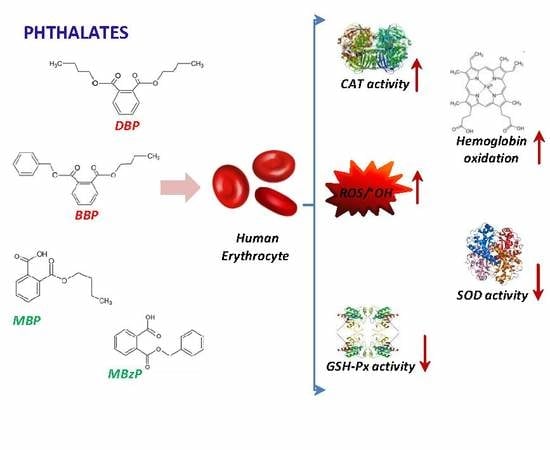
Human Erythrocytes Exposed to Phthalates and Their Metabolites Alter Antioxidant Enzyme Activity and Hemoglobin Oxidation
Abstract
:1. Introduction
2. Results
2.1. Hemoglobin Oxidation
2.2. ROS Levels
2.3. Superoxide Dismutase Activity
2.4. Catalase Activity
2.5. Glutathione Peroxidase Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation and Treatment of Human Erythrocytes
4.3. Hemoglobin Oxidation
4.4. Oxidation of H2DCFDA and HPF
4.5. Superoxide Dismutase Activity
4.6. Catalase Activity
4.7. Glutathione Peroxidase Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BBP | Butylbenzyl phthalate |
| BHb | Bovine hemoglobin |
| CAT | Catalase |
| DBP | Di-n-butyl phthalate |
| DCF | Dichlorofluorescein |
| ECHA | European Chemicals Agency |
| EDCs | Endocrine-Disrupting Chemicals |
| EU | European Union |
| GSH- Px | Glutathione peroxidase |
| H2DCFDA | 6-carboxy 2′,7′-dichlorodihydrofluorescein diacetate |
| Hb | Hemoglobin |
| hHb | Human hemoglobin |
| HPF | 3′-(p-hydroxyphenyl)-fluorescein |
| HTC | Hematocrit |
| Kow | Octanol–water partition coefficient |
| MBP | Mono-n-butylphthalate |
| MBzP | Monobenzylphthalate |
| metHb | Methemoglobin |
| NADPH | Reduced form of Nicotinamide Adenine Dinucleotide Phosphate |
| PAEs | Phthalates |
| PBMCs | Peripheral Blood Mononuclear Cells |
| RBCs | Red Blood Cells |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide dismutase |
| SVHC | Substances of Very High Concern |
| •OH | Hydroxyl radical |
References
- European Chemical Agency (ECHA). Available online: https://echa.europa.eu/-/chemicals-in-our-life-chemicals-of-concern-svhc (accessed on 16 May 2020).
- Bahadar, H.; Maqbool, F.; Abdollahi, M. Consumption of phthalates coated pharmaceutical tablets: An unnoticed threat. Int. J. Pharmacol. 2014, 10, 78–81. [Google Scholar]
- Lin, J.; Chen, W.; Zhu, H.; Wang, C. Determination of free and total phthalates in commercial whole milk products in different packaging materials by gas chroma-tography-mass spectrometry. J. Dairy Sci. 2015, 98, 8278–8284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Guo, J.; Liu, X.; Li, J.; Yang, X.; Ma, P.; Wu, Y. Oxidative stress mediates dibutyl phthalateinduced anxiety-like behavior in Kunming mice. Environ. Toxicol. Pharmacol. 2016, 45, 45–51. [Google Scholar] [CrossRef]
- Ashworth, M.J.; Chappell, A.; Ashmore, E.; Fowles, J. Analysis and Assessment of Exposure to Selected Phthalates Found in Children’s Toys in Christchurch, New Zealand. Int. J. Environ. Res. Public Health 2018, 15, 200. [Google Scholar] [CrossRef] [Green Version]
- Xiaowei, L.; Jianghong, S.; Ting, B.; Huiyuana, L.; Crittenden, J.C. Occurrence and risk assessment of selected phthalates in drinking water from waterworks in Chin. Environ. Sci. Pollut. Res. Int. 2015, 22, 10690–10698. [Google Scholar]
- Pie, X.Q.; Song, M.; Guo, M.; Mo, F.F.; Shen, X.Y. Concentration and risk assessment of phthalates present in indoor air from newly decorated apartments. Atmos. Environ. 2013, 68, 17–23. [Google Scholar]
