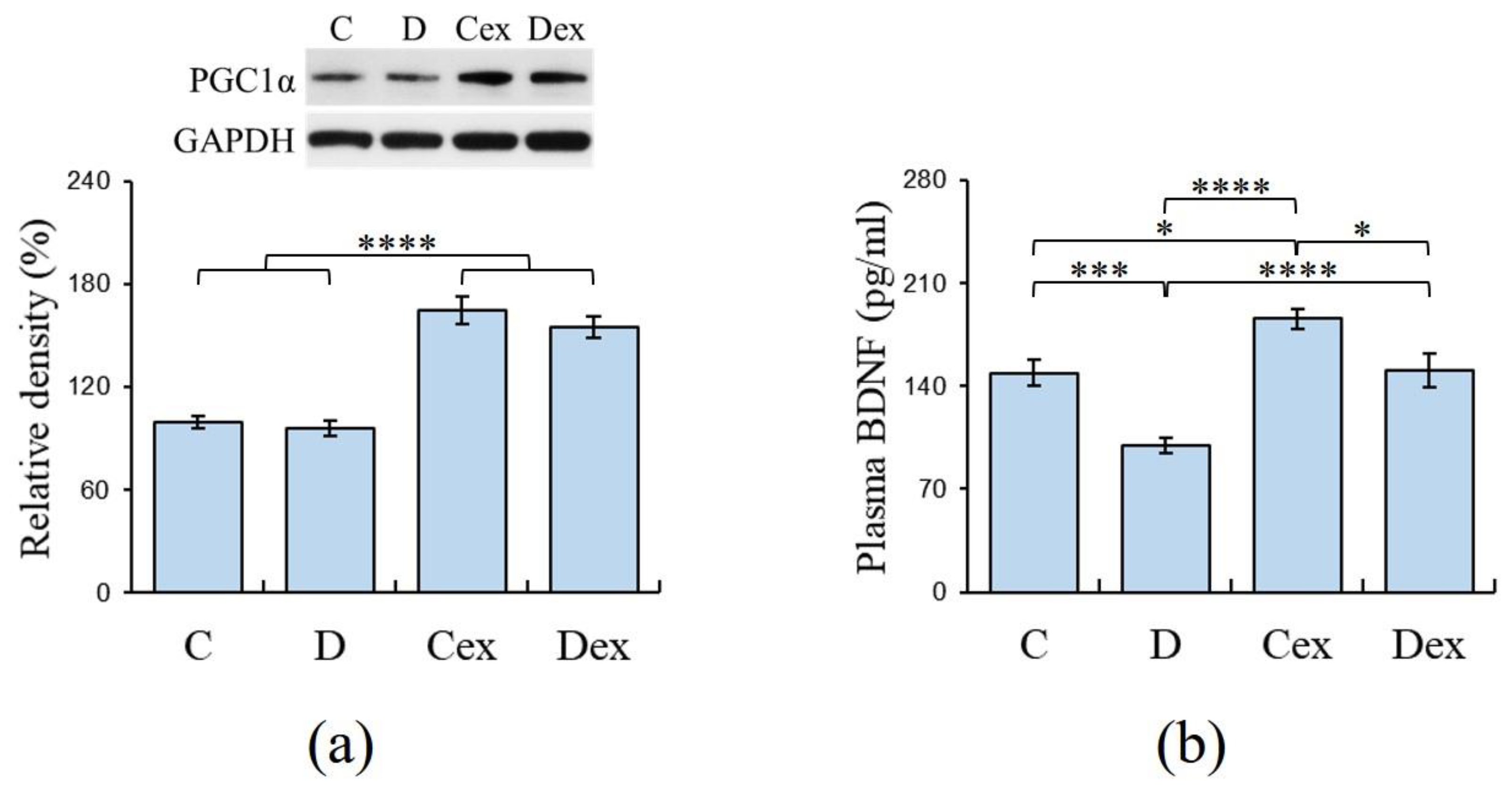
2.3. Efficacy of Exercise Regimen
2.4. Plasma Levels of BDNF
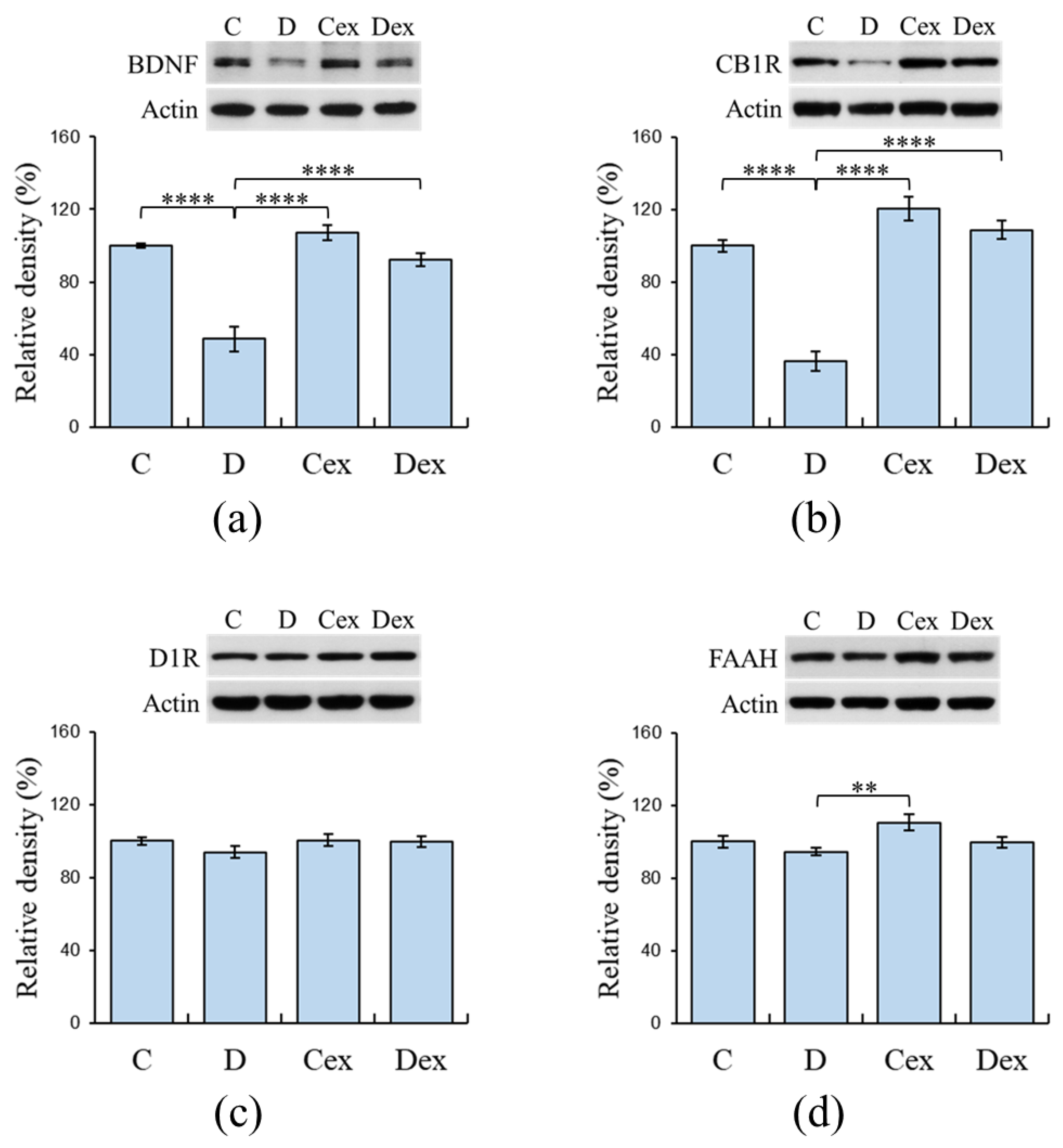
2.5. Protein Levels in the Prefrontal Cortex
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Gestational Administration of DEHP
4.4. Treadmill Running
4.5. Open Field Test
4.6. Delayed Non-Match-to-Sample Task
4.7. Blood and Tissue Sample Collection
4.8. Western Blot
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | attention-deficit hyperactivity disorder |
| BDNF | brain-derived neurotrophic factor |
| CB1R | cannabinoid receptor 1 |
| D1R | dopamine D1 receptor |
| DEHP | di-(2-ethylhexyl)-phthalate |
| FAAH | fatty acid amide hydrolase |
| GABA | gamma-aminobutyric acid |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
References
- Johns, L.E.; Cooper, G.S.; Galizia, A.; Meeker, J.D. Exposure assessment issues in epidemiology studies of phthalates. Environ. Int. 2015, 85, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Kim, M.S.; Lim, Y.H.; Lee, N.; Hong, Y.C. Prenatal and postnatal exposure to di-(2-Ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environ. Res. 2018, 167, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Yang, Y.; Xu, X.; Hu, Y. Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Horm. Behav. 2015, 71, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Quinnies, K.M.; Harris, E.P.; Snyder, R.W.; Sumner, S.S.; Rissman, E.F. Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS ONE 2017, 12, e0171977. [Google Scholar] [CrossRef]
- Wang, D.C.; Chen, T.J.; Lin, M.L.; Jhong, Y.C.; Chen, S.C. Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus-pituitary-adrenal axis. Horm. Behav. 2014, 66, 674–684. [Google Scholar] [CrossRef]
- Factor-Litvak, P.; Insel, B.; Calafat, A.M.; Liu, X.; Perera, F.; Rauh, V.A.; Whyatt, R.M. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS ONE 2014, 9, e114003. [Google Scholar]
- Huang, H.B.; Kuo, P.H.; Su, P.H.; Sun, C.W.; Chen, W.J.; Wang, S.L. Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study. Environ. Res. 2019, 172, 569–577. [Google Scholar] [CrossRef]
- Lien, Y.J.; Ku, H.Y.; Su, P.H.; Chen, S.J.; Chen, H.Y.; Liao, P.C.; Chen, W.J.; Wang, S.L. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ. Health Perspect. 2015, 123, 95–100. [Google Scholar] [CrossRef]
- Engel, S.M.; Villanger, G.D.; Nethery, R.C.; Thomsen, C.; Sakhi, A.K.; Drover, S.S.M.; Hoppin, J.A.; Zeiner, P.; Knudsen, G.P.; Reichborn-Kjennerud, T.; et al. Prenatal phthalates, maternal thyroid function, and risk of attention-deficit hyperactivity disorder in the Norwegian Mother and Child Cohort. Environ. Health Perspect. 2018, 126, 057004. [Google Scholar] [CrossRef] [Green Version]
