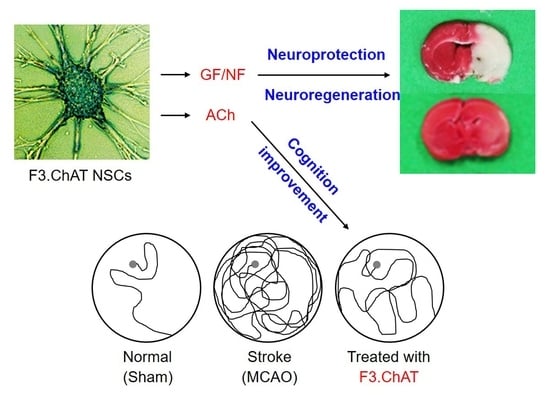
Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Aβ Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice
Abstract
:1. Introduction
2. Results
2.1. Cholinergic Properties of NSCs
2.2. Distribution and Differentiation of NSCs
2.3. Recovery of Cholinergic and Cognitive Functions
2.4. Aβ Elimination via Microglial Function Restoration
2.5. Neuroregeneration Mediated by GFs/NFs
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Human NSC Lines
4.3. Animal Model and NSC Transplantation
4.4. Cognitive Functions
4.5. Neurobehavioral Functions
4.6. Analysis of Aβ Peptides in Brain Tissues
4.7. Analysis of ACh in Brain Tissues
4.8. RT-PCR Analysis in NSCs and Brain Tissues
4.9. Western Blot Analysis in Brain Tissues
4.10. Immunohistochemistry in Brain Sections
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Terry, R.D.; Davies, P. Dementia of the Alzheimer type. Ann. Rev. Neurosci. 1980, 3, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Kasa, P.; Rakonczay, Z.; Gulya, K. The cholinergic system in Alzheimer’s disease. Progr. Neurobiol. 1997, 52, 511–535. [Google Scholar] [CrossRef]
- Musial, A.; Bajda, M.; Malawska, B. Recent developments in cholinesterases inhibitors for Alzheimer’s disease treatment. Curr. Med. Chem. 2007, 14, 2654–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hao, J.; Liu, R.; Zhang, Z.; Lei, G.; Su, C.; Miao, J.; Li, Z. Soluble Aβ levels correlate with cognitive deficits in the 12-month-old APPswe/PS1dE9 mouse model of Alzheimer’s disease. Behav. Brain Res. 2011, 222, 342–350. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Park, D.; Joo, S.S.; Kim, T.K.; Lee, S.H.; Kang, H.; Lee, H.J.; Lim, I.; Matsuo, A.; Tooyama, I.; Kim, Y.B.; et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Park, D.; Lee, H.J.; Joo, S.S.; Bae, D.K.; Yang, G.; Yang, Y.H.; Lim, I.; Matsuo, A.; Tooyama, I.; Kim, Y.B.; et al. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp. Neurol. 2012, 234, 521–526. [Google Scholar] [CrossRef]
- Shin, K.; Cha, Y.; Kim, K.S.; Choi, E.K.; Choi, Y.; Guo, H.; Ban, Y.H.; Kim, J.C.; Park, D.; Kim, Y.B. Human neural stem cells overexpressing choline acetyltransferase restore unconditioned fear in rats with amygdala injury. Behav. Neurol. 2016, 2016, 8521297. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Yang, Y.H.; Bae, D.K.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.K.; Lee, S.W.; Kim, G.H.; et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol. Aging 2013, 34, 2639–2646. [Google Scholar] [CrossRef]
- Kim, J.; Shin, K.; Cha, Y.; Ban, Y.H.; Park, S.K.; Jeong, H.S.; Park, D.; Choi, E.K.; Kim, Y.B. Neuroprotective effects of human neural stem cells over-expressing choline acetyltransferase in a middle cerebral artery occlusion model. J. Chem. Neuroanat. 2020, 103, 101730. [Google Scholar] [CrossRef]
- Park, D.; Lee, S.H.; Bae, D.K.; Yang, Y.H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.K.; Hong, J.T.; Shin, I.S.; et al. Transplantation of human adipose tissue-derived mesenchymal stem cells restores the neurobehavioral disorders of rats with neonatal hypoxic-ischemic encephalopathy. Cell Med. 2013, 5, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluchino, S.; Gritti, A.; Blezer, E.; Amadio, S.; Brambilla, E.; Borsellino, G.; Cossetti, C.; Del Carro, U.; Comi, G.; Bert’t, H.; et al. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann. Neurol. 2009, 66, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Machova, E.; Jakubik, J.; Michal, P.; Oksman, M.; Iivonen, H.; Tanila, H.; Dolezal, V. Impairment of muscarinic transmission in transgenic APPswe/PS1dE9 mice. Neurobiol. Aging 2008, 29, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V. Choline transporters, cholinergic transmission and cognition. Nat. Rev. Neurosci. 2005, 6, 48–56. [Google Scholar] [CrossRef]
