Functional Expression of Adenosine A3 Receptor in Yeast Utilizing a Chimera with the A2AR C-Terminus
Abstract
:1. Introduction
2. Results
2.1. Construction of an A3/A2AR Chimera to Aid in Receptor Expression
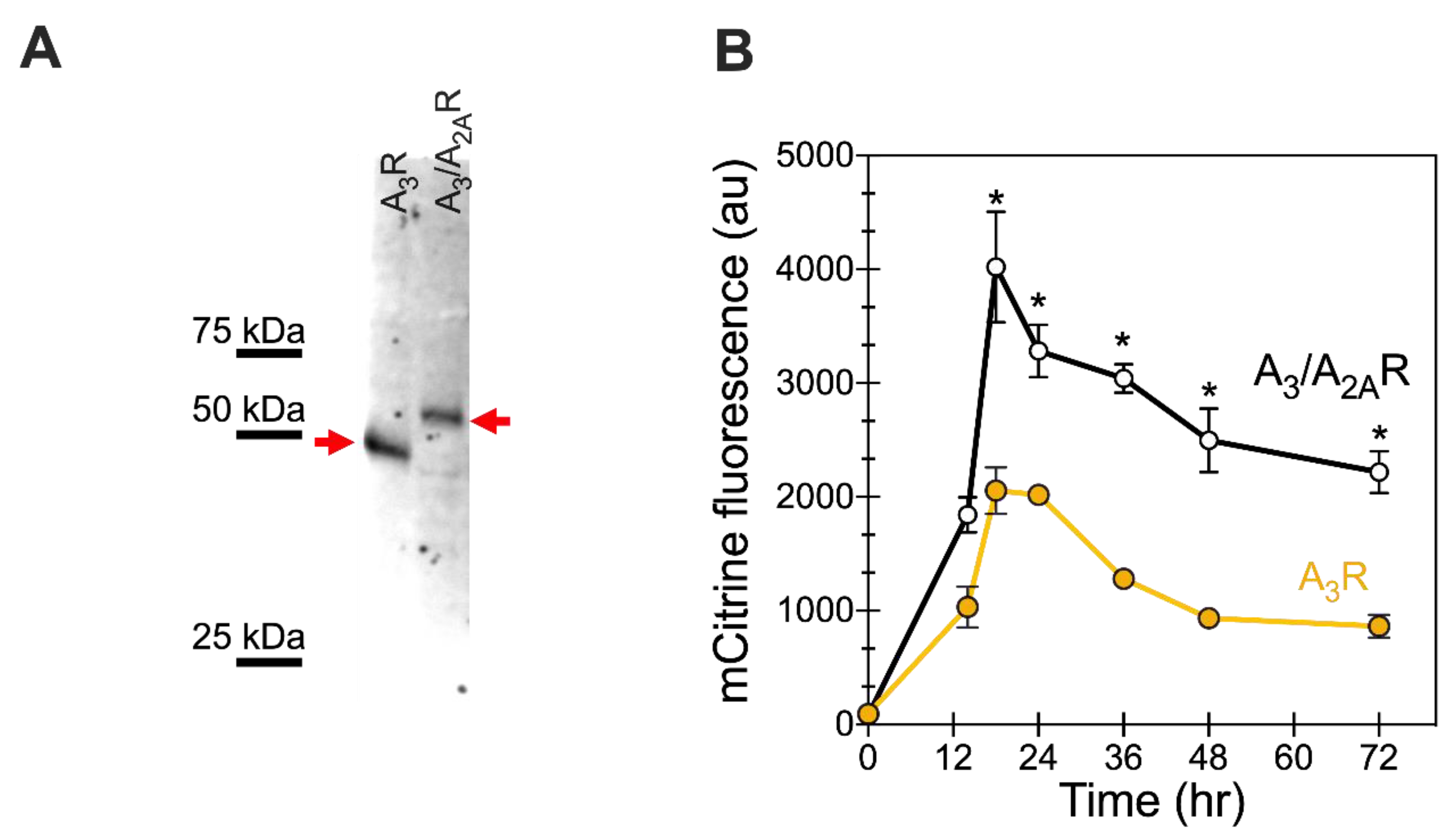
2.2. Improved Expression Using the Chimeric A3/A2AR
2.3. Decreased Unfolded Protein Response for the Chimeric A3/A2AR
2.4. Increasing Chimeric Receptor Expression by Varying Culture Conditions
2.5. Improved Receptor Trafficking to the Plasma Membrane
2.6. Chimeric A3/A2A Receptor Was Capable of Downstream Signaling Activity in Yeast
2.7. Chimeric Receptor Shows Preferential Coupling with the Inhibitory Gα Protein Family
3. Discussion
4. Materials and Methods
4.1. Cell and Culture Conditions
4.2. Plasmid Construction
4.3. Whole Cell Fluorescence Assay
4.4. Western Blotting
4.5. Confocal Microscope
4.6. Pheromone Response Signaling
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A3/A2AR | Chimeric receptor comprised of N-terminus and transmembrane domains from A3R (residues 1–284) and the cytoplasmic C-terminus of the A2AR (residues 291–412) |
| A3R | Adenosine A3 receptor |
| DMSO | Dimethyl sulfoxide |
| NECA | 5′-N-ethylcarboxamidoadenosine |
| PP | Pre-pro |
References
- Borea, P.A.; Varani, K.; Vincenzi, F.; Baraldi, P.G.; Tabrizi, M.A.; Merighi, S.; Gessi, S. The A3 adenosine receptor: History and perspectives. Pharmacol. Rev. 2015, 67, 74–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Merighi, S.; Varani, K.; Borea, P.A.; Baraldi, S.; Aghazadeh Tabrizi, M.; Romagnoli, R.; Baraldi, P.G.; Ciancetta, A.; Tosh, D.K.; et al. A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy. Med. Res. Rev. 2017. [Google Scholar] [CrossRef]
- Borea, P.A. A3 Adenosine Receptors from Cell Biology to Pharmacology and Therapeutics; Springer: Dordrecht, The Netherlands, 2010; p. xv. 322p. [Google Scholar]
- Fredholm, B.B.; AP, I.J.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar] [PubMed]
- Jacobson, K.A.; Tosh, D.K.; Jain, S.; Gao, Z.G. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front. Cell. Neurosci. 2019, 13, 124. [Google Scholar] [CrossRef] [Green Version]
- Fishman, P.; Bar-Yehuda, S.; Liang, B.T.; Jacobson, K.A. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov. Today 2012, 17, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Fishman, P.; Salhab, A.; Cohen, S.; Amer, J.; Itzhak, I.; Barer, F.; Safadi, R. The anti-inflammatory and anto-fibrogenic effects of namodenoson in NAFLD/NASH animal models. J. Hepatol. 2018, 68, S349–S350. [Google Scholar] [CrossRef]
- Stemmer, S.M.; Benjaminov, O.; Medalia, G.; Ciuraru, N.B.; Silverman, M.H.; Bar-Yehuda, S.; Fishman, S.; Harpaz, Z.; Farbstein, M.; Cohen, S.; et al. CF102 for the Treatment of Hepatocellular Carcinoma: A Phase I/II, Open-Label, Dose-Escalation Study. Oncologist 2013, 18, 25–26. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Gospodinov, D.K.; Gheorghe, N.; Mateev, G.S.; Rusinova, M.V.; Hristakieva, E.; Solovastru, L.G.; Patel, R.V.; Giurcaneanu, C.; Hitova, M.C.; et al. Treatment of Plaque-Type Psoriasis With Oral CF101: Data from a Phase II/III Multicenter, Randomized, Controlled Trial. J. Drugs Dermatol. 2016, 15, 931–938. [Google Scholar] [PubMed]