- Ku, H.Y.; Tsai, T.L.; Wang, P.L.; Su, P.H.; Sun, C.W.; Wang, C.J.; Wang, S.L. Prenatal and childhood phthalate exposure and attention deficit hyperactivity disorder traits in child temperament: A 12-year follow-up birth cohort study. Sci. Total. Environ. 2020, 699, 134053. [Google Scholar] [CrossRef]
- Kofler, M.J.; Sarver, D.E.; Harmon, S.L.; Moltisanti, A.; Aduen, P.A.; Soto, E.F.; Ferretti, N. Working memory and organizational skills problems in ADHD. J. Child. Psychol. Psychiatry 2018, 59, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hiser, J.; Koenigs, M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol. Psychiatry 2018, 83, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Reneaux, M.; Gupta, R. Prefronto-cortical dopamine D1 receptor sensitivity can critically influence working memory maintenance during delayed response tasks. PLoS ONE 2018, 13, e0198136. [Google Scholar]
- Bäckman, L.; Karlsson, S.; Fischer, H.; Karlsson, P.; Brehmer, Y.; Rieckmann, A.; MacDonald, S.W.; Farde, L.; Nyberg, L. Dopamine D1 receptors and age differences in brain activation during working memory. Neurobiol. Aging. 2011, 32, 1849–1856. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Kolb, B.; Forssberg, H. Motor inhibitory role of dopamine D1 receptors: Implications for ADHD. Physiol. Behav. 2007, 92, 155–160. [Google Scholar]
- Slifstein, M.; van de Giessen, E.; Van Snellenberg, J.; Thompson, J.L.; Narendran, R.; Gil, R.; Hackett, E.; Girgis, R.; Ojeil, N.; Moore, H.; et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: A positron emission tomographic functional magnetic resonance imaging study. Jama Psychiatry 2015, 72, 316–324. [Google Scholar] [CrossRef]
- Kodama, T.; Kojima, T.; Honda, Y.; Hosokawa, T.; Tsutsui, K.I.; Watanabe, M. Oral administration of methylphenidate (Ritalin) affects dopamine release differentially between the prefrontal cortex and striatum: A microdialysis study in the monkey. J. Neurosci. 2017, 37, 2387–2394. [Google Scholar]
- Robison, L.S.; Ananth, M.; Hadjiargyrou, M.; Komatsu, D.E.; Thanos, P.K. Chronic oral methylphenidate treatment reversibly increases striatal dopamine transporter and dopamine type 1 receptor binding in rats. J. Neural. Transm. 2017, 124, 655–667. [Google Scholar]
- Colizzi, M.; Fazio, L.; Ferranti, L.; Porcelli, A.; Masellis, R.; Marvulli, D.; Bonvino, A.; Ursini, G.; Blasi, G.; Bertolino, A. Functional genetic variation of the cannabinoid receptor 1 and cannabis use interact on prefrontal connectivity and related working memory behavior. Neuropsychopharmacology 2015, 40, 640–649. [Google Scholar]
