Exosomes as Messengers between Mother and Fetus in Pregnancy
Abstract
:1. Introduction
2. Exosomes and Other Extracellular Vesicles
3. The Human Pregnancy
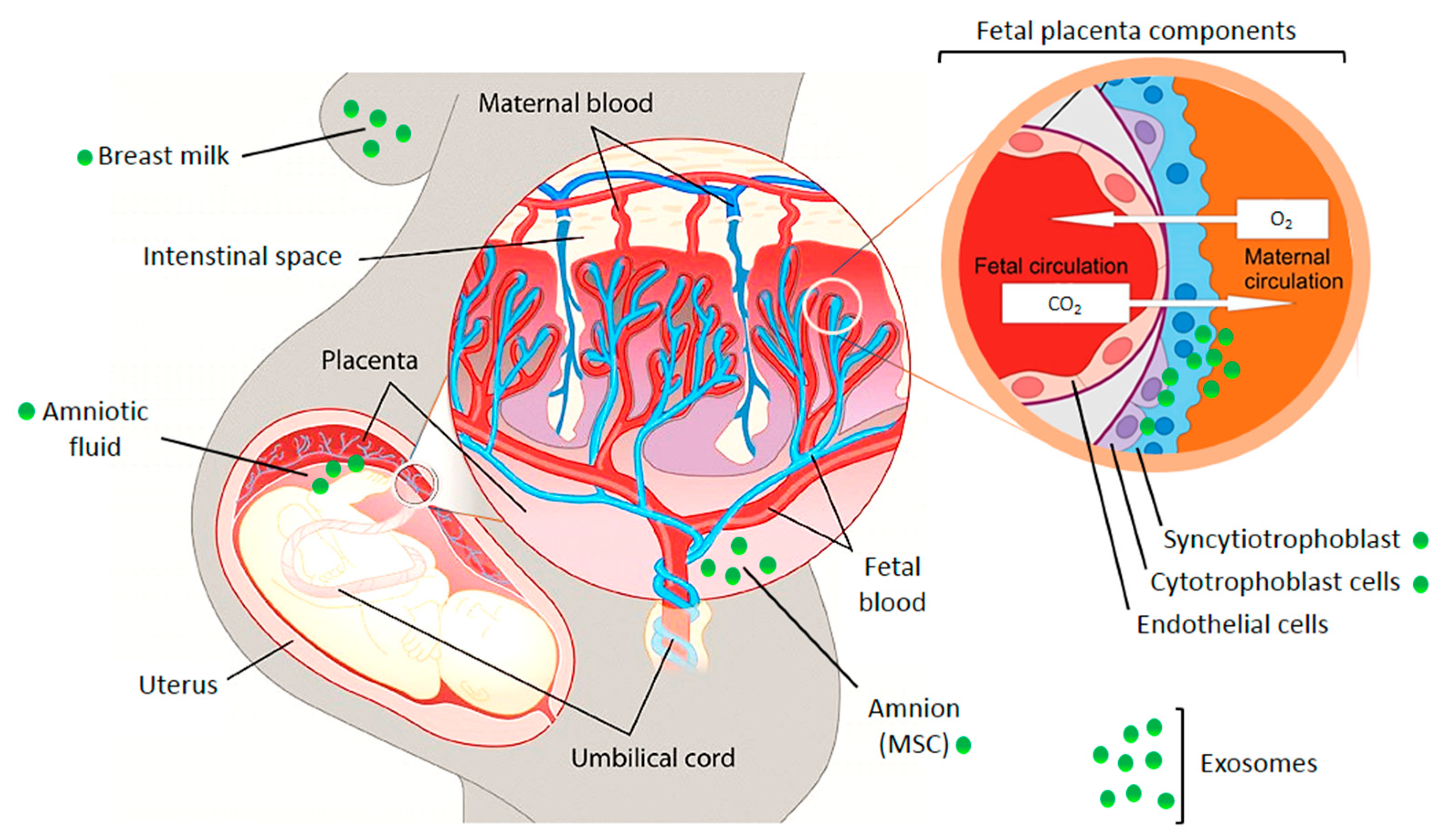
4. The Placenta as the Interface Between Maternal and Fetal Organisms
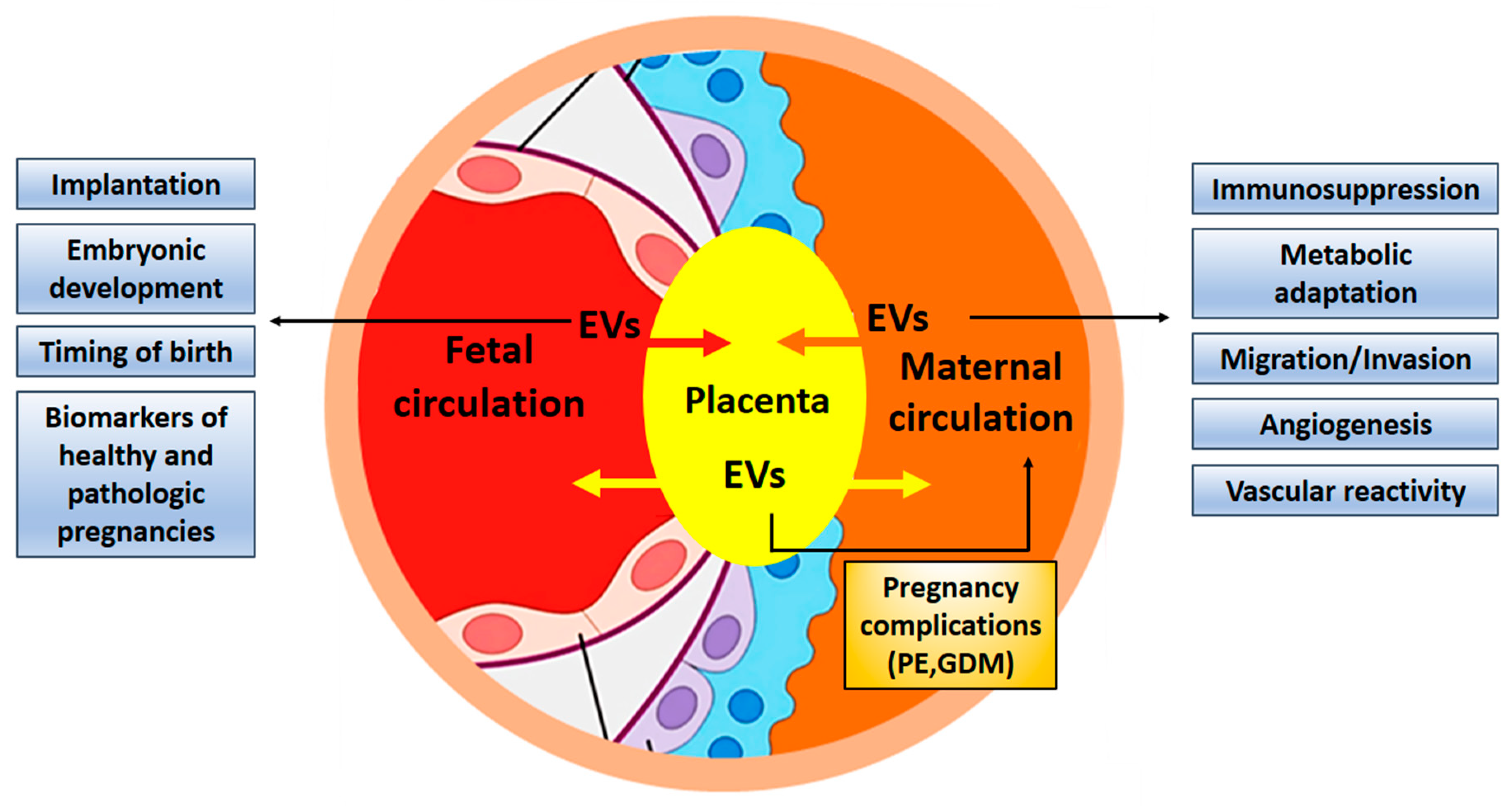
4.1. EVs Are Exchanged Between Maternal and Embryonic Tissues
4.2. Exosomes in Pregnancy Transfer miRNAs to Regulate Gene Expression in Target Cells
4.3. Exosomes Support the Implantation of the Embryo
4.4. The Influence of Pregnancy-Associated EVs on the Maternal Immune System
4.5. The Angiogenic Potential of Exosomes in Pregnancy
5. Exosomes in Pathological Pregnancies
5.1. Preeclampsia
5.2. Pre-Tterm Birth
5.3. Gestational Diabetes Mellitus (GDM)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EV | extracellular vesicle |
| CT | cytotrophoblast |
| VCT | villous cytotrophoblasts |
| SCT | syncytiotrophoblast |
| PLAP | placental alkaline phosphatase |
| HLA-G | human leukocyte antigen G |
| MSC | mesenchymal stem cells |
| C19MC | chromosome 19 miRNA cluster |
| TNFα | tumor necrosis factor α |
| ECM | extra-cellular matrix |
| VEGF | vascular endothelial growth factor |
| EVT | extravillous trophoblast |
| LC-MS | liquid chromatography–mass spectrometry |
| FasL | Fas ligand |
| PD-L1 | programmed death ligand-1 |
| TRAIL | TNF- Related Apoptosis Inducing Ligand |
| PBMCs | peripheral blood mononuclear cells |
| HSPE1 | heat shock 10kDa protein 1 |
| GSL | glycosphingolipid |
| IL-4 | interleukin-4 |
| IFN-γ | interferon-γ |
| HUVEC | human umbilical vein endothelial cell |
| cfDNA | cell-free DNA |
| eNOS | endothelial nitric oxide synthase |
| NO | nitric oxide |
| PE | preeclampsia |
| GDM | gestational diabetes mellitus |
| DAMP | damage associated molecular pattern |
References
- McNanley, T.; Woods, J. Placental Physiology. Glob. Libr. Women’s Med. 2008. [Google Scholar] [CrossRef]
- Linzer, D.I.; Fisher, S.J. The placenta and the prolactin family of hormones: Regulation of the physiology of pregnancy. Mol. Endocrinol. 1999, 13, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Menon, R. Placental exosomes: A proxy to understand pregnancy complications. Am. J. Reprod. Immunol. 2018, 79, e12788. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Regan, L. Recurrent miscarriage. Lancet 2006, 368, 601–611. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 3, 676–705. [Google Scholar] [CrossRef] [Green Version]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; Andaloussi, S.E.L.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef] [Green Version]
- Sheller-Miller, S.; Lei, J.; Saade, G.; Salomon, C.; Burd, I.; Menon, R. Feto-Maternal Trafficking of Exosomes in Murine Pregnancy Models. Front. Pharmacol. 2016, 7, 432. [Google Scholar] [CrossRef] [Green Version]
- Tannetta, D.; Masliukaite, I.; Vatish, M.; Redman, C.; Sargent, I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J. Reprod. Immunol. 2017, 119, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Kshirsagar, S.K.; Alam, S.M.; Jasti, S.; Hodes, H.; Nauser, T.; Gilliam, M.; Billstrand, C.; Hunt, J.S.; Petroff, M.G. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 2012, 33, 982–990. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.; Paules, C.; Nair, S.; Lai, A.; Palma, C.; Scholz-Romero, K.; Rice, G.E.; Gratacos, E.; Crispi, F.; Salomon, C. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction—Liquid biopsies to monitoring fetal growth. Placenta 2018, 64, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz-Bloom, R.D.; Crews, F.T.; Porrino, L.J.; Friedman, D.P.; Morrow, A.L.; Sulik, K.K. The Alcohol Pharmacology Education Partnership—Module 5. Available online: https://sites.duke.edu/apep/module-5-alcohol-and-babies (accessed on 1 June 2020).