- Notardonato, I.; Passarella, S.; Ianiri, G.; Di Fiore, K.; Russo, M.V.; Avino, P. Analytical Method Development and Chemometric Approach for Evidencing Presence of Plasticizer Residues in Nectar Honey Samples. Int. J. Environ. Res. Public Health 2020, 17, 1692. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, G.; Zhang, L.; Gu, Z.; Lou, C.; Zhang, H.; Liu, L. Phthalate Esters (PAEs) in Soil and Vegetables in Solar Greenhouses Irrigated with Reclaimed Water. Environ. Sci. Pollut. Res. Int. 2020, 27, 22658–22669. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Elkhatib, R. Screening of phthalate esters in 47 branded perfumes. Environ. Sci. Pollut. Res. Int. 2016, 23, 455–468. [Google Scholar] [CrossRef]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef]
- Tranfo, G.; Paci, E.; Pigini, D.; Bonanni, C.; Capanna, S.; Carolis, C.; Iavicoli, S. Phthalate Metabolites in Amniotic Fluid and Maternal Urine Samples. J. Environ. Prot. Ecol. 2014, 5, 1411–1418. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.A.; Liu, H.; Qiu, Z.; Shu, W. Analysis of di-n-butyl phthalate and other organic pollutants in Chongqing women undergoing parturition. Environ. Pollut. 2008, 156, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zheng, L.X.; Gu, Y.P.; Wang, J.Y.; Zhang, Y.H.; Song, W.M. Levels of environmental endocrine disruptors in umbilical cord blood and maternal blood of low-birth-weight infants. Mar Zhonghua Yufang Yixue Zazhi 2008, 42, 177–180. (In Chinese) [Google Scholar] [PubMed]
- Wan, H.T.; Leung, P.Y.; Zhao, Y.G.; Wei, X.; Wong, M.H.; Wong, C.K.C. Blood plasma concentrations of endocrine disrupting chemicals in Hong Kong populations. J. Hazard. Mater. 2013, 261, 763–769. [Google Scholar] [CrossRef]
- Tang, S.; He, C.; Thai, P.; Vijayasarathy, S.; Mackie, R.; Toms, L.; Thompson, K.; Hobson, P.; Tscharke, B.; O’Brien, J.; et al. Concentrations of Phthalate Metabolites in Australian Urine Samples and Their Contribution to the Per Capita Loads in Wastewater. Environ. Int. 2020, 137, 105534. [Google Scholar] [CrossRef]
- Weng, T.; Chen, M.H.; Lien, G.; Chen, P.; Lin, J.C.; Fang, C.C.; Chen, P. Effects of Gender on the Association of Urinary Phthalate Metabolites with Thyroid Hormones in Children: A Prospective Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2017, 14, 123. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Ku, H.S.; Su, P.H.; Chen, J.W.; Huang, P.C.; Angere, J.; Wang, S.L. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere 2011, 82, 947–955. [Google Scholar] [CrossRef]
- Kolena, B.; Petrovičová, I.; Šidlovská, M.; Hlisníková, H.; Bystričanová, L.; Wimmerová, S.; Trnovec, T. Occupational Hazards and Risks Associated with Phthalates among Slovakian Firefighters. Int. J. Environ. Res. Public Health 2020, 17, 2483. [Google Scholar] [CrossRef] [Green Version]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Jőnsson, B.A.G.; Lindh, C.H.; Giwercman, A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ. Int. 2015, 85, 8554–8560. [Google Scholar] [CrossRef]
- Hartmann, C.; Uhl, M.; Weiss, S.; Koch, H.M.; Scharf, S.; Konig, J. Human biomonitoring of phthalate exposure in Austrian children and adults and cumulative risk assessment. Int. J. Hyg. Environ. Health 2015, 218, 489–499. [Google Scholar] [CrossRef]