- Zanettini, C.; Panlilio, L.V.; Alicki, M.; Goldberg, S.R.; Haller, J.; Yasar, S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. 2011, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Elevating endocannabinoid levels: Pharmacological strategies and potential therapeutic applications. Proc. Nutr. Soc. 2014, 73, 96–105. [Google Scholar] [PubMed] [Green Version]
- Pazos, M.R.; Núñez, E.; Benito, C.; Tolón, R.M.; Romero, J. Functional neuroanatomy of the endocannabinoid system. Pharmacol. Biochem. Behav. 2005, 81, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Borgan, F.; Beck, K.; Butler, E.; McCutcheon, R.; Veronese, M.; Vernon, A.; Howes, O.D. The effects of cannabinoid 1 receptor compounds on memory: A meta-analysis and systematic review across species. Psychopharmacology 2019, 236, 3257–3270. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, D.C.; Cortes-Briones, J.A.; Ranganathan, M.; Thurnauer, H.; Creatura, G.; Surti, T.; Planeta, B.; Neumeister, A.; Pittman, B.; Normandin, M.; et al. Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Hirvonen, J.; Goodwin, R.S.; Li, C.T.; Terry, G.E.; Zoghbi, S.S.; Morse, C.; Pike, V.W.; Volkow, N.D.; Huestis, M.A.; Innis, R.B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 2012, 17, 642–649. [Google Scholar]
- Bonnin, A.; de Miguel, R.; Hernández, M.L.; Ramos, J.A.; Fernández-Ruiz, J.J. The prenatal exposure to delta 9-tetrahydrocannabinol affects the gene expression and the activity of tyrosine hydroxylase during early brain development. Life Sci. 1995, 56, 2177–2184. [Google Scholar] [CrossRef]
- Bosier, B.; Muccioli, G.G.; Mertens, B.; Sarre, S.; Michotte, Y.; Lambert, D.M.; Hermans, E. Differential modulations of striatal tyrosine hydroxylase and dopamine metabolism by cannabinoid agonists as evidence for functional selectivity in vivo. Neuropharmacology 2012, 62, 2328–2336. [Google Scholar] [CrossRef]
- Campolongo, P.; Trezza, V.; Cassano, T.; Gaetani, S.; Morgese, M.G.; Ubaldi, M.; Soverchia, L.; Antonelli, T.; Ferraro, L.; Massi, M.; et al. Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict. Biol. 2007, 12, 485–495. [Google Scholar]
- de Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. J. Sci. Med. Sport. 2018, 21, 501–507. [Google Scholar]
- Verburgh, L.; Königs, M.; Scherder, E.J.; Oosterlaan, J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis. Br. J. Sports Med. 2014, 48, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [PubMed]
- Walsh, J.J.; Tschakovsky, M.E. Exercise and circulating BDNF: Mechanisms of release and implications for the design of exercise interventions. Appl. Physiol. Nutr. Metab. 2018, 43, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E. Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, E.I.; Smith, L.; Northey, J.; Rattray, B.; Cherbuin, N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: A review of associations and dosage. Ageing Res. Rev. 2020, 11, 101044. [Google Scholar] [CrossRef] [PubMed]