- Baltos, J.A.; Paoletta, S.; Nguyen, A.T.; Gregory, K.J.; Tosh, D.K.; Christopoulos, A.; Jacobson, K.A.; May, L.T. Structure-Activity Analysis of Biased Agonism at the Human Adenosine A3 Receptor. Mol. Pharmacol. 2016, 90, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundstrom, K.; Wagner, R.; Reinhart, C.; Desmyter, A.; Cherouati, N.; Magnin, T.; Zeder-Lutz, G.; Courtot, M.; Prual, C.; Andre, N.; et al. Structural genomics on membrane proteins: Comparison of more than 100 GPCRs in 3 expression systems. J. Struct. Funct. Genom. 2006, 7, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, A.N.; McNeely, P.M.; Katsaras, J.; Robinson, A.S. Impact of purification conditions and history on A2A adenosine receptor activity: The role of CHAPS and lipids. Protein Expr. Purif. 2016, 124, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Malley, M.A.; Lazarova, T.; Britton, Z.T.; Robinson, A.S. High-level expression in Saccharomyces cerevisiae enables isolation and spectroscopic characterization of functional human adenosine A2a receptor. J. Struct. Biol. 2007, 159, 166–178. [Google Scholar]
- Fowlkes, D.M.C.H.; Broach, J.R.P.; Manfredi, J.P.O.; Paul, J.I.N.; Trueheart, J.S.N.; Klein, C.A.O.; Murphy, A.J.M.M. Yeast Cells Expressing Modified G Proteins and Methods of Use Therefor. U.S. Patent US19970946298, 7 October 1997. [Google Scholar]
- King, K.; Dohlman, H.G.; Thorner, J.; Caron, M.G.; Lefkowitz, R.J. Control of yeast mating signal transduction by a mammalian beta 2-adrenergic receptor and Gs alpha subunit. Science 1990, 250, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Dyos, S.L.; Whiteway, M.S.; White, J.H.; Watson, M.A.; Marzioch, M.; Clare, J.J.; Cousens, D.J.; Paddon, C.; Plumpton, C.; et al. Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein alpha-subunit chimeras. Yeast 2000, 16, 11–22. [Google Scholar] [CrossRef]
- Knight, A.; Hemmings, J.L.; Winfield, I.; Leuenberger, M.; Frattini, E.; Frenguelli, B.G.; Dowell, S.J.; Lochner, M.; Ladds, G. Discovery of Novel Adenosine Receptor Agonists That Exhibit Subtype Selectivity. J. Med. Chem. 2016, 59, 947–964. [Google Scholar] [CrossRef] [Green Version]
- Stewart, G.D.; Valant, C.; Dowell, S.J.; Mijaljica, D.; Devenish, R.J.; Scammells, P.J.; Sexton, P.M.; Christopoulos, A. Determination of adenosine A1 receptor agonist and antagonist pharmacology using Saccharomyces cerevisiae: Implications for ligand screening and functional selectivity. J. Pharmacol. Exp. Ther. 2009, 331, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Groenewoud, N.J.; Peeters, M.C.; Lenselink, E.B.; AP, I.J. A yeast screening method to decipher the interaction between the adenosine A2B receptor and the C-terminus of different G protein alpha-subunits. Purinergic Signal. 