- Perng, W.; Watkins, D.J.; Cantoral, A.; Mercado-García, A.; Meeker, J.D.; Tellez-Rojo, M.M.; Peterson, K.E. Exposure to phthalates is associated with lipid profile in peripubertal Mexican youth. Environ. Res. 2017, 154, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Wu, Y.; Zhao, F.; Lv, Y.; Huang, D.; Wei, J.; Ruan, C.; Huang, M.; Deng, J.; Huang, D.; et al. The risk of missed abortion associated with the levels of tobacco, heavy metals and phthalate in hair of pregnant woman: A case control study in Chinese women. Medicine 2017, 96, e9388. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Chen, Y.C.; Chen, M.J.; Chang, C.W.; Lai, G.L.; Tzeng, C.R. Exposure to Mono-n-Butyl Phthalate in Women with Endometriosis and Its Association with the Biological Effects on Human Granulosa Cells. Int. J. Mol. Sci. 2020, 21, 1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, I.; Karmaus, W.J.J. Oxidative Stress-Related Genetic Variants May Modify Associations of Phthalate Exposures with Asthma. Int. J. Environ. Res. Public Health 2017, 14, 162. [Google Scholar] [CrossRef] [Green Version]
- Rotondo, E.; Chiarelli, F. Endocrine-Disrupting Chemicals and Insulin Resistance in Children. Biomedicines 2020, 8, 137. [Google Scholar] [CrossRef]
- Predieri, B.; Bruzzi, P.; Bigi, E.; Ciancia, S.; Madeo, S.F.; Lucaccioni, L.; Iughetti, L. Endocrine Disrupting Chemicals and Type 1 Diabetes. Int. J. Mol. Sci. 2020, 21, 2937. [Google Scholar] [CrossRef]
- Zabłocka, A.; Janusz, M. The two faces of reactive oxygen species. Postepy Hig. Med. Dosw. 2008, 62, 118–124. (In Polish) [Google Scholar]
- Gao, M.; Dong, Y.; Zhang, Z.; Song, W.; Qi, Y. Growth and antioxidant defense responses of wheat seedlings to di-nbutyl phthalate and di (2-ethylhexyl) phthalate stress. Chemosphere 2017, 172, 418–428. [Google Scholar] [CrossRef]
- Gu, S.; Zheng, H.; Xu, Q.; Sun, C.; Shi, M.; Wang, Z. Comparative toxicity of the plasticizer dibutyl phthalate to two freshwater algae. Aquat. Toxicol. 2017, 191, 122–130. [Google Scholar] [CrossRef]
- Lee, E.; Ahn, M.Y.; Kim, H.J.; Kim, Y.I.; Han, S.Y.; Kang, T.S.; Hong, J.H.; Park, K.L.; Lee, B.M.; Kim, H.S. Effect of Di(n-Butyl) Phthalate on Testicular Oxidative Damage and Antioxidant Enzymes in Hyperthyroid Rats. Environ. Toxicol. 2007, 22, 245–255. [Google Scholar] [CrossRef]
- Ma, T.; Chen, L.; Wu, L.; Zhang, H.; Luo, Y. Oxidative stress, cytotoxicity and genotoxicity in Earthworm Eisenia fetida at different Di-n-Butyl phthalate exposure. PLoS ONE 2016, 11, e0151128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Wang, J.; Zhu, L.; Wang, J.; Li, H.; Zhang, Y.; Liu, W.; Gao, J. Oxidative Damage and Genetic Toxicity Induced by DBP in Earthworms (Eisenia fetida). Arch. Environ. Contam. Toxicol. 2018, 74, 527–538. [Google Scholar] [CrossRef]
- Yin, N.; Liang, S.; Liang, S.; Hu, B.; Yang, R.; Zhou, Q.; Jiang, G.; Faiola, F. DEP and DBP induce cytotoxicity in mouse embryonic stem cells and abnormally enhance neural ectoderm development. Environ. Pollut. 2018, 236, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, J.; Cheng, J.; Wu, Z. Dibutyl phthalate-induced activation of ROS and ERK1/2 causes hepatic and renal damage in Kunming mice. Hum. Exp. Toxicol. 2019, 38, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Gao, J.; Li, X.; Zhang, C.; Zhu, L.; Wang, J.; Wang, J. Phthalate induced oxidative stress and DNA damage in earthworms (Eisenia fetida). Environ. Int. 2019, 129, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sicińska, P. Di-n-butyl phthalate, butylbenzyl phthalate and their metabolites induce haemolysis and eryptosis in human erythrocytes. Chemosphere 2018, 203, 44–53. [Google Scholar]