- Decker, M.W.; Majchrzak, M.J. Effects of systemic and intracerebroventricular administration of mecamylamine, a nicotinic cholinergic antagonist, on spatial memory in rats. Psychopharmacology 1992, 107, 530–534. [Google Scholar] [CrossRef]
- Wess, J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Ann. Rev. Pharmacol. Toxicol. 2004, 44, 423–450. [Google Scholar] [CrossRef]
- Li, Y.; Chigurupati, S.; Holloway, H.W.; Mughal, M.; Tweedie, D.; Bruestle, D.A.; Mattson, M.P.; Wang, Y.; Harvey, B.K.; Ray, B.; et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e32008. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Takata, K.; Takada, T.; Ito, A.; Asai, M.; Tawa, M.; Saito, Y.; Ashihara, E.; Tomimoto, H.; Kitamura, Y.; Shimohama, S. Microglial amyloid-β1-40 phagocytosis dysfunction is caused by high mobility group box protein-1: Implications for the pathological progression of Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 685739. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Alloza, M.; Robbins, E.M.; Zhang-Nunes, S.X.; Purcell, S.M.; Betensky, R.A.; Raju, S.; Prada, C.; Greenberg, S.M.; Bacskai, B.J.; Frosch, M.P. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006, 24, 516–524. [Google Scholar] [CrossRef]
- El Khoury, J.B.; Moore, K.J.; Means, T.K.; Leung, J.; Terada, K.; Toft, M.; Freeman, M.W.; Luster, A.D. CD36 mediates the innate host response to β-amyloid. J. Exp. Med. 2003, 197, 1657–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasko, I.; Marx, F.; Steiner, E.; Hartmann, T.; Grubeck-Loebenstein, B. TNFα plus IFNγ induce the production of Alzheimer β-amyloid peptides and decrease the secretion of APPs. FASEB J. 1999, 13, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. The β-secretase, BACE. A prime drug target for Alzheimer’s disease. J. Mol. Neurosci. 2001, 17, 157–170. [Google Scholar] [CrossRef]
- Sastre, M.; Dewachter, I.; Landreth, G.E.; Willson, T.M.; Klockgether, T.; van Leuven, F.; Heneka, M.T. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-γ agonists modulate immunostimulated processing of amyloid precursor protein through regulation of β-secretase. J. Neurosci. 2003, 23, 9796–9804. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Lee, J.K.; Lee, H.; Carter, J.E.; Chang, J.W.; Oh, W.; Yang, Y.S.; Suh, J.G.; Lee, B.H.; Jin, H.K.; et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol. Aging 2012, 33, 588–602. [Google Scholar] [CrossRef]
- Ben-Hur, T. Immunomodulation by neural stem cells. J. Neurol. Sci. 2008, 265, 102–104. [Google Scholar] [CrossRef]
- Krady, J.K.; Lin, H.W.; Liberto, C.M.; Basu, A.; Kremlev, S.G.; Levison, S.W. Ciliary neurotrophic factor and interleukin-6 differentially activate microglia. J. Neurosci. Res. 2008, 86, 1538–1547. [Google Scholar] [CrossRef]
- Nagai, A.; Kim, W.K.; Lee, H.J.; Jeong, H.S.; Kim, K.S.; Hong, S.H.; Park, I.H.; Kim, S.U. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS ONE 2007, 2, e1272. [Google Scholar] [CrossRef]
- Zhang, D.K.; He, F.Q.; Li, T.K.; Pang, X.H.; Cui, D.J.; Xie, Q.; Huang, X.L.; Gan, H.T. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J. Pathol. 2010, 222, 213–222. [Google Scholar] [CrossRef]
- Burke, M.A.; Mobley, W.C.; Cho, J.; Wiegand, S.J.; Lindsay, R.M.; Mufson, E.J.; Kordower, J.H. Loss of developing cholinergic basal forebrain neurons following excitotoxic lesions of the hippocampus: Rescue by neurotrophins. Exp. Neurol. 1994, 130, 178–195. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Ann. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef] [PubMed]