- Wigal, S.B.; Emmerson, N.; Gehricke, J.G.; Galassetti, P. Exercise: Applications to childhood ADHD. J. Atten. Disord. 2013, 17, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Ashdown-Franks, G.; Firth, J.; Carney, R.; Carvalho, A.F.; Hallgren, M.; Koyanagi, A.; Rosenbaum, S.; Schuch, F.B.; Smith, L.; Solmi, M.; et al. Exercise as medicine for mental and substance use disorders: A meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med. 2020, 50, 151–170. [Google Scholar] [CrossRef]
- Mercurio, L.Y.; Amanullah, S.; Gill, N.; Gjelsvik, A. Children with ADHD engage in less physical activity. J. Atten. Disord. 2019. [Google Scholar] [CrossRef]
- Jung, S.; Kim, K. Exercise-induced PGC-1α transcriptional factors in skeletal muscle. Integr. Med. Res. 2014, 3, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Masuo, Y.; Ishido, M.; Morita, M.; Oka, S. Effects of neonatal treatment with 6-hydroxydopamine and endocrine disruptors on motor activity and gene expression in rats. Neural Plast. 2004, 11, 59–76. [Google Scholar] [CrossRef] [Green Version]
- Barakat, R.; Lin, P.C.; Park, C.J.; Best-Popescu, C.; Bakry, H.H.; Abosalem, M.E.; Abdelaleem, N.M.; Flaws, J.A.; Ko, C. Prenatal exposure to DEHP induces neuronal degeneration and neurobehavioral abnormalities in adult male mice. Toxicol. Sci. 2018, 164, 439–452. [Google Scholar]
- Lee, K.I.; Chiang, C.W.; Lin, H.C.; Zhao, J.F.; Li, C.T.; Shyue, S.K.; Lee, T.S. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch. Toxicol. 2016, 90, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.M.; Sucharski, I.L.; Finlay, J.M. Desipramine attenuates working memory impairments induced by partial loss of catecholamines in the rat medial prefrontal cortex. Psychopharmacology 2006, 183, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, T.; Okada, J.; Jung, M.W.; Kim, J.J. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn. Mem. 2008, 15, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Gu, X.; Zhu, J.; Zhang, X.; Han, Z.; Yan, W.; Cheng, Q.; Hao, J.; Fan, H.; Hou, R.; et al. Medial prefrontal activity during delay period contributes to learning of a working memory task. Science 2014, 346, 458–463. [Google Scholar] [CrossRef]
- Ozdemir, A.T.; Lagler, M.; Lagoun, S.; Malagon-Vina, H.; Lasztóczi, B.; Klausberger, T. Unexpected rule-changes in a working memory task shape the firing of histologically identified delay-tuned neurons in the prefrontal cortex. Cell Rep. 2020, 30, 1613–1626. [Google Scholar]
