Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications
Abstract
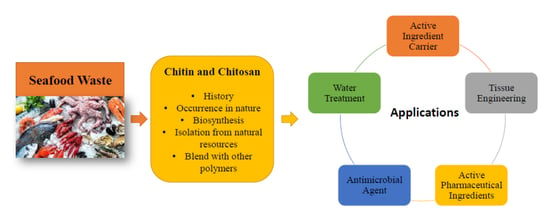
:1. Introduction
2. Chitin and Chitosan History
3. Occurrence of Chitin in Nature
4. Biosynthesis of Chitin
5. Chitin Isolation from Natural Resources
5.1. Chemical Extraction
5.1.1. Chemical Deproteinization
5.1.2. Chemical Demineralization
5.1.3. Discoloration
5.2. Biological Extraction
5.2.1. Enzymatic Deproteinization
5.2.2. Fermentation
6. Chitin to Chitosan Conversion
7. Chitin and Chitosan Blend with Other Polymers
8. Chitin and Chitosan Applications
8.1. Active Ingredient Carrier
8.2. Tissue Engineering
8.3. Active Pharmaceutical Applications
8.4. Antimicrobial Agent
8.5. Water Treatment
8.6. Chitosan Applications in Food Technology
9. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.L.; Nguyen, V.B. Production of potent antidiabetic compounds from shrimp head powder via Paenibacillus conversion. Process. Chem. 2019, 76, 18–24. [Google Scholar] [CrossRef]
- Liu, C.; Cai, W.; Cuixia, Z.; Ma, M.; Rao, W.; Li, W.; He, K.; Gao, M. Developing the ecological compensation criterion of industrial solid waste based on energy for sustainable development. Energy 2018, 157, 940–948. [Google Scholar] [CrossRef]
- Bedoić, R.; Ćosić, B.; Duić, N. Technical potential and geographic distribution of agricultural residues, co-products and by-products in the European Union. Sci. Total Environ. 2019, 686, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Mohanty, U.; Pattanaik, S.S.; Panda, A.; Jena, A.K. Future prospects and trends for effective utilization of fish processing wastes in India. Innovat. Farm. 2018, 3, 1–5. [Google Scholar]
- Tsakanika, A.; Clauzet, M.; May, P.H. Envolvendo os pescadores artesanais no desenvolvimento sustentável urbano e periurbano no Brasil. Revibec. 2018, 28, 1–20. [Google Scholar]
- Sivaraman, I.; Krishnan, M.; Radhakrishnan, K. Better Management Practices for sustainable small-scale shrimp farming. J. Clean. Prod. 2019, 214, 559–572. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, I.; Rodríguez, C.; Gillet, D.; Moerschbacher, B.M. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Arasukumar, B.; Prabakaran, G.; Gunalan, B.; Moovendhan, M. Chemical composition, structural features, surface morphology and bioactivities of chitosan derivatives from lobster (Thenus unimaculatus) shells. Int. J. Biol. Macromol. 2019, 135, 1237–1245. [Google Scholar] [CrossRef]
- Lucas-Bautista, J.A.; Bautista-Baños, S.; Ventura-Aguilar, R.I.; Gómez-Ramírez, M. Determinación de quitina en hongos postcosecha y de quitinasas en frutos de papaya “Maradol”. Rev. Mex. Fit. 2019, 37, 1–7. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial Coatings Based On Chitosan For Pharmaceutical And Biomedical Applications. Curr. Pharm. 2018, 24, 866–885. [Google Scholar] [CrossRef]
- Knorr, D. Recovery and utilization of chitin and chitosan in food processing waste management. Food Technol. 1991, 45, 114–120. [Google Scholar]
- Mehebub, M.S.; Mahmud, N.U.; Rahman, M.; Surovy, M.Z.; Gupta, D.R.; Hasanuzzaman, M.; Rahman, M.; Islam, M.T. Chitosan biopolymer improves the fruit quality of litchi (Litchi chinensis Sonn). Acta Agrobot. 2019, 72, 1–9. [Google Scholar] [CrossRef]
- Campos-Takaki, G.M.; Beakes, G.W.; Dietrich, S.M.C. Electron microscopic X-ray microprobe and cytochemical study of isolated cell walls of mucoralean fungi. T. Brit. Mycol. Soc. 1983, 80, 536–541. [Google Scholar] [CrossRef]
- Craveiro, A.A.; Craveiro, A.C.; Queiroz, D.C. Quitosana: A fibra do futuro, 2 ed.; Fortaleza: Padetec-Disal, Brazil, 2004. [Google Scholar]