- Tan, S.; Wang, D.; Chi, Z.; Li, W.; Shan, Y. Study on the interaction between typical phthalic acid esters (PAEs) and human haemoglobin (hHb) by molecular docking. Environ. Toxicol. Pharmacol. 2017, 53, 206–211. [Google Scholar] [CrossRef]
- Sicińska, P. Di-n-butyl phthalate, butylbenzyl phthalate, and their metabolites exhibit different apoptotic potential in human peripheral blood mononuclear cells. Food Chem Toxicol. 2019, 133, 110750. [Google Scholar] [CrossRef]
- Hansen, J.F.; Nielsen, C.H.; Brorson, M.M.; Frederiksen, H.; Hartoft-Nielsen, M.L.; Rasmussen, Å.K.; Bendtzen, K.; Feldt-Rasmussen, U. Influence of phthalates on in vitro innate and adaptive immune responses. PLoS ONE 2015, 10, e0131168. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem. Biol. Interact. 2018, 5, 73–83. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Wu, X.; Yang, G.; Wang, J.; Ding, J.; Cai, L.; Su, D. Oxidative stress-mediated signalling pathways: A review. J. Food Agric. Environ. 2013, 11, 132–139. [Google Scholar]
- Maćczak, A.; Bukowska, B.; Michałowicz, J. Comparative Study of the Effect of BPA and Its Selected Analogues on Hemoglobin Oxidation, Morphological Alterations and Hemolytic Changes in Human Erythrocytes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 176–177, 62–70. [Google Scholar]
- Kwack, S.J.; Kim, K.B.; Kim, H.S.; Lee, B.M. Comparative toxicological evaluation of phthalate diesters and metabolites in Sprague-Dawley male rats for risk assessment. J. Toxicol. Environ. Health A 2009, 72, 1446–1454. [Google Scholar] [CrossRef]
- Chi, Z.; Zhao, J.; You, H.; Wang, M. Study on the Mechanism of Interaction between Phthalate Acid Esters and Bovine Hemoglobin. J. Agric. Food Chem. 2016, 3, 6035–6041. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. The Second Face of Oxygen: Free Radical in Nature, 2nd ed.; PWN: Warszawa, Poland, 2009. (In Polish) [Google Scholar]
- Pagano, M.; Faggio, C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity, cell biochemistry and function. Cell Biochem. Funct. 2015, 33, 351–355. [Google Scholar] [CrossRef]
- Farombi, E.O.; Abarikwu, S.O.; Adedara, I.A.; Oyeyemi, M.O. Curcumin and kolaviron ameliorate di-n-butyl phthalate-induced testicular damage in rats. Basic Clin. Pharmacol. Toxicol. 2006, 100, 43–48. [Google Scholar] [CrossRef]
- Arif, A.; Salam, S.; Mahmood, R. Bioallethrin-induced generation of reactive species and oxidative damage in isolated human erythrocytes. Toxicol. In Vitro 2020, 22, 104810. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease. Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Silva, M.J.; Samandar, E.; Preau, J.L., Jr.; Reidy, J.A.; Needham, L.L.; Calafat, A. Automated solid-phase extraction and quantitative analysis of 14 phthalate metabolites in human serum using isotope dilution-high-performance liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2005, 29, 819–824. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Zhu, B.B.; Gao, H.; Huang, K.; Xu, Y.Y.; Yan, S.Q.; Zhou, S.S.; Cai, X.X.; Zhang, Q.F.; Qi, J.; et al. Repeated measures of prenatal phthalate exposure and maternal hemoglobin concentration trends: The Ma’anshan birth cohort (MABC) study. Environ. Pollut. 2018, 242, 1033–1041. [Google Scholar] [CrossRef]