- Porter, M.C.; Burk, J.A.; Mair, R.G. A comparison of the effects of hippocampal or prefrontal cortical lesions on three versions of delayed non-matching-to-sample based on positional or spatial cues. Behav. Brain Res. 2000, 109, 69–81. [Google Scholar] [CrossRef]
- Kougias, D.G.; Sellinger, E.P.; Willing, J.; Juraska, J.M. Perinatal exposure to an environmentally relevant mixture of phthalates results in a lower number of neurons and synapses in the medial prefrontal cortex and decreased cognitive flexibility in adult male and female rats. J. Neurosci. 2018, 38, 6864–6872. [Google Scholar]
- Lin, H.; Yuan, K.; Li, L.; Liu, S.; Li, S.; Hu, G.; Lian, Q.Q.; Ge, R.S. In utero exposure to diethylhexyl phthalate affects rat brain development: A behavioral and genomic approach. Int. J. Environ. Res. Public Health. 2015, 12, 13696–13710. [Google Scholar]
- Ran, D.; Luo, Y.; Gan, Z.; Liu, J.; Yang, J. Neural mechanisms underlying the deficit of learning and memory by exposure to di(2-ethylhexyl) phthalate in rats. Ecotoxicol. Environ. Saf. 2019, 174, 58–65. [Google Scholar] [CrossRef]
- Avery, M.C.; Krichmar, J.L. Improper activation of D1 and D2 receptors leads to excess noise in prefrontal cortex. Front. Comput. Neurosci. 2015, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Holahan, M.R.; Smith, C.A.; Luu, B.E.; Storey, K.B. Preadolescent phthalate (DEHP) exposure is associated with elevated locomotor activity and reward-related behavior and a reduced number of tyrosine hydroxylase positive neurons in post-adolescent male and female rats. Toxicol. Sci. 2018, 165, 512–530. [Google Scholar] [PubMed]
- Wang, R.; Xu, X.; Zhu, Q. Pubertal exposure to di-(2-ethylhexyl) phthalate influences social behavior and dopamine receptor D2 of adult female mice. Chemosphere 2016, 144, 1771–1779. [Google Scholar] [PubMed]
- Ishido, M.; Morita, M.; Oka, S.; Masuo, Y. Alteration of gene expression of G protein-coupled receptors in endocrine disruptors-caused hyperactive rats. Regul. Pept. 2005, 126, 145–153. [Google Scholar]
- Andersen, S.L.; Thompson, A.P.; Krenzel, E.; Teicher, M.H. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology 2002, 27, 683–691. [Google Scholar]
- Andersen, S.L.; Thompson, A.T.; Rutstein, M.; Hostetter, J.C.; Teicher, M.H. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 2000, 37, 167–169. [Google Scholar] [CrossRef]
- Ceccarini, J.; Ahmad, R.; Van de Vliet, L.; Casteels, C.; Vandenbulcke, M.; Vandenberghe, W.; Van Laere, K. Behavioral symptoms in premanifest Huntington disease correlate with reduced frontal CB1R levels. J. Nucl. Med. 2019, 60, 115–121. [Google Scholar]
