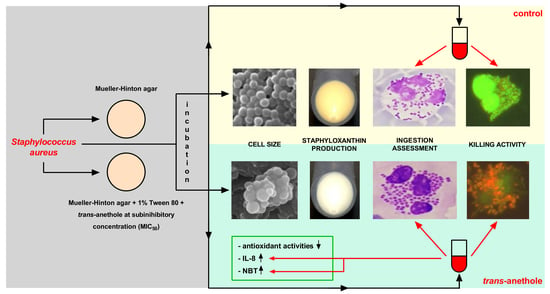
Innate Immune Response against Staphylococcus aureus Preincubated with Subinhibitory Concentration of trans-Anethole
Abstract
:1. Introduction
2. Results
2.1. The Antimicrobial Activity of trans-Anethole Against S. aureus Newman Strain
2.2. Measurement of Antioxidant Activities
2.3. Pigment Measurements
2.4. Analysis of Cells Size
2.5. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.6. Complete Blood Count (CBC) Analysis
2.7. Killing Assay
2.8. Phagocytosis Assay
2.9. Nitroblue Tetrazolium (NBT) Dye Reduction Analysis
2.10. The Influence of S. aureus Preincubated with the Subinhibitory Concentration of trans-Anethole on the Production of Interleukin-8 (IL-8)
3. Discussion
4. Materials and Methods
4.1. S. aureus Newman Susceptibility to trans-Anethole
4.1.1. Culture Media Preparation
4.1.2. Determination of MIC50 of trans-Anethole
4.1.3. Preparation of Ready-to-Use Culture Media
4.2. Preparation of S. aureus Newman Suspensions
4.3. Determination of Antioxidant Capacity of S. aureus Cells Treated with trans-Anethole
4.3.1. Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
4.3.2. Reducing Power (RP) Assay
4.3.3. Determination of Total Polyphenolic Content (TPC)
4.4. Staphyloxanthin Production Assessment
4.5. Staphylococcal Cell Analysis Under trans-Anethole Pressure
4.5.1. Measurements of the Particle Size Distribution
4.5.2. Scanning Electron Microscopy (SEM)
4.5.3. Determination of Functional Groups in Staphylococcal Cells by the Use of Fourier Transform Infrared (FTIR) Spectroscopy
4.6. Whole Blood Model of Staphylococcal Sepsis
4.6.1. Killing Assay
4.6.2. Phagocytosis Assay
4.6.3. Stimulated Nitroblue Tetrazolium (NBT) Dye Reduction
4.6.4. IL-8 Detection—ELISA Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CBC | complete blood count |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EOs | essential oils |
| EOCs | essential oil compounds |
| EEO | eucalyptus essential oil |
| FEO | fennel essential oil |
| KI | killing index |
| MRSA | methicillin-resistant Staphylococcus aureus |
| PI | phagocytic index |
| RP | reducing power |
| TPC | total polyphenolic content |
| TTO | tea tree oil |
References
- Marshall, J.H.; Wilmoth, G.J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J. Bacteriol. 1981, 147, 900–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.H.; Cho, M.H.; Lee, J. Flavone reduces the production of virulence factors, staphyloxanthin and α-hemolysin, in Staphylococcus aureus. Curr. Microbiol. 2012, 65, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Leejae, S.; Hasap, L.; Vorvauthikunchai, S.P. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J. Med. Microbiol. 2013, 62, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Di, H.; Wang, Y.; Cao, Q.; Xu, B.; Zhang, X.; Yang, N.; Liu, G.; Yang, C.G.; Xu, Y.; et al. Small-molecule targeting of a diapophytoene desaturase inhibits S. aureus virulence. Nat. Chem. Biol. 2016, 12, 174–179. [Google Scholar] [CrossRef]
- Cueno, M.E.; Imai, K. Network analytics approach towards identifying potential antivirulence drug targets within the Staphylococcus aureus staphyloxanthin biosynthetic network. Arch. Biochem. Biophys. 2018, 645, 81–86. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.I.; Lin, F.Y.; No, J.H.; Hensler, M.; Liu, Y.L.; Jeng, W.Y.; Low, J.; Liu, G.Y.; Nizet, V.; et al. Inhibition of staphyloxanthin virulence factor biosynthesis in Staphylococcus aureus: In vitro, in vivo, and crystallographic results. J. Med. Chem. 2009, 52, 3869–3880. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.Y.; Zhang, Y.; Hensler, M.; Liu, Y.L.; Chow, O.A.; Zhu, W.; Wang, K.; Pang, R.; Thienphrapa, W.; Nizet, V.; et al. Dual dehydrosqualene/squalene synthase inhibitors: Leads for innate immune system-based therapeutics. ChemMedChem 2012, 7, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Uribe-Querol, E.; Rosales, C. Control of phagocytosis by microbial pathogens. Front. Immunol. 2017, 8, 1368. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Neutrophils and bacterial immune evasion. J. Innate Immun. 2018, 10, 432–441. [Google Scholar] [CrossRef]
- Horn, J.; Stelzner, K.; Rudel, T.; Fraunholz, M. Inside job: Staphylococcus aureus host-pathogen interactions. Int. J. Med. Microbiol. 2018, 308, 607–624. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Giedrys-Kalemba, S.; Mizielińska, M.; Bartkowiak, A. Antibacterial activity of rosemary, caraway and fennel essential oils. Herba Pol. 2015, 61, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, M.; Nagasaki, S.; Ohta, T. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to beta-lactams in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2007, 59, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widianingrum, D.C.; Noviandi, C.T.; Salasia, S.I.O. Antibacterial and immunomodulator activities of virgin coconut oil (VCO) against Staphylococcus aureus. Heliyon 2019, 5, 02612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, R.; Cañigueral, S. Effect of some essential oils on phagocytosis and complement system activity. J. Agric. Food Chem. 2015, 63, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Committee of Experts on Cosmetic Products. Active Ingredients Used in Cosmetics: Safety Survey; Council of Europe Pub.: Strasbourg, France, 2008; p. 143. [Google Scholar]
- Moradi, J.; Abbasipour, F.; Zaringhalam, J.; Maleki, B.; Ziaee, N.; Khodadoustan, A.; Janahmadi, M. Anethole, a medicinal plant compound, decreases the production of pro-inflammatory TNF-α and IL-1β in a rat model of LPS-induced periodontitis. Iran. J. Pharm. Res. 2014, 13, 1319–1325. [Google Scholar] [PubMed]
- Ryu, J.; Seo, J.; Lee, Y.; Lim, Y.; Ahn, J.H.; Hur, H.G. Identification of syn- and anti-anethole-2,3-epoxides in the metabolism of trans-anethole by the newly isolated bacterium Pseudomonas putida JYR-1. J. Agric. Food Chem. 2005, 53, 5954–5958. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, E.; Baasov, T.; Ravid, U.; Shoham, Y. The trans-anethole degradation pathway in an Arthrobacter sp. J. Biol. Chem. 2002, 277, 11866–11872. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Grygorcewicz, B.; Pruss, A.; Wojciuk, B.; Dołęgowska, B.; Giedrys-Kalemba, S.; Sienkiewicz, M.; Wojciechowska-Koszko, I. The effect of subinhibitory concentrations of trans-anethole on antibacterial and antibiofilm activity of mupirocin against mupirocin-resistant Staphylococcus aureus strains. Microb. Drug Resist. 2019, 25, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Costache, I.I.; Miron, A. Anethole and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 247–267. [Google Scholar] [PubMed]
- Kim, K.Y.; Lee, H.S.; Seol, G.H. Anti-inflammatory effects of trans-anethole in a mouse model of chronic obstructive pulmonary disease. Biomed. Pharm. 2017, 91, 925–930. [Google Scholar] [CrossRef]