- Batista, A.C.L.; Souza Neto, F.E.; Paiva, W.S. Review of fungal chitosan: Past, present and perspectives in Brazil. Polímeros 2018, 28, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Gómez, D.; Nagel-Hassemer, M.E.; Lapolli, F.R.; Lobo-Recio, M.A. Potencial dos resíduos do processamento de camarão para remediação de águas contaminadas com drenagem ácida mineral. Polímeros 2016, 26, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Mao, J.; Penh, N.; Luo, X.; Chang, C. Chitin/clay microspheres with hierarchical architecture for highly efficient removal of organic dyes. Carbohydr. Polym. 2018, 188, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, D.; Yano, H.; Abe, K. Insect cuticle-mimetic hydrogels with high mechanical properties achieved via the combination of chitin nanofiber and gelatin. J. Agric. Food Chem. 2019, 67, 5571–5578. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Anwar, W.; Javed, M.A.; Shahid, A.A.; Nawaz, K.; Akhter, A.; Rehman, M.Z.U.; Hameed, U.; Iftikhar, S.; Haider, M.S. Chitinase genes from Metarhizium anisopliae for the control of whitefly in cotton. R. Soc. Open Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef]
- Ru, G.; Wu, S.; Yan, X.; Liu, B.; Gong, P.; Wang, L.; Feng, J. Inverse solubility of chitin/chitosan in aqueous alkali solvents at low temperature. Carbohydr. Polym. 2019, 206, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.Y.; Rejinold, N.S.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef]
- Arrouze, F.; Desbrieres, J.; Rhazi, M.; Essahli, M.; Tolaimate, A. Valorization of chitins extracted from North Morocco shrimps: Comparison of chitin reactivity and characteristics. J. Appl. Polym. Sci. 2019, 136, 1–10. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Brigham, C.J. Chitin and chitosan: Sustainable, medically relevant biomaterials. Int. J. Biotech. Well. Indus. 2017, 6, 41–47. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 1–12. [Google Scholar] [CrossRef] [Green Version]
- BenBettaieb, N.; Karbowiak, T.; Bornaz, S. Spectroscopic analyses of the influence of electron beam irradiation doses on mechanical, transport properties and microstructure of chitosan-fish gelatin blend films. Food Hydrocoloids 2014, 48, 37–51. [Google Scholar] [CrossRef]
- Xu, W.; Mohan, A.; Pitts, N.L.; Udenigwe, C.; Mason, B. Bile acid-binding capacity of lobster shell-derived chitin, chitosan and chitooligosaccharides. Food Biosci. 2020, 33, 100476. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Souza, F.M.; Ferreira, R.M.S.; Barbosa, R.C. Utilização da casca de camarão para produção de quitina. Rev. Scir. 2015, 7, 1–11. [Google Scholar]
- Abreu, F.L.; Vasconcelos, F.P.; Albuquerque, M.F.C. A Diversidade no Uso e Ocupação da Zona Costeira do Brasil: A Sustentabilidade como Necessidade. Conex. Cienc. Tec. 2017, 11, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [Green Version]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 2, 2–025022. [Google Scholar] [CrossRef] [Green Version]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajed, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Henry García, Y.; Troncoso-Rojas, R.; Tiznado-Hernández, M.E.; Báez-Flores, M.E.; Carvajal-Millan, E.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Martínez-Robinson, K.G. Enzymatic treatments as alternative to produce chitin fragments of low molecular weight from Alternaria alternata. J. Appl. Polym. Sci. 2019, 136, 47339. [Google Scholar] [CrossRef]
- Ali, M.E.A.; Aboelfadl, M.M.S.; Selim, A.M.; Khalil, H.F.; Elkady, G.M. Chitosan nanoparticles extracted from shrimp shells, application for removal of Fe (II) and Mn (II) from aqueous phases. Sep. Sci. Technol. 2018, 53, 2870–2881. [Google Scholar] [CrossRef]