- Albrecht, C.; Institute of Biochemistry and Molecular Medicine, University of Bern. Available online: https://www.ibmm.unibe.ch/research/group_albrecht/index_eng.html (accessed on 1 June 2020).
- Menon, R.; Mesiano, S.; Taylor, R.N. Programmed fetal membrane senescence and exosome-mediated signaling: A mechanism associated with timing of human parturition. Front. Endocrinol. 2017, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Sabapatha, A.; Gercel-Taylor, C.; Taylor, D.D. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am. J. Reprod. Immunol. 2006, 56, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomon, C.; Kobayashi, M.; Ashman, K.; Sobrevia, L.; Mitchell, M.D.; Rice, G.E. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS ONE 2013, 8, e79636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.D.; Peiris, H.N.; Kobayashi, M.; Koh, Y.Q.; Duncombe, G.; Illanes, S.E.; Rice, G.E.; Salomon, C. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015, 213, S173–S181. [Google Scholar] [CrossRef]
- Salomon, C.; Torres, M.J.; Kobayashi, M.; Scholz-Romero, K.; Sobrevia, L.; Dobierzewska, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS ONE 2014, 9, e98667. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Chen, Q.; James, J.L.; Wise, M.R.; Stone, P.R.; Chamley, L.W. In vivo targets of human placental micro-vesicles vary with exposure time and pregnancy. Reproduction 2017, 153, 835–845. [Google Scholar] [CrossRef]
- Sheller-Miller, S.; Choi, K.; Choi, C.; Menon, R. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am. J. Obstet. Gynecol. 2019, 221, 502. [Google Scholar] [CrossRef]
- Sayed, D.; Abdellatif, M. MicroRNAs in development and disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef] [PubMed]
- Kluszczyńska, K.; Czernek, L.; Cypryk, W.; Pęczek, Ł.; Düchler, M. Methods for the Determination of the Purity of Exosomes. Curr. Pharm. Des. 2019, 25, 4464–4485. [Google Scholar] [CrossRef] [PubMed]
- Chim, S.S.; Shing, T.K.; Hung, E.C.; Leung, T.Y.; Lau, T.K.; Chiu, R.W.; Lo, Y.M. Detection and characterization of placental microRNAs in maternal plasma. Clin. Chem. 2008, 54, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.; Mouillet, J.F.; Coyne, C.B.; Sadovsky, Y. Review: Placenta-specific microRNAs in exosomes-good things come in nano-packages. Placenta 2014, 35, S69–S73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguer-Dance, M.; Abu-Amero, S.; Al-Khtib, M.; Lefèvre, A.; Coullin, P.; Moore, G.E.; Cavaillé, J. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum. Mol. Genet. 2010, 19, 3566–3582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.; Sun, X.; Jiang, D.; Ding, Y.; Lu, Z.; Gong, L.; Liu, H.; Xie, J. Origin and evolution of a placental-specific microRNA family in the human genome. BMC Evol. Biol. 2010, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Donker, R.B.; Mouillet, J.F.; Chu, T.; Hubel, C.A.; Stolz, D.B.; Morelli, A.E.; Sadovsky, Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol. Hum. Reprod. 2012, 18, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.S.; Ishibashi, O.; Ishikawa, G.; Ishikawa, T.; Katayama, A.; Mishima, T.; Takizawa, T.; Shigihara, T.; Goto, T.; Izumi, A.; et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol. Reprod. 2009, 81, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Kambe, S.; Yoshitake, H.; Yuge, K.; Ishida, Y.; Ali, M.M.; Takizawa, T.; Kuwata, T.; Ohkuchi, A.; Matsubara, S.; Suzuki, M.; et al. Human exosomal placenta-associated miR-517a-3p modulates the expression of PRKG1 mRNA in Jurkat cells. Biol. Reprod. 2014, 91, 129. [Google Scholar] [CrossRef] [Green Version]