- Yea, S.S.; Jeong, H.S.; Choi, C.Y.; Park, K.R.; Oh, S.; Shin, J.G.; Yun, C.H. Inhibitory effect of anethole on T-lymphocyte proliferation and interleukin-2 production through down-regulation of the NF-AT and AP-1. Toxicol. In Vitro 2006, 20, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, X.; Devshilt, I.; Yun, Q.; Huang, C.; An, L.; Dorjbat, S.; He, X. Fennel main constituent, trans-anethole treatment against LPS-induced acute lung injury by regulation of Th17/Treg function. Mol. Med. Rep. 2018, 18, 1369–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmondson, M.; Newall, N.; Carville, K.; Smith, J.; Riley, T.V.; Carson, C.F. Uncontrolled, open-label, pilot study of tea tree (Melaleuca alternifolia) oil solution in the decolonisation of methicillin-resistant Staphylococcus aureus positive wounds and its influence on wound healing. Int. Wound J. 2011, 8, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.; Thomazzi, S.M. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, K.S.; Prosise, W.E.; Corring, R. An Alcohol-Free Slightly-Alcoholic Oral Care Composition and a Process for Preparing Same. WO2012018519A1 2012. [Google Scholar]
- Australian Government Department of Health TGA. Australian Regulatory Information. 2017. Available online: https://www.tga.gov.au/book-page/213-<italic>trans</italic>-anethole#aus (accessed on 3 June 2020).
- Özek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Özek, T.; Başer, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar]
- Serafino, A.; Sinibaldi Vallebona, P.; Andreola, F.; Zonfrillo, M.; Mercuri, L.; Federici, M.; Rasi, G.; Garaci, E.; Pierimarchi, P. Stimulatory effect of eucalyptus essential oil on innate cell-mediated immune response. BMC Immunol. 2008, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Silva-Comar, F.M.; Wiirzler, L.A.; Silva-Filho, S.E.; Kummer, R.; Pedroso, R.B.; Spironello, R.A.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K. Effect of estragole on leukocyte behavior and phagocytic activity of macrophages. Evid. Based Complement. Altern. Med. 2014, 2014, 784689. [Google Scholar] [CrossRef]
- Tareb, R.; Bernardeau, M.; Amiel, C.; Vernoux, J.P. Usefulness of FTIR spectroscopy to distinguish rough and smooth variants of Lactobacillus farciminis CNCM-I-3699. Fems Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [Green Version]
- Güler, G.; Gärtner, R.M.; Ziegler, C.; Mäntele, W. Lipid-protein interactions in the regulated betaine symporter BetP probed by infrared spectroscopy. J. Biol. Chem. 2016, 291, 4295–4307. [Google Scholar] [CrossRef] [Green Version]
- Pelz, A.; Wieland, K.P.; Putzbach, K.; Hentschel, P.; Albert, K.; Götz, F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J. Biol. Chem. 2005, 280, 32493–32498. [Google Scholar] [CrossRef] [Green Version]
- Orsini, F.; Ami, D.; Villa, A.M.; Sala, G.; Bellotti, M.G.; Doglia, S.M. FT-IR microspectroscopy for microbiological studies. J. Microbiol. Methods 2000, 42, 17–27. [Google Scholar] [CrossRef]
- Yadav, N.; Chandra, H. Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFĸB. PLoS ONE 2017, 12, 0188232. [Google Scholar] [CrossRef] [Green Version]
- Fournier, B. The function of TLR2 during staphylococcal diseases. Front. Cell. Infect. Microbiol. 2013, 2, 167. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Ha, J.M.; Kim, H.S.; Lee, H.; Kurokawa, K.; Lee, B.L. The role of phagocytosis in IL-8 production by human monocytes in response to lipoproteins on Staphylococcus aureus. Biochem. Biophys. Res. Commun. 2011, 406, 449–453. [Google Scholar] [CrossRef]
- Methods for Dilution Antimicrobial Susceptibility Test. for Bacteria that Grow Aerobically, Approved Standard. M07-A7; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2009.