- Küçükgülmez, A. Extraction of Chitin from Crayfish (Astacus leptodactylus) Shell Waste. Alint. Zir. Bilim. Derg. 2018, 33, 99–104. [Google Scholar] [CrossRef]
- Santos, V.P.; Maia, P.; Alencar, N.S.; Farias, L.; Andrade, R.F.S.; Souza, D.; Ribeaux, D.R.; Franco, L.O.; Campos-Takaki, G.M. Recovery of chitin and chitosan from shrimp waste with microwave technique and versatile application. Arq. Inst. Biol. 2019, 86, 1–7. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresources 2019, 6, 8–28. [Google Scholar] [CrossRef]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Marques, S.C.; Nunes, P.M.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Li, P.P.; Chen, X.; Kang, Y.; Xie, Y.; Li., X.; Xie, T.; Zhang, Y. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2019, 126, 867–876. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Clean. Prod. 2018, 172, 4140–4151. [Google Scholar] [CrossRef] [Green Version]
- Broquá, J.; Zanin, B.G.; Flach, A.M.; Mallmann, C.; Taborda, F.G.D.; Machado, L.E.L.; Alves, S.M.L.; Silva, M.M.; Dias, R.J.S.P.; Reis, O.V.; et al. Different aspects of chemical and biochemical methods for chitin production a short review. Nanomed. Res. J. 2018, 10, 1477–2577. [Google Scholar]
- Sugiyanti, D.; Darmadji, P.; Anggrahini, S.; Anwar, C.; Santoso, U. Preparation and Characterization of Chitosan from Indonesian Tambak Lorok Shrimp Shell Waste and Crab Shell Waste. Pak. J. Nutr. 2018, 17, 446–453. [Google Scholar] [CrossRef]
- Samrot, A.V.; Burman, U.; Philip, S.A.; Sobrana, N.; Chandrasekaran, K. Synthesis of curcumin loaded polymeric nanoparticles from crab shell derived chitosan for drug delivery. Inform. Med. Unlocked 2018, 10, 159–182. [Google Scholar] [CrossRef]
- Ali, M.; Shakeel, M.; Mehmood, K. Extraction and characterization of high purity chitosan by rapid and simple techniques from mud crabs taken from Abbottabad. Pak. J. Pharm. Sci. 2019, 32, 171–175. [Google Scholar] [PubMed]
- Tanabtabzadeh, M.S.; Javanbakht, V.; Golshirazi, A.H. Extraction of Betacyanin and Betaxanthin Pigments from Red Beetroots by Chitosan Extracted from Shrimp Wastes. Waste Biomass Valor. 2019, 10, 641–653. [Google Scholar] [CrossRef]
- Buanasari, B.; Sugiyo, W.; Fitriani, N.; Suryaningsih, S. Potential of Chitosan From Local Crab (Portunus Pelagicus) to Enhance Storability of Musa Paradisiaca, L. J. Bahan. Alam. Terb. 2019, 8, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Chakravarty, J.; Yang, C.L.; Palmer, J.; Brigham, C.J. Chitin extraction from lobster shell waste using microbial culture-based methods. Appl. Food Biotech. 2018, 5, 141–154. [Google Scholar] [CrossRef]
- Aranday-García, R.; Saimoto, H.; Shirai, K.; Ifuku, S. Chitin biological extraction from shrimp wastes and its fibrillation for elastic nanofiber sheets preparation. Carbohydr. Polym. 2019, 213, 112–120. [Google Scholar] [CrossRef]
- Gachhi, D.B.; Hungund, B.S. Two-phase extraction, characterization, and biological evaluation of chitin and chitosan from Rhizopus oryzae. J. Appl. Pharm. Sci. 2018, 8, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Dun, Y.; Li, Y.; Xu, J.; Hu, Y.; Zhang, C.; Liang, Y.; Zhao, S. Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. Int. J. Biol. Macromol. 2019, 123, 420–426. [Google Scholar] [CrossRef]
- Marzieh, M.N.; Zahra, F.; Tahereh, E.; Sara, K.N. Comparison of the physicochemical and structural characteristics of enzymatic produced chitin and commercial chitin. Int. J. Biol. Macromol. 2019, 139, 270–276. [Google Scholar] [CrossRef]