- Ceccarini, J.; Casteels, C.; Ahmad, R.; Crabbé, M.; Van de Vliet, L.; Vanhaute, H.; Vandenbulcke, M.; Vandenberghe, W.; Van Laere, K. Regional changes in the type 1 cannabinoid receptor are associated with cognitive dysfunction in Parkinson’s disease. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2348–2357. [Google Scholar]
- Eggan, S.M.; Hashimoto, T.; Lewis, D.A. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch. Gen. Psychiatry 2008, 65, 772–784. [Google Scholar] [CrossRef]
- Ruiz-Contreras, A.E.; Carrillo-Sánchez, K.; Ortega-Mora, I.; Barrera-Tlapa, M.A.; Román-López, T.V.; Rosas-Escobar, C.B.; Flores-Barrera, L.; Caballero-Sánchez, U.; Muñoz-Torres, Z.; Romero-Hidalgo, S.; et al. Performance in working memory and attentional control is associated with the rs2180619 SNP in the CNR1 gene. Genes Brain Behav. 2014, 13, 173–178. [Google Scholar] [CrossRef]
- Castelli, M.; Federici, M.; Rossi, S.; De Chiara, V.; Napolitano, F.; Studer, V.; Motta, C.; Sacchetti, L.; Romano, R.; Musella, A.; et al. Loss of striatal cannabinoid CB1 receptor function in attention-deficit/hyperactivity disorder mice with point-mutation of the dopamine transporter. Eur. J. Neurosci. 2011, 34, 1369–1377. [Google Scholar] [PubMed]
- Chiodi, V.; Uchigashima, M.; Beggiato, S.; Ferrante, A.; Armida, M.; Martire, A.; Potenza, R.L.; Ferraro, L.; Tanganelli, S.; Watanabe, M.; et al. Unbalance of CB1 receptors expressed in GABAergic and glutamatergic neurons in a transgenic mouse model of Huntington’s disease. Neurobiol. Dis. 2012, 45, 983–991. [Google Scholar] [PubMed]
- Bisset, K.M.; Dhopeshwarkar, A.S.; Liao, C.; Nicholson, R.A. The G protein-coupled cannabinoid-1 (CB1) receptor of mammalian brain: Inhibition by phthalate esters in vitro. Neurochem. Int. 2011, 59, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Szwarcfarb, B.; Ponzo, O.; Reynoso, R.; Cardoso, N.; Deguiz, L.; Moguilevsky, J.A.; Scacchi, P. Impact of gestational and lactational phthalate exposure on hypothalamic content of amino acid neurotransmitters and FSH secretion in peripubertal male rats. Neurotoxicology 2010, 31, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; de Miguel, R.; Fernández-Ruiz, J.; Gutiérrez-López, D.; Carai, M.A.; Ramos, J.A. Effects of a short-term exposure to alcohol in rats on FAAH enzyme and CB1 receptor in different brain areas. Drug Alcohol Depend. 2009, 99, 354–358. [Google Scholar] [CrossRef]
- Rubio, M.; McHugh, D.; Fernández-Ruiz, J.; Bradshaw, H.; Walker, J.M. Short-term exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci. Lett. 2007, 421, 270–274. [Google Scholar]