2014, 10, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Takemoto, N.; Ishii, J.; Kondo, A. Simultaneous method for analyzing dimerization and signaling of G-protein-coupled receptor in yeast by dual-color reporter system. Biotechnol. Bioeng. 2014, 111, 586–596. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Youkey, R.L.; Ghartey, K.; Leonard, M.; Linden, J.; Tucker, A.L. The allosteric enhancer PD81,723 increases chimaeric A1/A2A adenosine receptor coupling with Gs. Biochem. J. 2006, 396, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Tucker, A.L.; Jia, L.G.; Holeton, D.; Taylor, A.J.; Linden, J. Dominance of G(s) in doubly G(s)/G(i)-coupled chimaeric A(1)/A(2A) adenosine receptors in HEK-293 cells. Biochem. J. 2000, 352, 203–210. [Google Scholar] [CrossRef]
- O’Malley, M.A.; Mancini, J.D.; Young, C.L.; McCusker, E.C.; Raden, D.; Robinson, A.S. Progress toward heterologous expression of active G-protein-coupled receptors in Saccharomyces cerevisiae: Linking cellular stress response with translocation and trafficking. Protein Sci. 2009, 18, 2356–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, A. A Systems Pharmacology Approach to the Adenosine A1 Receptor. Ph.D. Thesis, University of Warick, Coventry, UK, 2015. [Google Scholar]
- Jain, A.R.; Stradley, S.H.; Robinson, A.S. The A2aR C-terminus provides improved total and active expression yields for adenosine receptor chimeras. AIChE J. 2018, 64, 4297–4307. [Google Scholar] [CrossRef]
- Jain, A.R.; Britton, Z.T.; Markwalter, C.E.; Robinson, A.S. Improved ligand-binding- and signaling-competent human NK2R yields in yeast using a chimera with the rat NK2R C-terminus enable NK2R-G protein signaling platform. Protein Eng. Des. Sel. 2019, 32, 459–469. [Google Scholar] [CrossRef]
- Hauser, A.S.; Chavali, S.; Masuho, I.; Jahn, L.J.; Martemyanov, K.A.; Gloriam, D.E.; Babu, M.M. Pharmacogenomics of GPCR Drug Targets. Cell 2018, 172, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Nufer, O.; Guldbrandsen, S.; Degen, M.; Kappeler, F.; Paccaud, J.P.; Tani, K.; Hauri, H.P. Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J. Cell. Sci. 2002, 115, 619–628. [Google Scholar]
- Sikorski, R.S.; Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 1989, 122, 19–27. [Google Scholar] [PubMed]
- Bitter, G.A.; Egan, K.M. Expression of interferon-gamma from hybrid yeast GPD promoters containing upstream regulatory sequences from the GAL1-GAL10 intergenic region. Gene 1988, 69, 193–207. [Google Scholar] [CrossRef]
- Arnold, C.E.; Parekh, R.N.; Yang, W.; Wittrup, K.D. Leader peptide efficiency correlates with signal recognition particle dependence in Saccharomyces cerevisiae. Biotechnol. Bioeng. 1998, 59, 286–293. [Google Scholar] [CrossRef]
- Kimata, Y.; Kimata, Y.I.; Shimizu, Y.; Abe, H.; Farcasanu, I.C.; Takeuchi, M.; Rose, M.D.; Kohno, K. Genetic evidence for a role of BiP/Kar2 that regulates Ire1 in response to accumulation of unfolded proteins. Mol. Biol. Cell. 2003, 14, 2559–2569. [Google Scholar] [CrossRef] [Green Version]