- Doan, C.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of squid pens to chitosanases and proteases via Paenibacillus sp. TKU042. Mar. Drugs 2018, 16, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Guerrero-Legarreta, I.; Bórquez, R. Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol. Rep. 2018, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Abirami, S.; Nagarajan, D. Extraction of Chitin from Shrimp Shell Wastes by Using Bacillus licheniformis and Lactobacillus plantarum. Inter. J. Recent Res. Aspects 2018, 307–315. [Google Scholar]
- Ghorbel-Bellaaj, O.; Maalej, H.; Nasri, M.; Jellouli, K. Fermented Shrimp Waste Hydrolysates: Promising Source of Functional Molecules with Antioxidant Properties. J. Culin. Sci. Technol. 2018, 16, 357–377. [Google Scholar] [CrossRef]
- Gong, X.; Tian, W.; Bai, J.; Qiao, K.; Zhao, J.; Wang, L. Highly efficient deproteinization with an ammonifying bacteria Lysinibacillus fusiformis isolated from brewery spent diatomite. J. Biosci. Bioeng. 2019, 127, 326–332. [Google Scholar] [CrossRef]
- Navarrete-Bolaños, J.L.; González-Torres, I.; Vargas-Bermúdez, V.H.; Jiménez-Islas, H. A biotechnological insight to recycle waste: Analyzing the spontaneous fermentation of shrimp waste to design the hydrolysis process of chitin into n-acetylglucosamine. Rev. Mex. Ing. Quim. 2020, 19, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Dilarri, G.; Mendes, C.R.; Martins, A.O. Síntese de biofilmes de quitosana reticulados com tripolifosfato atuando como agente quelante na fixação de nanopartículas de prata. Cienc. Eng. 2016, 25, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Kidibule, P.E.; Santos-Moriano, P.; Jiménez-Ortega, E.; Ramírez-Escudero, M.; Limón, M.C.; Ramavha, M.; Plou, F.J.; Sanz-Aparicio, J.; Fernández-Lobato, M. Use of chitin and chtosan to produce new chitooligosaccharides by chitinase Chit42: Enzymatic activity and strutural basis of protein specificity. Microb. Cell. Fact. 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.F.P.; Cumba, L.R.; Andrade, R.D.A.; Carmo, D.R. Chemical modifications of cyclodextrin and chitosan for biological and environmental applications: Metals and organic pollutants adsorption and removal. J. Polym. Environ. 2019, 27, 1352–1366. [Google Scholar] [CrossRef]
- Younes, I.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Cytotoxicity of chitosans with different acetylation degrees and molecular weights on bladder carcinoma cells. Int. J. Biol. Macromol. 2016, 84, 200–207. [Google Scholar] [CrossRef]
- Baroudi, A.; García-Payo, C.; Khayet, M. Structural, mechanical, and transport properties of electron beam-irradiated chitosan membranes at different doses. Polymers 2018, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, S.A.; Veiga, I.G.; Moraes, A.M. Desenvolvimento de dispositivo de quitosana e xantana para a liberação tópica ou em tecidos moles de indometacina. Bluch. Chem. Eng. Proc. 2015, 1, 13205–13212. [Google Scholar]
- Monteiro, A.A.S.; Richter, A.R.; Maciel, J.S.; Feitosa, J.P.A.; Paula, H.C.B.; Monteiro-Paula, R.C. Effect of chemical modification on the solubility and swelling of microspheres based on carboxymethyl cashew gum and chitosan. Polímeros 2015, 25, 31–39. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. The improved antiviral activities of amino-modified chitosan derivatives on Newcastle virus. Drug Chem. Toxicol. 2019, 1, 1–6. [Google Scholar] [CrossRef]
- Rolim, A.E.H.; Carvalho, F.A.; Costa, R.C.C.; Rosa, F.P. Arcabouços de quitosana-propriedades físico-químicas e biológicas para o reparo ósseo. Rev. Virtual. Quim. 2018, 10. [Google Scholar]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Tahriri, M.; Rasoulianboroujeni, M.; Koturi, H.; Tayeb, L. Preparation of natural chitosan from shrimp shell with different deacetylation degree. Mater. Res. Innov. 2018, 22, 177–181. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Zhao, L.; Mehwish, H.M.; Wu, Y.; Mahmood, S. Chitosan and its derivatives: Synthesis, biotechnological applications, and future challenges. Appli. Microbiol. Biotechnol. 2019, 103, 1557–1571. [Google Scholar] [CrossRef]