- Qiu, F.; Zhou, Y.; Deng, Y.; Yi, J.; Gong, M.; Liu, N.; Wei, C.; Xiang, S. Knockdown of TNFAIP1 prevents di-(2-ethylhexyl) phthalate-induced neurotoxicity by activating CREB pathway. Chemosphere 2020, 241, 125114. [Google Scholar] [PubMed]
- Smith, C.A.; Holahan, M.R. Reduced hippocampal dendritic spine density and BDNF expression following acute postnatal exposure to di(2-ethylhexyl) phthalate in male Long Evans rats. PLoS ONE 2014, 9, e109522. [Google Scholar] [CrossRef] [Green Version]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001, 15, 1748–1757. [Google Scholar] [CrossRef]
- Fumagalli, F.; Racagni, G.; Colombo, E.; Riva, M.A. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol. Psychiatry 2003, 8, 898–899. [Google Scholar]
- Kim, H.; Heo, H.I.; Kim, D.H.; Ko, I.G.; Lee, S.S.; Kim, S.E.; Kim, B.K.; Kim, T.W.; Ji, E.S.; Kim, J.D.; et al. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci. Lett. 2011, 504, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, H.A.; Nelson, M.E.; Granholm, A.C. Age-related deficits as working memory load increases: Relationships with growth factors. Neurobiol. Aging 2003, 24, 37–48. [Google Scholar] [CrossRef]
- Islam, R.; Matsuzaki, K.; Sumiyoshi, E.; Hossain, M.E.; Hashimoto, M.; Katakura, M.; Sugimoto, N.; Shido, O. Theobromine improves working memory by activating the CaMKII/CREB/BDNF pathway in rats. Nutrients 2019, 11, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, F.F.; Ribeiro, F.F.; Rodrigues, R.S.; Sebastião, A.M.; Xapelli, S. Brain-derived neurotrophic factor (BDNF) role in cannabinoid-mediated neurogenesis. Front. Cell. Neurosci. 2018, 12, 441. [Google Scholar] [CrossRef] [Green Version]
- Butovsky, E.; Juknat, A.; Goncharov, I.; Elbaz, J.; Eilam, R.; Zangen, A.; Vogel, Z. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Delta-tetrahydrocannabinol. J. Neurochem. 2005, 93, 802–811. [Google Scholar] [CrossRef]
- Aso, E.; Ozaita, A.; Valdizán, E.M.; Ledent, C.; Pazos, A.; Maldonado, R.; Valverde, O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J. Neurochem. 2008, 105, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Maison, P.; Walker, D.J.; Walsh, F.S.; Williams, G.; Doherty, P. BDNF regulates neuronal sensitivity to endocannabinoids. Neurosci. Lett. 2009, 467, 90–94. [Google Scholar] [CrossRef]
- Chang, Y.K.; Etnier, J.L. Exploring the dose-response relationship between resistance exercise intensity and cognitive function. J. Sport Exerc. Psychol. 2009, 31, 640–656. [Google Scholar] [CrossRef] [Green Version]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef]