- Stefanski, A.L.; Martinez, N.; Peterson, L.K.; Callahan, T.J.; Treacy, E.; Luck, M.; Friend, S.F.; Hermesch, A.; Maltepe, E.; Phang, T.; et al. Murine trophoblast-derived and pregnancy-associated exosome-enriched extracellular vesicle microRNAs: Implications for placenta driven effects on maternal physiology. PLoS ONE 2019, 14, e0210675. [Google Scholar] [CrossRef] [Green Version]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E.; et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12048–12053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, W.R.; Cribbs, A.; Zhang, W.; Kandzija, N.; Motta-Mejia, C.; Dombi, E.; Ri, R.; Cerdeira, A.S.; Redman, C.; Vatish, M. Maternal circulating syncytiotrophoblast-derived extracellular vesicles contain biologically active 5′-tRNA halves. Biochem. Biophys. Res. Commun. 2019, 518, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, B.; Sochacka, E.; Düchler, M. tRNA structural and functional changes induced by oxidative stress. Cell. Mol. Life Sci. 2011, 68, 4023–4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef]
- Nakamura, K.; Kusama, K.; Bai, R.; Sakurai, T.; Isuzugawa, K.; Godkin, J.D.; Suda, Y.; Imakawa, K. Induction of IFNT-Stimulated Genes by Conceptus-Derived Exosomes during the Attachment Period. PLoS ONE 2016, 11, e0158278. [Google Scholar] [CrossRef] [Green Version]
- Hemmatzadeh, M.; Shomali, N.; Yousefzadeh, Y.; Mohammadi, H.; Ghasemzadeh, A.; Yousefi, M. MicroRNAs: Small molecules with a large impact on pre-eclampsia. J. Cell. Physiol. 2019, 235, 3235–3248. [Google Scholar] [CrossRef]
- Chang, G.; Mouillet, J.F.; Mishima, T.; Chu, T.; Sadovsky, E.; Coyne, C.B.; Parks, W.T.; Surti, U.; Sadovsky, Y. Expression and trafficking of placental microRNAs at the feto-maternal interface. FASEB J. 2017, 31, 2760–2770. [Google Scholar] [CrossRef] [Green Version]
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef] [Green Version]
- Greening, D.W.; Nguyen, H.P.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions. Biol. Reprod. 2016, 94, 38. [Google Scholar] [CrossRef]
- Vilella, F.; Moreno-Moya, J.M.; Balaguer, N.; Grasso, A.; Herrero, M.; Martínez, S.; Marcilla, A.; Simón, C. Hsa-mir-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 2015, 142, 3210–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Ohkuchi, A.; Kuwata, T.; Usui, R.; Baba, Y.; Suzuki, H.; Chaw Kyi, T.T.; Matsubara, S.; Saito, S.; Takizawa, T. Endogenous and exogenous miR-520c-3p modulates CD44-mediated extravillous trophoblast invasion. Placenta 2017, 50, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.; Brooks, K.; Wildung, M.; Navakanitworakul, R.; Christenson, L.K.; Spencer, T.E. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE 2014, 9, e90913. [Google Scholar] [CrossRef] [Green Version]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Tolerance, suppression and the fetal allograft. J. Mol. Med. 2005, 83, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Czernek, L.; Düchler, M. Functions of Cancer-Derived Extracellular Vesicles in Immunosuppression. Arch. Immunol. Ther. Exp. (Warsz.) 2017, 65, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Düchler, M.; Czernek, L.; Peczek, L.; Cypryk, W.; Sztiller-Sikorska, M.; Czyz, M. Melanoma-Derived Extracellular Vesicles Bear the Potential for the Induction of Antigen-Specific Tolerance. Cells 2019, 8, 665. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, V.L.; Ellwanger, J.H.; Chies, J.A.B. Extracellular vesicles in host-pathogen interactions and immune regulation-exosomes as emerging actors in the immunological theater of pregnancy. Heliyon 2019, 5, e02355. [Google Scholar] [CrossRef] [Green Version]