- Müller, K.; Zollfrank, C.; Schmid, M. Natural Polymers from Biomass Resources as Feedstocks for Thermoplastic Materials. Macromol. Mater. Eng. 2019, 304, 1800760–1800777. [Google Scholar] [CrossRef]
- Castel-Molieres, M.; Conzatti, G.; Torrisani, J.; Rouilly, A.; Cavalie, S.; Carrere, N.; Tourrette, A. Influence of homogenization technique and blend ratio on chitosan/alginate polyelectrolyte complex properties. J. Med. Biol. Eng. 2018, 38, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, P.N.; Montembault, A.; Sudre, G.; Alcouffe, P.; Marcon, L.; Gehan, H.; Lux, F.; Albespy, K.; Centis, V.; Campos, D.; et al. Self-crosslinked fibrous collagen/chitosan blends: Processing, properties evaluation and monitoring of degradation by bi-fluorescence imaging. Int. J. Biol. Macromol. 2019, 131, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Kasai, D.; Chougale, R.; Masti, S.; Chalannavar, R.; Malabadi, R.; Gani, R.; Gouripur, G. An Investigation into the Influence of Filler Piper nigrum Leaves Extract on Physicochemical and Antimicrobial Properties of Chitosan/Poly (Vinyl Alcohol) Blend Films. J. Polym. Environ. 2019, 27, 472–488. [Google Scholar] [CrossRef]
- Gopi, S.; Pius, A.; Kargl, R.; Kleinschek, K.S.; Thomas, S. Fabrication of cellulose acetate/chitosan blend films as efficient adsorbent for anionic water pollutants. Polym. Bull. 2019, 76, 1557–1571. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.L.L.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thamilarasan, V.; Sethuraman, V.; Gopinath, K.; Balalakshmi, C.; Govindarajan, M.; Mothana, R.A.; Siddiqui, N.A.; Khaled, J.M.; Benelli, G. Single step fabrication of chitosan nanocrystals using Penaeus semisulcatus: Potential as new insecticides, antimicrobials and plant growth promoters. J. Clust. Sci. 2018, 29, 375–384. [Google Scholar] [CrossRef]
- Mirzaie, A.; Hasanzadeh, M.; Jouyban, A. Cross-linked chitosan/thiolated graphene quantum dots as a biocompatible polysaccharide towards aptamer immobilization. Int. J. Biol. Macromol. 2019, 123, 1091–1105. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Li, M.; Wu, H.; Zhen, T.; Xiong, L.; Sun, Q. pH-Sensitive Chitosan–Sodium Phytate Core–Shell Hollow Beads and Nanocapsules for the Encapsulation of Active Ingredients. J. Agric. Food Chem. 2019, 67, 2894–2905. [Google Scholar] [CrossRef]
- Morganti, P.; Coltelli, M.B. A New Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Pascoli, M.; Lopes-Oliveira, P.J.; Fraceto, L.F.; Seabra, A.B.; Oliveira, H.C. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energ. Ecol. Environ. 2018, 3, 137–148. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Melville, C.C.; Vechia, J.F.D.; Andrade, D.J.; Fraceto, L.F. Chitosan nanoparticles functionalized with β-cyclodextrin: A promising carrier for botanical pesticides. Sci. Rep. 2018, 8, 2067–2082. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.O.; Barbosa, R.C.; Buriti, J.S.; Bezerra Junior, A.G.; Sousa, W.J.B.; Barros, S.M.C.; Oliveira, R.J.; Fook, M.V.L. Thermal, chemical, biological and mechanical properties of chitosan films with powder of eggshell membrane for biomedical applications. J. Therm. Anal. Calorim. 2018, 136, 725–735. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ali, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Soundarya, S.P.; Menon, A.H.; Chandran, S.V.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Baranwal, A.; Kumar, A.; Priyadharshini, A.; Oggu, G.S.; Bhatnagar, I.; Srivastava, A.; Chandra, P. Chitosan: An undisputed bio-fabrication material for tissue engineering and bio-sensing applications. Int. J. Biol. Macromol. 2018, 110, 110–123. [Google Scholar] [CrossRef]