- Rose, M.D.; Misra, L.M.; Vogel, J.P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell 1989, 57, 1211–1221. [Google Scholar] [CrossRef]
- Young, C.L.; Yuraszeck, T.; Robinson, A.S. Decreased secretion and unfolded protein response upregulation. Methods Enzym. 2011, 491, 235–260. [Google Scholar]
- Wedekind, A.; O’Malley, M.A.; Niebauer, R.T.; Robinson, A.S. Optimization of the human adenosine A2a receptor yields in Saccharomyces cerevisiae. Biotechnol. Prog. 2006, 22, 1249–1255. [Google Scholar] [CrossRef]
- Attrill, H.; Harding, P.J.; Smith, E.; Ross, S.; Watts, A. Improved yield of a ligand-binding GPCR expressed in E. coli for structural studies. Protein Expr. Purif. 2009, 64, 32–38. [Google Scholar] [CrossRef]
- Naranjo, A.N.; Chevalier, A.; Cousins, G.D.; Ayettey, E.; McCusker, E.C.; Wenk, C.; Robinson, A.S. Conserved disulfide bond is not essential for the adenosine A2A receptor: Extracellular cysteines influence receptor distribution within the cell and ligand-binding recognition. Biochim. Biophys. Acta 2015, 1848, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wong, W.; AP, I.J. Human G protein-coupled receptor studies in Saccharomyces cerevisiae. Biochem. Pharmacol. 2016, 114, 103–115. [Google Scholar] [CrossRef]
- Jain, A.R. G Protein-Coupled Receptor Expression and Signaling in Yeast: Design and Optimization of Host/Protein Platform for Therapeutic Development. Ph.D. Thesis, Tulane University, Ann Arbor, MI, USA, 2019. [Google Scholar]
- Britton, Z.T. Novel Approaches to the Expression and Purification of G Protein-Coupled Receptors; University of Delaware, ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2012; UMI 3526401. [Google Scholar]
- Bjorkskov, F.B.; Krabbe, S.L.; Nurup, C.N.; Missel, J.W.; Spulber, M.; Bomholt, J.; Molbaek, K.; Helix-Nielsen, C.; Gotfryd, K.; Gourdon, P.; et al. Purification and functional comparison of nine human Aquaporins produced in Saccharomyces cerevisiae for the purpose of biophysical characterization. Sci. Rep. 2017, 7, 16899. [Google Scholar] [CrossRef] [Green Version]
- Peeters, M.C.; Wisse, L.E.; Dinaj, A.; Vroling, B.; Vriend, G.; Ijzerman, A.P. The role of the second and third extracellular loops of the adenosine A1 receptor in activation and allosteric modulation. Biochem. Pharmacol. 2012, 84, 76–87. [Google Scholar] [CrossRef]
- Bertheleme, N.; Singh, S.; Dowell, S.J.; Hubbard, J.; Byrne, B. Loss of constitutive activity is correlated with increased thermostability of the human adenosine A2A receptor. Br. J. Pharmacol. 2013, 169, 988–998. [Google Scholar] [CrossRef] [Green Version]
- Mundell, S.J.; Kelly, E. Evidence for co-expression and desensitization of A2a and A2b adenosine receptors in NG108-15 cells. Biochem. Pharmacol. 1998, 55, 595–603. [Google Scholar] [CrossRef]
- Burke, D.; Dawson, D.; Stearns, T. Cold Spring Harbor Laboratory. In Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 2000 ed.; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 2000; p. xvii. 205p. [Google Scholar]