- Lokossou, A.G.; Toudic, C.; Nguyen, P.T.; Elisseeff, X.; Vargas, A.; Rassart, É.; Lafond, J.; Leduc, L.; Bourgault, S.; Gilbert, C.; et al. Endogenous retrovirus-encoded syncytin-2 contributes to exosome-mediated immunosuppression of t cells. Biol. Reprod. 2020, 102, 185–198. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod. Immunol. 2014, 72, 440–457. [Google Scholar] [CrossRef]
- Stenqvist, A.C.; Nagaeva, O.; Baranov, V.; Mincheva-Nilsson, L. Exosomes secreted by human placenta carry functional fas ligand and trail molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 2013, 191, 5515–5523. [Google Scholar] [CrossRef] [Green Version]
- Mincheva-Nilsson, L.; Nagaeva, O.; Chen, T.; Stendahl, U.; Antsiferova, J.; Mogren, I.; Hernestål, J.; Baranov, V. Placenta-derived soluble MHC class I, chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: A possible novel immune escape mechanism for fetal survival. J. Immunol. 2006, 176, 3585–3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.Y.; Chiang, C.H.; Chen, F.P.; Yu, C.L. The alteration of placental-derived soluble MHC class I chain-related protein A and B during pregnancy. Acta. Obstet. Gynecol. Scand. 2011, 90, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.F.; Fekete, N.; Turiák, L.; Ács, A.; Kőhidai, L.; Buzás, E.I.; Pállinger, É. Unravelling the Role of Trophoblastic-Derived Extracellular Vesicles in Regulatory T Cell Differentiation. Int. J. Mol. Sci. 2019, 20, 3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Yang, C.; Yang, J.; Liu, P.; Jiang, K.; Shaukat, A.; Wu, H.; Deng, G. Placental exosome-mediated bta-mir-499-lin28b/let-7 axis regulates inflammatory bias during early pregnancy. Cell Death Dis. 2018, 9, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H. Roles of exosome-associated glycosphingolipids in immune tolerance of embryo implantation and pregnancy. Fertil. Steril. 2018, 110, e238. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomon, C.; Scholz-Romero, K.; Sarker, S.; Sweeney, E.; Kobayashi, M.; Correa, P.; Longo, S.; Duncombe, G.; Mitchell, M.D.; Rice, G.E.; et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes 2016, 65, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Holder, B.; Jones, T.; Sancho Shimizu, V.; Rice, T.F.; Donaldson, B.; Bouqueau, M.; Forbes, K.; Kampmann, B. Macrophage exosomes induce placental inflammatory cytokines: A novel mode of maternal-placental messaging. Traffic 2016, 17, 168–178. [Google Scholar] [CrossRef]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef]
- Massimiani, M.; Vecchione, L.; Piccirilli, D.; Spitalieri, P.; Amati, F.; Salvi, S.; Ferrazzani, S.; Stuhlmann, H.; Campagnolo, L. Epidermal growth factor-like domain 7 promotes migration and invasion of human trophoblast cells through activation of MAPK, PI3K and NOTCH signaling pathways. Mol. Hum. Reprod. 2015, 21, 435–451. [Google Scholar] [CrossRef] [Green Version]
- Patton, A.L.; McCallie, B.; Parks, J.C.; Schoolcraft, W.B.; Katz-Jaffe, M. Exosome bound microRNAs transcriptionally regulate embryo-endometrial dialogue impacting implantation potential for AMA patients. Fertil. Steril. 2015, 104, e308. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, X.; Huang, X.; Xu, X.; Jia, Y.; Wu, Y.; Yao, J.; Wu, Y.; Wang, K. Maternal and umbilical cord serum-derived exosomes enhance endothelial cell proliferation and migration. FASEB J. 2018, 32, 4534–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Fan, Y.; Shen, L.; Niu, L.; Zhao, Y.; Jiang, D.; Zhu, L.; Jiang, A.; Tang, Q.; Ma, J.; et al. The pro-angiogenesis of exosomes derived from umbilical cord blood of intrauterine growth restriction pigs was repressed associated with miRNAs. Int. J. Biol. Sci. 2018, 14, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Kan, F.W.; Koti, M.; Tayade, C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci. Rep. 2017, 7, 40476. [Google Scholar] [CrossRef]