- Ways, M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Heise, K.; Hobisch, M.; Sacarescu, L.; Maver, U.J.; Hobisch, J.; Reichelt, T.; Sega, M.; Fischer, S.; Spirk, S. Low-molecular-weight sulfonated chitosan as template for anticoagulant nanoparticles. Int. J. Nanomed. 2018, 13, 4881–4894. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Ji, X.; Xing, R.; Liu, S.; Guo, Z.; Chen, X.; Li, P. The preparation and antioxidant activity of the sulfanilamide derivatives of chitosan and chitosan sulphates. Bioorg. Med. Chem. 2007, 15, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Vikhoreva, G.; Bannikova, G.; Stolbushkina, P.; Panov, A.; Drozd, N.; Makarov, V.; Gal’braikh, L. Preparation and anticoagulant activity of a low molecular weight sulfated chitosan. Carbohydr. Polym. 2005, 62, 327–332. [Google Scholar] [CrossRef]
- Desai, U.R. New antithrombin-based anticoagulants. Med. Res. Rev. 2004, 24, 151–181. [Google Scholar] [CrossRef]
- Drozd, N.N.; Sher, A.I.; Makarov, V.A.; Vikhoreva, G.A.; Gorbachiova, I.N.; Galbraich, L.S. Comparison of antithrombin activity of the polysulphate chitosan derivatives in vitro and in vivo system. Thromb. Res. 2001, 102, 445–455. [Google Scholar] [CrossRef]
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asife, M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm. J 2020, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Subhapradha, N.; Suman, S.; Ramasamy, P.; Saravanan, R.; Shanmugam, V.; Srinivasan, A.; Shanmugam, A. Anticoagulant and antioxidant activity of sulfated chitosan from the shell of donacid clam Donax scortum (Linnaeus, 1758). IJNPND 2013, 3, 39–45. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential–a critical literature survey. Microb. Biotechnol. 2009, 33, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohyd. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Marine Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Gomes, L.P.; Anjo, S.I.; Manadas, B.; Coelho, A.V.; Paschoalin, V.M.F. Proteomic analyses reveal new insights on the antimicrobial mechanisms of chitosan biopolymers and their nanosized particles against Escherichia coli. Int. J. Mol. Sci. 2020, 21, 225. [Google Scholar] [CrossRef] [Green Version]
- Khaneghah, A.M.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, M.K.J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Ferreira, N.; Olievira, C.M.; Pinhero, A.S.B.; Nunes, J.R.S.; Castrillon, S.K.I. Escassez hídrica: Estudo de caso em uma comunidade rural do pantanal mato-grossense. Rev. Ibero-Am. Ciênc. Ambient. 2018, 9, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Vidal, R.R.L.; Moraes, J.S. Removal of organic pollutants from wastewater using chitosan: A literature review. Int. J. Environ. Sci. Technol. 2019, 16, 1741–1754. [Google Scholar] [CrossRef]
- Richards, S.; Dawson, J.; Stutter, M. The potential use of natural vs commercial biosorbent material to remediate stream waters by removing heavy metal contaminants. J. Environ. Manage. 2019, 231, 275–281. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saud. Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Jawad, R.J.; Ismail, M.H.S.; Siajam, S.I. Adsorption of heavy metals and residual oil from palm oil mill effluent using novel adsorbent of alginate and mangrove composite beads coated by chitosan in a packed bed column. Engineer. J. 2018, 19, 1–14. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Fourmentin, M.; Zemmouri, H.; Nascimento, I.O.C.; Queiroz, L.M.; Tadza, M.Y.M.; Pico-Corrales, L.A.; Pei, H.; Wilsom, L.D.; et al. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019, 17, 1603–1621. [Google Scholar] [CrossRef] [Green Version]