- Giardina, B.; Messana, I.; Catena, R.; Cascagnola, M. The multiple function of hemoglobin. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Skold, A.; Cosco, D.L.; Klein, R. Methemoglobinemia: Pathogenesis, diagnosis, and management. South Med. J. 2011, 104, 757–761. [Google Scholar] [CrossRef]
- Jarosiewicz, M.; Duchnowicz, P.; Włuka, A.; Bukowska, B. Evaluation of the effect of brominated flame retardants on hemoglobin oxidation and hemolysis in human erythrocytes. Food Chem. Toxicol. 2017, 109, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Chrest, F.J.; Rifkind, J.M. Hydrogen-peroxide-induced heme degradation in red blood cells: The protective roles of catalase and glutathione peroxidase. Biochim. Biophys. Acta 2003, 16201, 211–217. [Google Scholar] [CrossRef]
- Umbreit, J. Methemoglobin it’s not just blue: A concise review. Am. J. Hematol. 2007, 82, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waris, S.; Patel, A.; Ali, A.; Mahmood, R. Acetaldehyde-induced oxidative modifications and morphological changes in isolated human erythrocytes: An in vitro study. Environ. Sci. Pollut. Res. Int. 2020, 27, 16268–16281. [Google Scholar] [CrossRef]
- Ansari, F.A.; Mahmood, R. Sodium nitrite enhances generation of reactive oxygen species that decrease antioxidant power and inhibit plasma membrane redox system of human erythrocytes. Cell Biol. Int. 2016, 40, 887–894. [Google Scholar] [CrossRef]
- Liu, C.; Duan, P.; Chen, Y.; Deng, Y.; Luo, Q.; Miao, Y.; Cui, S.; Liu, E.; Wang, Q.; Wang, W.; et al. Mediation of the Relationship Between Phthalate Exposure and Semen Quality by Oxidative Stress Among 1034 Reproductive-Aged Chinese Men. Environ. Res. 2019, 17, 108778. [Google Scholar] [CrossRef]
- Wu, H.; Estill, S.M.; Shershebnev, A.; Suvorov, A.; Krawetz, A.S.; Whitcomb, W.B.; Dinnie, H.; Rahil, T.; Sites, K.C.; Pilsner, R.J. Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: A cross-sectional study. Hum. Reprod. 2017, 32, 2159–2169. [Google Scholar] [CrossRef]
- Chu, D.P.; Tian, S.; Sun, D.G.; Hao, C.J.; Xia, H.F.; Ma, X. Exposure to mono-n-butyl phthalate disrupts the development of preimplantation embryos. Reprod. Fertil. Dev. 2013, 25, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Hepatotoxicity of di-(2-ethylhexyl) phthalate is attributed to calcium aggravation, ROS-mediated mitochondrial depolarization, and ERK/NF- κB pathway activation. Free Radical. Biol. Med. 2010, 49, 1779–1791. [Google Scholar] [CrossRef]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Guemouri, L.; Artur, Y.; Herbeth, B.; Jeandel, C.; Cuny, G.; Siest, G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin. Chem. 1991, 37, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Michalowicz, J.; Pieniazek, D.; Sicinska, P.; Duda, D. Superoxide Dismutases and their Inhibitors-the role in some diseases. Curr. Enzym. Inhib. 2006, 2, 379–397. [Google Scholar] [CrossRef]
- Prasanth, G.K.; Divya, L.M.; Sadasivan, C. Effects of mono and di(n-butyl) phthalate on superoxide dismutase. Toxicology 2009, 28, 38–42. [Google Scholar] [CrossRef]