- van der Post, J.A.; Lok, C.A.; Boer, K.; Sturk, A.; Sargent, I.L.; Nieuwland, R. The functions of microparticles in pre-eclampsia. Semin. Thromb. Hemost. 2011, 37, 146–152. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of extracellular vesicles on placentation and pregnancy disorders. Reproduction 2019, 158, R189–R196. [Google Scholar] [CrossRef] [Green Version]
- Tannetta, D.S.; Dragovic, R.A.; Gardiner, C.; Redman, C.W.; Sargent, I.L. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: Expression of Flt-1 and endoglin. PLoS ONE 2013, 8, e56754. [Google Scholar] [CrossRef]
- Repiská, G.; Konečná, B.; Shelke, G.V.; Lässer, C.; Vlková, B.I.; Minárik, G.F.F. Is the DNA of placental origin packaged in exosomes isolated from plasma and serum of pregnant women? Clin. Chem. Lab. Med. 2018, 56, e150–e153. [Google Scholar] [CrossRef]
- Konečná, B.; Tóthová, L.; Repiská, G. Exosomes-Associated DNA-New Marker in Pregnancy Complications? Int. J. Mol. Sci. 2019, 20, 2890. [Google Scholar] [CrossRef] [Green Version]
- Ananth, C.V.; Keyes, K.M.; Wapner, R.J. Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. BMJ 2013, 347, f6564. [Google Scholar] [CrossRef] [Green Version]
- Kuklina, E.V.; Ayala, C.; Callaghan, W.M. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet. Gynecol. 2009, 113, 1299–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Wang, C.; Chen, Y.; Wang, J.; Xu, X.; Hilton, T.; Cai, W.; Zhao, Z.; Wu, Y.; Li, K.; et al. Placenta-derived extracellular vesicles induce preeclampsia in mouse models. Haematologica 2020, 105, 1686–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragovic, R.A.; Southcombe, J.H.; Tannetta, D.S.; Redman, C.W.; Sargent, I.L. Multicolor flow cytometry and nanoparticle tracking analysis of extracellular vesicles in the plasma of normal pregnant and pre-eclamptic women. Biol. Reprod. 2013, 89, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redman, C.W.; Tannetta, D.S.; Dragovic, R.A.; Gardine, C.; Southcombe, J.H.; Collett, G.P.; Sargent, I.L. Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012, 33, S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Gaynullina, D.K.; Schubert, R.; Tarasova, O.S. Changes in Endothelial Nitric Oxide Production in Systemic Vessels during Early Ontogenesis—A Key Mechanism for the Perinatal Adaptation of the Circulatory System. Int. J. Mol. Sci. 2019, 20, 1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motta-Mejia, C.; Kandzija, N.; Zhang, W.; Mhlomi, V.; Cerdeira, A.S.; Burdujan, A.; Tannetta, D.; Dragovic, R.; Sargent, I.L.; Redman, C.W.; et al. Placental Vesicles Carry Active Endothelial Nitric Oxide Synthase and Their Activity is Reduced in Preeclampsia. Hypertension 2017, 70, 372–381. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Li, R.; Diao, Z.; Yany, M.; Wu, M.; Sun, H.; Yan, G.; Hu, Y. Placenta associated serum exosomal miR155 derived from patients with preeclampsia inhibits eNOS expression in human umbilical vein endothelial cells. Int. J. Mol. Med. 2018, 41, 1731–1739. [Google Scholar]