- Bugajska, P.; Filipkowska, U.; Jóźwiak, T.; Kuczajowska-Zadrożna, M. The influence of chitosan flake deacetylation degree on orthophosphate sorption efficiency from aqueous solutions. Prog. Chem. Appl. Chitin. Deriv. 2018, 23, 33–44. [Google Scholar] [CrossRef]
- Hirano, S.; Itakura, C.; Seino, H.; Akiyama, Y.; Nonaka, I.; Kanbara, N.; Kawakami, T. Chitosan as an ingredient for domestic animal feeds. J. Agric. Food Chem. 1990, 38, 1214–1217. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and properties of chitosan. J. Bioact. Comp. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Butler, B.L.; Vergano, P.J.; Testin, R.F.; Bunn, J.M.; Wiles, J.L. Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J. Food Sci. 1996, 61, 953–955, 961. [Google Scholar]
- Nadarajah, K.; Prinyawiwatkul, W.; No, H.K.; Sathivel, S.; Xu, Z. Sorption behavior of crawfish chitosan films as affected by chitosan extraction processes and solvent types. J. Food Sci. 2006, 71, E33–E39. [Google Scholar] [CrossRef]
- Campos-Takaki, G.M.; Dietrich, S.M.C. Characterization of cell walls from Mucoralean fungi: Biochemical composition, Electron microscopy and X--Ray microanalysis. In Current Research Topics in Applied Microbiology and Microbial Biotechnology; Mendez-vilas, A., Ed.; World Scientific Publishing: Toh Tuck Link, Singapore, 2009; pp. 121–125. [Google Scholar]
- Berger, L.R.R.; Stamford, T.C.M.; Stamford-Arnaud, T.M.; de Alcântara, S.R.C.; da Silva, A.C.; da Silva, A.M.; Nascimento, A.E.; Campos-Takaki, G.M. Green Conversion of Agroindustrial Wastes into Chitin and Chitosan by Rhizopus arrhizus and Cunninghamella elegans Strains. Int. J. Mol. Sci. 2014, 15, 9082–9102. [Google Scholar] [CrossRef] [Green Version]
- Vilar Junior, J.C.; Ribeaux, D.R.; Alves da Silva, C.A.; Campos-Takaki, G.M. Physicochemical and Antibacterial Properties of Chitosan Extracted from Waste Shrimp Shells. Inter. J. Microbiol. 2018, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Sobczak-Kupiec, A. Sustainable Production of Chitosan. In Sustainable Production: Novel Trends in Energy, Environment and Material Systems. Studies in Systems, Decision and Control; Królczyk, G., Wzorek, M., Król, A., Kochan, O., Su, J., Kacprzyk, J., Eds.; Springer: Cham, NY, USA, 2020; Volume 198, pp. 45–60. [Google Scholar]
- Liua, Y.; Yuana, Y.; Duan, S.; Liu, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.; Chia, C.H.; Wan Ahmad, W.H.; Kaco, H.; Chook, S.W.; Chan, C.H. Mechanical and Antibacterial Properties of Paper Coated with Chitosan. Sains Malaysiana 2015, 44, 905–911. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Lima, M.A.B.d.; Franco, L.d.O.; Campos-Takaki, G.M.d. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124290
Santos VP, Marques NSS, Maia PCSV, Lima MABd, Franco LdO, Campos-Takaki GMd. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. International Journal of Molecular Sciences. 2020; 21(12):4290. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124290
Chicago/Turabian StyleSantos, Vanessa P., Nathália S. S. Marques, Patrícia C. S. V. Maia, Marcos Antonio Barbosa de Lima, Luciana de Oliveira Franco, and Galba Maria de Campos-Takaki. 2020. "Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications" International Journal of Molecular Sciences 21, no. 12: 4290. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124290