- Amiri, A.; Torabi Parizi, G.; Kousha, M.; Saadat, F.; Modabbernia, M.J.; Najafi, K.; Atrkar Roushan, Z. Changes in plasma brain-derived neurotrophic factor (BDNF) levels induced by methylphenidate in children with attention deficit-hyperactivity disorder (ADHD). Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 47, 20–24. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur. J. Appl. Physiol. 2013, 113, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Titterness, A.K.; Morrish, A.C.; Carrier, E.J.; Lee, T.T.; Gil-Mohapel, J.; Gorzalka, B.B.; Hillard, C.J.; Christie, B.R. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 2010, 20, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyman, E.; Gamelin, F.X.; Goekint, M.; Piscitelli, F.; Roelands, B.; Leclair, E.; Di Marzo, V.; Meeusen, R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Bastos, C.P.; Pereira, G.S.; Moreira, F.A.; Massensini, A.R. A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus 2014, 24, 79–88. [Google Scholar] [CrossRef]
- Raubertas, R.F.; Davis, B.A.; Bowen, W.H.; Pearson, S.K.; Watson, G.E. Litter effects on caries in rats and implications for experimental design. Caries Res. 1999, 33, 164–169. [Google Scholar] [CrossRef]
- Shelby, M.D. NTP-CERHR monograph on the potential human reproductive and developmental effects of di (2-ethylhexyl) phthalate (DEHP). Ntp Cerhr Mon 2006, 18, v–vii. [Google Scholar]
- Campioli, E.; Martinez-Arguelles, D.B.; Papadopoulos, V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr. Diabetes. 2014, 4, e115. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.C.; Liu, P.C.; Hung, H.S.; Chen, T.J. Both PKMζ and KIBRA are closely related to reference memory but not working memory in a T-maze task in rats. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2014, 200, 77–82. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.-C.; Lin, H.-T.; Lee, Y.-J.; Yu, H.-F.; Wu, S.-R.; Qamar, M.U. Recovery of BDNF and CB1R in the Prefrontal Cortex Underlying Improvement of Working Memory in Prenatal DEHP-Exposed Male Rats after Aerobic Exercise. Int. J. Mol. Sci. 2020, 21, 3867. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113867
Wang D-C, Lin H-T, Lee Y-J, Yu H-F, Wu S-R, Qamar MU. Recovery of BDNF and CB1R in the Prefrontal Cortex Underlying Improvement of Working Memory in Prenatal DEHP-Exposed Male Rats after Aerobic Exercise. International Journal of Molecular Sciences. 2020; 21(11):3867. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113867
Chicago/Turabian StyleWang, Dean-Chuan, Hwai-Ting Lin, Yi-Ju Lee, Hsien-Fu Yu, Sin-Ru Wu, and Muhammad Usama Qamar. 2020. "Recovery of BDNF and CB1R in the Prefrontal Cortex Underlying Improvement of Working Memory in Prenatal DEHP-Exposed Male Rats after Aerobic Exercise" International Journal of Molecular Sciences 21, no. 11: 3867. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113867