- Gill, M.; Motta-Mejia, C.; Kandzija, N.; Cooke, W.; Zhang, W.; Cerdeira, A.S.; Bastie, C.; Redman, C.; Vatish, M. Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in Preeclampsia. Hypertension 2019, 73, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Kumar, S.; Hyett, J.; Salomon, C. Molecular Targets of Aspirin and Prevention of Preeclampsia and Their Potential Association with Circulating Extracellular Vesicles during Pregnancy. Int. J. Mol. Sci. 2019, 20, 4370. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, K.S.; Kim, J.H.; Lee, D.K.; Park, M.; Choi, S.; Park, W.; Kim, S.; Choi, Y.K.; Hwang, J.Y.; et al. Aspirin prevents TNF-a-induced endothelial cell dysfunction by regulating the NF-kB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radic Biol. Med. 2017, 104, 185–198. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Dvorakova, L.; Kotlabova, K.; Krofta, L. The Prediction of Gestational Hypertension, Preeclampsia and Fetal Growth Restriction via the First Trimester Screening of Plasma Exosomal C19MC microRNAs. Int. J. Mol. Sci. 2019, 20, 2972. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Treacy, R.; Herrero, T.; Olsen, R.; Leonardo, T.R.; Zhang, X.; DeHoff, P.; To, C.; Poling, L.G.; Fernando, A.; et al. Discovery and Verification of Extracellular miRNA Biomarkers for Non-invasive Prediction of Pre-eclampsia in Asymptomatic Women. Cell Rep. Med. 2020, 1, 100013. [Google Scholar] [CrossRef]
- Salomon, C.; Guanzon, D.; Scholz-Romero, K.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. J. Clin. Endocrinol. Metab. 2017, 102, 3182–3194. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Kothandaraman, N.; Manikandan, J.; Rong, L.; Ee, K.H.; Hill, J.; Lai, C.W.; Tan, W.Y.; Yeoh, F.; Kale, A.; et al. Proteomic analysis of human placental syncytiotrophoblast microvesicles in preeclampsia. Clin. Proteom. 2014, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, A.; Zhou, S.; Éthier-Chiasson, M.; Flipo, D.; Lafond, J.; Gilbert, C.; Barbeau, B. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 2014, 28, 3703–3719. [Google Scholar] [CrossRef]
- Tan, K.H.; Tan, S.S.; Ng, M.J.; Tey, W.S.; Sim, W.K.; Allen, J.C.; Lim, S.K. Extracellular vesicles yield predictive pre-eclampsia biomarkers. J. Extracell. Vesicles 2017, 6, 1408390. [Google Scholar] [CrossRef] [PubMed]
- Jadli, A.; Ghosh, K.; Satoskar, P.; Damania, K.; Bansal, V.; Shetty, S. Combination of copeptin, placental growth factor and total annexin V microparticles for prediction of preeclampsia at 10–14 weeks of gestation. Placenta 2017, 58, 67–73. [Google Scholar] [CrossRef]
- Klumper, J.; Breebaart, W.; Roos, C.; Naaktgeboren, C.A.; van der Post, J.; Bosmans, J.; van Kaam, A.; Schuit, E.; Mol, B.W.; Baalman, J.; et al. Study protocol for a randomised trial for atosiban versus placebo in threatened preterm birth: The APOSTEL 8 study. BMJ Open 2019, 9, e029101. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, S.L.; Greenberg, J.W.; Wang, H.; Collaer, B.W.; Wang, J.; Petroff, M.G. Quantifying murine placental extracellular vesicles across gestation and in preterm birth data with tidyNano: A computational framework for analyzing and visualizing nanoparticle data in R. PLoS ONE 2019, 14, e0218270. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.; Dixon, C.L.; Sheller-Miller, S.; Fortunato, S.J.; Saade, G.R.; Palma, C.; Lai, A.; Guanzon, D.; Salomon, C. Quantitative Proteomics by SWATH-MS of Maternal Plasma Exosomes Determine Pathways Associated With Term and Preterm Birth. Endocrinology 2019, 160, 639–650. [Google Scholar] [CrossRef] [Green Version]