- Ding, W.; Wang, L.; Zhang, J.; Ke, W.; Zhou, J.; Zhu, J.; Guo, X.; Long, R. Characterization of antioxidant properties of lactic acid bacteria isolated from spontaneously fermented yak milk in the Tibetan Plateau. J. Funct. Foods 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, P.; Pruss, A.; Wojciuk, B.; Dołęgowska, B.; Wajs-Bonikowska, A.; Sienkiewicz, M.; Mężyńska, M.; Łopusiewicz, Ł. The influence of essential oil compounds on antibacterial activity of mupirocin-susceptible and induced low-level mupirocin-resistant MRSA strains. Molecules 2019, 24, 3105. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Li, J.; Yang, L.; Shi, F.; Yang, L.; Ye, M. Antibacterial activity and a membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control. 2017, 73, 1445–1451. [Google Scholar] [CrossRef]
- Krumholz, W.; Endrass, J.; Hempelmann, G. Inhibition of phagocytosis and killing of bacteria by anaesthetic agents in vitro. Br. J. Anaesth. 1995, 75, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Porter, A.R.; Kennedy, A.D.; Kobayashi, S.D.; DeLeo, F.R. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J. Innate Immun. 2014, 6, 639–649. [Google Scholar] [CrossRef]
- Williams, J.C.; Craven, R.R.; Earp, H.S.; Kawula, T.H.; Matsushima, G.K. TAM receptors are dispensable in the phagocytosis and killing of bacteria. Cell. Immunol. 2009, 259, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Glasser, L.; Fiederlein, R.L. The effect of various cell separation procedures on assays of neutrophil function. A critical appraisal. Am. J. Clin. Pathol. 1990, 93, 662–669. [Google Scholar] [CrossRef]
- Skjeflo, E.W.; Christiansen, D.; Espevik, T.; Nielsen, E.W.; Mollnes, T.E. Combined inhibition of complement and CD14 efficiently attenuated the inflammatory response induced by Staphylococcus aureus in a human whole blood model. J. Immunol. 2014, 192, 2857–2864. [Google Scholar] [CrossRef] [Green Version]








| Medium | DPPH 1 Free Radical scavenging Activity (%) | Total Polyphenolic Content (mg GAE/g DM 2) | Reducing Power (%) |
|---|---|---|---|
| A | 16.60 ± 0.24 b | 0.0158 ± 0.000 a | 3.37 ± 1.35 b |
| B | 27.52 ± 0.15 a | 0.0141 ± 0.005 b | 6.96 ± 1.11 a |
| C | 6.09 ± 0.83 c | 0.0101 ± 0.002 c | 2.73 ± 1.48 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, P.; Wojciuk, B.; Wojciechowska-Koszko, I.; Łopusiewicz, Ł.; Grygorcewicz, B.; Pruss, A.; Sienkiewicz, M.; Fijałkowski, K.; Kowalczyk, E.; Dołęgowska, B. Innate Immune Response against Staphylococcus aureus Preincubated with Subinhibitory Concentration of trans-Anethole. Int. J. Mol. Sci. 2020, 21, 4178. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114178
Kwiatkowski P, Wojciuk B, Wojciechowska-Koszko I, Łopusiewicz Ł, Grygorcewicz B, Pruss A, Sienkiewicz M, Fijałkowski K, Kowalczyk E, Dołęgowska B. Innate Immune Response against Staphylococcus aureus Preincubated with Subinhibitory Concentration of trans-Anethole. International Journal of Molecular Sciences. 2020; 21(11):4178. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114178
Chicago/Turabian StyleKwiatkowski, Paweł, Bartosz Wojciuk, Iwona Wojciechowska-Koszko, Łukasz Łopusiewicz, Bartłomiej Grygorcewicz, Agata Pruss, Monika Sienkiewicz, Karol Fijałkowski, Edward Kowalczyk, and Barbara Dołęgowska. 2020. "Innate Immune Response against Staphylococcus aureus Preincubated with Subinhibitory Concentration of trans-Anethole" International Journal of Molecular Sciences 21, no. 11: 4178. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114178