- Ito, K.; Sugawara, T.; Shiroishi, M.; Tokuda, N.; Kurokawa, A.; Misaka, T.; Makyio, H.; Yurugi-Kobayashi, T.; Shimamura, T.; Nomura, N.; et al. Advanced method for high-throughput expression of mutated eukaryotic membrane proteins in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2008, 371, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Woods, R.A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzym. 2002, 350, 87–96. [Google Scholar]
- Young, C.L. Interrogation of Quality Control Mechanisms and Protein Trafficking in Saccharomyces Cerevisiae; University of Delaware, ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2012; UMI 3543550. [Google Scholar]
- McNeely, P.M. Receptor-Receptor, Ligand, and Membrane Interactions of the Adenosine A2a Receptor. Ph.D. Thesis, University of Delaware, Ann Arbor, MI, USA, 2016. [Google Scholar]
- McNeely, P.M.; Naranjo, A.N.; Forsten-Williams, K.; Robinson, A.S. A2AR Binding Kinetics in the Ligand Depletion Regime. Slas Discov. 2017, 22, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Yeast Strain | G Protein | Last 5 Amino Acids at C-Terminus | Equivalent Human Gα |
|---|---|---|---|
| MMY12, BY4741 | Gpa1 | KIGIICOOH | GPA1 (yeast) |
| MMY14, CY13397 | Gpa1-Gαq(5) | EYNLVCOOH | GNAQ, GNA11 |
| MMY16, CY13395 | Gpa1-Gα16(5) | EINLLCOOH | GNA15, GNA16 |
| MMY19 | Gpa1-Gα12(5) | DIMLQCOOH | GNA12 |
| MMY20 | Gpa1-Gα13(5) | QLMLQCOOH | GNA13 |
| MMY21 | Gpa1-Gα14(5) | EFNLVCOOH | GNA14 |
| MMY22 | Gpa1-Gαo(5) | GCGLYCOOH | GNAO |
| MMY23, CY13393 | Gpa1-Gαi1(5) | DCGLFCOOH | GNAI1, GNAI2, GNAT1, GNAT2, GNAT3 |
| MMY24 | Gpa1-Gαi3(5) | ECGLYCOOH | GNAI3 |
| MMY25 | Gpa1-Gαz(5) | YIGLCCOOH | GNAZ |
| MMY28, CY13399 | Gpa1-Gαs(5) | QYELLCOOH | GNAS, GNAL |
| Primer Name | Sequence | Receptor Variants |
|---|---|---|
| PP_A3_F | CGGTTCCGCTGCAGAAGGCTCTTTGGACAAGAGAGAAGCTATGCCCAACAACAGCACTGC | A3R and A3/A2AR |
| A3_mCit_R | TTGGGACAACACCAGTGAATAATTCTTCACCTTTAGACATCTCAGAATTCTTCTCAATGC | A3R |
| A3_A2A_F | CCATGATGAACCCTATCGTCTATGCCTATCGTATCCGCGAGTTCCGCCAGACCTTCCGCA | A3/A2AR |
| A3_A2A_R | TGCGGAAGGTCTGGCGGAACTCGCGGATACGATAGGCATAGACGATAGGGTTCATCATGG | A3/A2AR |
| A2A_mCit_R | TTGGGACAACACCAGTGAATAATTCTTCACCTTTAGACATGGACACTCCTGCTCCATCCT | A3/A2AR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, A.R.; Robinson, A.S. Functional Expression of Adenosine A3 Receptor in Yeast Utilizing a Chimera with the A2AR C-Terminus. Int. J. Mol. Sci. 2020, 21, 4547. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124547
Jain AR, Robinson AS. Functional Expression of Adenosine A3 Receptor in Yeast Utilizing a Chimera with the A2AR C-Terminus. International Journal of Molecular Sciences. 2020; 21(12):4547. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124547
Chicago/Turabian StyleJain, Abhinav R., and Anne S. Robinson. 2020. "Functional Expression of Adenosine A3 Receptor in Yeast Utilizing a Chimera with the A2AR C-Terminus" International Journal of Molecular Sciences 21, no. 12: 4547. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124547