- Sheller-Miller, S.; Trivedi, J.; Yellon, S.M.; Menon, R. Exosomes Cause Preterm Birth in Mice: Evidence for Paracrine Signaling in Pregnancy. Sci. Rep. 2019, 9, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.; Bennett, P.R.; Kim, S.H.; Teoh, T.G.; Sykes, L.; Kindinger, L.M.; Garrett, A.; Binkhamis, R.; MacIntyre, D.A.; Terzidou, V. First Trimester Circulating MicroRNA Biomarkers Predictive of Subsequent Preterm Delivery and Cervical Shortening. Sci. Rep. 2019, 9, 5861. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.A.; Anton, L.; Bastek, J.; Brown, A.G. Can microRNA profiling in maternal blood identify women at risk for preterm birth? Am. J. Obstet. Gynecol. 2015, 212, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; McCowan, L.M.; Patel, R.; Taylor, R.S.; Vickers, M.H. Maternal plasma miRNAs as biomarkers during mid-pregnancy to predict later spontaneous preterm birth: A pilot study. Sci. Rep. 2017, 7, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winger, E.E.; Reed, J.L.; Ji, X. Early first trimester peripheral blood cell microRNA predicts risk of preterm delivery in pregnant women: Proof of concept. PLoS ONE 2017, 12, e0180124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallen, S.; Baxter, D.; Wu, X.; Kim, T.K.; Shynlova, O.; Lee, M.Y.; Scherler, K.; Lye, S.; Hood, L.; Wang, K. Extracellular vesicle RNAs reflect placenta dysfunction and are a biomarker source for preterm labour. J. Cell. Mol. Med. 2018, 22, 2760–2773. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.; Sheller-Miller, S.; Salomon, C. Protein Profile Changes in Circulating Placental Extracellular Vesicles in Term and Preterm Births: A Longitudinal Study. Endocrinology 2020, 161, bqaa009. [Google Scholar] [CrossRef]
- Ornoy, A.; Reece, E.A.; Pavlinkova, G.; Kappen, C.; Miller, R.K. Effect of maternal diabetes on the embryo, fetus, and children: Congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res. C Embryo Today 2015, 105, 53–72. [Google Scholar] [CrossRef]
- Nair, S.; Jayabalan, N.; Guanzon, D.; Palma, C.; Scholz-Romero, K.; Elfeky, O.; Zuñiga, F.; Ormazabal, V.; Diaz, E.; Rice, G.E.; et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin. Sci. (Lond.) 2018, 132, 2451–2467. [Google Scholar] [CrossRef]
- Rice, G.E.; Scholz-Romero, K.; Sweeney, E.; Peiris, H.; Kobayashi, M.; Duncombe, G.; Mitchell, M.D.; Salomon, C. The Effect of Glucose on the Release and Bioactivity of Exosomes from First Trimester Trophoblast Cells. J. Clin. Endocrinol. Metab. 2015, 100, E1280–E1288. [Google Scholar] [CrossRef]
- Jayabalan, N.; Lai, A.; Ormazabal, V.; Adam, S.; Guanzon, D.; Palma, C.; Scholz-Romero, K.; Lim, R.; Jansson, T.; McIntyre, H.D.; et al. Adipose tissue exosomal proteomic profile reveals a role on placenta glucose metabolism in gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 1735–1752. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, N.; Lai, A.; Nair, S.; Guanzon, D.; Scholz-Romero, K.; Palma, C.; McIntyre, H.D.; Lappas, M.; Salomon, C. Quantitative Proteomics by SWATH-MS Suggest an Association Between Circulating Exosomes and Maternal Metabolic Changes in Gestational Diabetes Mellitus. Proteomics 2019, 19, e1800164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandrarao, S.P.; Hamlin, A.A.; Awdishu, L.; Overcash, R.; Zhou, M.; Proudfoot, J.; Ishaya, M.; Aghania, E.; Madrigal, A.; Kokoy-Mondragon, C.; et al. Proteomic analyses of Urine Exosomes reveal New Biomarkers of Diabetes in Pregnancy. Madr. J. Diabetes 2016, 1, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Almohammadi, D.; Casper, J.; Elfeky, O.; Chang, C.; Scholz-Romero, K.; Longo, S.; Duncombe, G.; Rice, G.; Salomon, C. C19MC miRNA Signatures of Placenta-Derived Exosomes in Women Diagnosed with Gestational Diabetes Mellitus. In Proceedings of the Endocrine Society’s 98th Annual Meeting and Expo, Boston, MA, USA, 1–4 April 2016. [Google Scholar]