- Yu, M.; Li, S.M.; Li, X.Y.; Zhang, B.J.; Wang, J.J. Acute effects of 1-octyl- 3 methylimidazolium bromide ionic liquid on the antioxidant enzyme system of mouse liver. Ecotoxicol. Environ. Saf. 2008, 71, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, G.; Liu, M.; Li, Y.; Yin, S.; Zhao, J.; Zhang, X. Evaluation of DNA damage and antioxidant system induced by di-n-Butyl phthalates exposure in earthworms (Eisenia fetida). Ecotoxicol. Environ. Saf. 2015, 115, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, G.; Liu, M.; Li, Y.; Yin, S.; Zhao, J. Biomarker responses in earthworms (Eisenia fetida) to soils contaminated with di-n-butyl phthalates. Environ. Sci. Pollut. Res. Int. 2015, 22, 4660–4669. [Google Scholar] [CrossRef]
- Paco, L.; Galarneau, A.; Drone, J.; Fajula, F.; Bailly, C.; Pulvin, S.; Thomas, D. Catalase-like activity of bovine Met-hemoglobin: Interaction with the pseudo-catalytic peroxidation of anthracene traces in aqueous medium. Biotechnol. J. 2009, 4, 1460–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Sánchez, M.I.; García-Carmona, F.; Macià, H.; Valero, E. Catalase-like activity of human methemoglobin: A kinetic and mechanistic study. Arch. Biochem. Biophys. 2011, 516, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ścibor, D.; Czeczot, H. Catalase: Structure, properties, functions. Postepy Hig. Med. Dosw. 2006, 60, 170–180. (In Polish) [Google Scholar]
- Eaton, J.W.; Ma, M. Acatalasemia. In The Metabolic and Molecular Bases of Inherited Disease; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill Inc.: New York, NY, USA, 1995; pp. 2371–2383. [Google Scholar]
- Zhou, D.; Wang, H.; Zhang, J. Di-n-butyl Phthalate (DBP) Exposure induces oxidative stress in epididymis of adult rats. Toxicol. Ind. Health 2011, 27, 65–71. [Google Scholar] [CrossRef]
- Blum, J.; Fridovich, I. Inactivation of glutathione peroxi-dase by superoxide radical. Arch. Biochem. Biophys. 1985, 240, 500–508. [Google Scholar] [CrossRef]
- Johnson, R.M.; Ho, Y.S.; Yu, D.Y.; Kuypers, F.A.; Ravindranath, Y.; Goyette, G.W. The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism. Free Radic. Biol. Med. 2010, 15, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.S.; Lee, S.; Lee, G.T.; Woo, H.A.; Choi, E.J.; Rhee, S.G. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid. Redox Signal. 2010, 12, 1235–1246. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, Z.; Nakanishi, T.; Wan, Y.; Hiromori, Y.; Nagase, H.; Hu, J. Structure dependent activity of phthalate esters and phthalate monoesters binding to human constitutive androstane receptor. Chem. Res. Toxicol. 2015, 28, 1196–1204. [Google Scholar] [CrossRef]
- Bissinger, R.; Bhuyan, A.; Qadri, S.M.; Lang, F. Oxidative Stress, Eryptosis and Anemia: A Pivotal Mechanistic Nexus in Systemic Diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef] [Green Version]
- Jarosiewicz, M.; Krokosz, A.; Marczak, A.; Bukowska, B. Changes in the activities of antioxidant enzymes and reduced glutathione level in human erythrocytes exposed to selected brominated flame retardants. Chemosphere 2019, 227, 93–99. [Google Scholar] [CrossRef]
- Woźniak, E.; Sicińska, P.; Michałowicz, J.; Woźniak, K.; Reszka, E.; Huras, B.; Zakrzewski, J.; Bukowska, B. The mechanism of DNA damage induced by Roundup 360 PLUS, glyphosate and AMPA in human peripheral blood mononuclear cells—Genotoxic risk assessement. Food Chem. Toxicol. 2018, 120, 510–522. [Google Scholar]
- Misra, H.; Fridovich, I. The role of superoxide anion in the autooxidation of epinephryne and a simple assai for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Rice-Evans, C.A.; Daplock, A.; Simonts, M.C.R. Techniques in free radical research. In Laboratory Techniques in Biochemistry and Molecular Biology; Diplock, A.T., Symons, M.C.R., Rice-Evans, C.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 22, pp. 206–280. [Google Scholar]