- Grosse, G.; Djalali, S.; Deng, D.R.; Holtje, M.; Hinz, B.; Schwartzkopff, K.; Cygon, M.; Rothe, T.; Stroh, T.; Hellweg, R.; et al. Area-specific effects of brain-derived neurotrophic factor (BDNF) genetic ablation on various neuronal subtypes of the mouse brain. Brain Res. Dev. Brain Res. 2005, 156, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.L.; Hagg, T. BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Exp. Neurol. 2000, 162, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, K.S.; Kim, E.J.; Choi, H.B.; Lee, K.H.; Park, I.H.; Ko, Y.; Jeong, S.W.; Kim, S.U. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007, 25, 1204–1212. [Google Scholar] [CrossRef]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Muller, F.J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef] [Green Version]
- Xuan, A.G.; Long, D.H.; Gu, H.G.; Yang, D.D.; Hong, L.P.; Leng, S.L. BDNF improves the effects of neural stem cells on the rat model of Alzheimer’s disease with unilateral lesion of fimbria-fornix. Neurosci. Lett. 2008, 440, 331–335. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, I.J.; Park, S.W.; Kim, Y.B.; Ko, Y.; Kim, S.U. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012, 21, 2487–2496. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kyung, J.; Park, D.; Choi, E.K.; Kim, K.S.; Shin, K.; Lee, H.; Shin, I.S.; Kang, S.K.; Ra, J.C.; et al. Healthspan-extending activity of human amniotic membrane- and adipose tissue-derived stem cells in F344 rats. Stem Cells Transl. Med. 2015, 4, 1144–1154. [Google Scholar] [CrossRef] [Green Version]
- Kan, I.; Barhum, Y.; Melamed, E.; Offen, D. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev. 2011, 7, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, H.N.; Park, H.J.; Shin, J.Y.; Lee, P.H. Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway in an Alzheimer’s disease model. Cell Transplant. 2015, 24, 1097–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.C.; Lee, D.C.; Chiu, I.M. Neural stem cells, neural progenitors, and neurotrophic factors. Cell Transplant. 2007, 16, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.R.; Sommer, C.; Zoder, W.; Kiessling, M.; Schwaninger, M.; Schwab, S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 2000, 31, 2212–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.U.; Nagai, A.; Nakagawa, E.; Choi, H.B.; Bang, J.H.; Lee, H.J.; Lee, M.A.; Lee, Y.B.; Park, I.H. Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol. Biol. 2008, 438, 103–121. [Google Scholar] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Choi, E.-K.; Cho, T.-H.; Joo, S.S.; Kim, Y.-B. Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Aβ Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2020, 21, 3958. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113958
Park D, Choi E-K, Cho T-H, Joo SS, Kim Y-B. Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Aβ Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice. International Journal of Molecular Sciences. 2020; 21(11):3958. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113958
Chicago/Turabian StylePark, Dongsun, Ehn-Kyoung Choi, Tai-Hyoung Cho, Seong Soo Joo, and Yun-Bae Kim. 2020. "Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Aβ Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice" International Journal of Molecular Sciences 21, no. 11: 3958. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21113958