- Gillet, V.; Ouellet, A.; Stepanov, Y.; Rodosthenous, R.S.; Croft, E.K.; Brennan, K.; Abdelouahab, N.; Baccarelli, A.; Takser, L. miRNA Profiles in Extracellular Vesicles From Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Yoffe, L.; Polsky, A.; Gilam, A.; Raff, C.; Mecacci, F.; Ognibene, A.; Crispi, F.; Gratacós, E.; Kanety, H.; Mazaki-Tovi, S.; et al. Early diagnosis of gestational diabetes mellitus using circulating microRNAs. Eur. J. Endocrinol. 2019, 181, 565–577. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, J.; Jiang, T.; Shi, Z.; Yu, B.; Zhu, Y.; Chen, D.; Xu, J.; Huo, R.; Dai, J.; et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS ONE 2011, 6, e23925. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, F.; Li, H.; Zhou, Y.; Lu, J.; Ge, Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int. J. Gynaecol. Obstet. 2015, 130, 49–53. [Google Scholar] [CrossRef]
- Floriano, J.F.; Willis, G.; Catapano, F.; Lima, P.R.; Reis, F.; Barbosa, A.; Rudge, M.; Emanueli, C. Exosomes Could Offer New Options to Combat the Long-Term Complications Inflicted by Gestational Diabetes Mellitus. Cells 2020, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Lai, A.; Elfeky, O.; Rice, G.E.; Salomon, C. Optimized Specific Isolation of Placenta-Derived Exosomes from Maternal Circulation. Methods Mol. Biol. 2018, 1710, 131–138. [Google Scholar]
- Burkova, E.E.; Grigor’eva, A.E.; Bulgakov, D.V.; Dmitrenok, P.S.; Vlassov, V.V.; Ryabchikova, E.I.; Sedykh, S.E.; Nevinsky, G.A. Extra Purified Exosomes from Human Placenta Contain An Unpredictable Small Number of Different Major Proteins. Int. J. Mol. Sci. 2019, 20, 2434. [Google Scholar] [CrossRef] [Green Version]
- Powe, C.E. Early Pregnancy Biochemical Predictors of Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Pillar, N.; Yoffe, L.; Hod, M.; Shomron, N. The possible involvement of microRNAs in preeclampsia and gestational diabetes mellitus. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 176–182. [Google Scholar] [CrossRef]
- Herrera-Van Oostdam, A.S.; Salgado-Bustamante, M.; López, J.A.; Herrera-Van Oostdam, D.A.; López-Hernández, Y. Placental exosomes viewed from an ‘omics’ perspective: Implications for gestational diabetes biomarkers identification. Biomark. Med. 2019, 13, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, H.; Heidarpour, M.; Lolicato, F.; Nouri, M.; Rahbarghazi, R.; Mahdipour, M. Physiological impact of extracellular vesicles on female reproductive system; highlights to possible restorative effects on female age-related fertility. BioFactors 2019, 45, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Than, U.T.T.; Le, H.T.; Hoang, D.H.; Nguyen, X.-H.; Pham, C.T.; Bui, K.T.V.; Bui, H.T.H.; Nguyen, P.V.; Nguyen, T.D.; Do, T.T.H.; et al. Induction of Antitumor Immunity by Exosomes Isolated from Cryopreserved Cord Blood Monocyte-Derived Dendritic Cells. Int. J. Mol. Sci. 2020, 21, 1834. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czernek, L.; Düchler, M. Exosomes as Messengers between Mother and Fetus in Pregnancy. Int. J. Mol. Sci. 2020, 21, 4264. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124264
Czernek L, Düchler M. Exosomes as Messengers between Mother and Fetus in Pregnancy. International Journal of Molecular Sciences. 2020; 21(12):4264. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124264
Chicago/Turabian StyleCzernek, Liliana, and Markus Düchler. 2020. "Exosomes as Messengers between Mother and Fetus in Pregnancy" International Journal of Molecular Sciences 21, no. 12: 4264. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124264