| Concentration | Methemoglobin (%) | |||
|---|---|---|---|---|
| (µg/mL) | DBP | BBP | MBP | MBzP |
| 0 | 1.21 ± 0.65 | 1.21 ± 0.65 | 1.21 ± 0.65 | 1.21 ± 0.65 |
| 0.5 | 3.19 ± 1.12 | 2.71 ± 0.76 | 2.11 ± 0.46 | 2.87 ± 0.73 |
| 1 | 3.10 ± 0.85 | 3.66 ± 0.77 | 2.45 ± 0.73 | 3.66 ± 0.43 |
| 2.5 | 6.61 ± 0.83 * | 3.73 ± 0.44 | 2.70 ± 0.96 | 3.32 ± 0.43 |
| 5 | 8.99 ± 0.94 * | 4.83 ± 0.81 * | 2.86 ± 0.97 | 4.08 ± 0.48 |
| 10 | 16.85 ± 1.67 * | 13.99 ± 1.10 * | 3.66 ± 0.81 | 4.70 ± 0.97 |
| 20 | 25.15 ± 1.87 * | 24.94 ± 2.79 * | 5.09 ± 0.65 | 5.68 ± 0.91 |
| 50 | - | - | 7.73 ± 0.80 * | 9.74 ± 1.09 * |
| 100 | - | - | 9.90 ± 0.89 * | 14.09 ± 2.23 * |
| Compound | Concentration (µg/mL) | Activity of Antioxidant Enzymes | ||
|---|---|---|---|---|
| SOD (U/g Hb) | CAT (µmol/min/mg Hb) | GSH-Px (µmol/min/gHb) | ||
| 0 | 1373.9 ± 139.2 | 161.97 ± 5.6 | 32.0 ± 3.6 | |
| DBP | 0.5 | 1220.1 ± 79.5 | 159.7 ± 11.8 | 31.7 ± 2.5 |
| 1 | 1145.1 ± 68.6 | 168.7 ± 21.5 | 27.3 ± 3.1 | |
| 2.5 | 1028.1 ± 79.6 * | 181.3 ± 16.0 | 23.5 ± 2.2 | |
| 5 | 920.5 ± 56.3 * | 215.9 ± 12.0 * | 21.6 ± 3.2 * | |
| 10 | 754.1 ± 44.6 * | 220.7 ± 18.6 * | 18.9 ± 5.4 * | |
| 20 | 662.5 ± 82.2 * | 238.8 ± 15.5 * | 17.9 ± 6.2 * | |
| BBP | 0.5 | 1279.1 ± 89.7 | 151.5 ± 8.5 | 30.8 ± 4.9 |
| 1 | 1165.1 ± 64.1 | 166.0 ± 14.3 | 30.4 ± 4.4 | |
| 2.5 | 904.6 ± 61.5 * | 179.2 ± 12.3 | 28.5 ± 4.2 | |
| 5 | 841.9 ± 75.5 * | 197.9 ± 13.2 * | 22.7 ± 3.8* | |
| 10 | 750.0 ± 95.3 * | 207.1 ± 15.4 * | 19.2 ± 5.1 * | |
| 20 | 549.7 ± 66.6 * | 231.5 ± 14.6 * | 18.4 ± 5.6 * | |
| MBP | 2.5 | 1270.2 ± 78.5 | 154.1 ± 15.9 | 31.3 ± 5.7 |
| 5 | 1177.7 ± 105.3 | 158.6 ± 17.1 | 26.1 ± 5.8 | |
| 10 | 837.1 ± 64.0 * | 165.1 ±16.8 | 24.6 ± 5.5 | |
| 20 | 759.1 ± 82.9 * | 184.6 ± 10.9 | 23.9 ± 1.7 | |
| 50 | 673.2 ± 89.4 * | 201.3 ± 22.5 * | 21.6 ± 1.9 * | |
| 100 | 436.9 ± 59.7 * | 217.2 ± 20.9 * | 20.6 ± 1.9 * | |
| MBzP | 2.5 | 1225.7 ± 122.5 | 156.8 ± 19.4 | 33.1 ± 4.4 |
| 5 | 1046.1 ± 149.7 | 160.4 ± 12.8 | 28.1 ± 6.3 | |
| 10 | 861.0 ± 95.2 * | 179.8 ± 17.8 | 27.6 ± 2.8 | |
| 20 | 815.1 ± 87.3 * | 185.4 ± 13.6 | 24.9 ± 3.1 | |
| 50 | 631.2 ± 98.1 * | 216.6 ± 14.5 * | 24.8 ± 6.1 * | |
| 100 | 577.8 ± 67.5 * | 222.0 ± 19.1 * | 23.7 ± 3.6 * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicińska, P.; Kik, K.; Bukowska, B. Human Erythrocytes Exposed to Phthalates and Their Metabolites Alter Antioxidant Enzyme Activity and Hemoglobin Oxidation. Int. J. Mol. Sci. 2020, 21, 4480. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124480
Sicińska P, Kik K, Bukowska B. Human Erythrocytes Exposed to Phthalates and Their Metabolites Alter Antioxidant Enzyme Activity and Hemoglobin Oxidation. International Journal of Molecular Sciences. 2020; 21(12):4480. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124480
Chicago/Turabian StyleSicińska, Paulina, Kinga Kik, and Bożena Bukowska. 2020. "Human Erythrocytes Exposed to Phthalates and Their Metabolites Alter Antioxidant Enzyme Activity and Hemoglobin Oxidation" International Journal of Molecular Sciences 21, no. 12: 4480. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124480