Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections
Abstract
:1. Introduction
2. General Concepts on Vimentin Structure and Assembly
3. Extracellular Vimentin
4. Vimentin in Tissue Damage and Repair
5. Vimentin in Immune Responses
6. Vimentin in Host-Pathogen Interactions
6.1. Bacterial Infections
6.2. Viral Infections
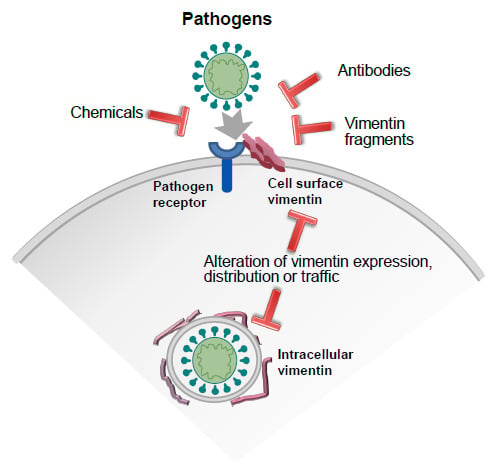
7. Strategies to Modulate Vimentin Function: Focus on Extracellular Vimentin-Pathogen Interactions
7.1. Anti-Vimentin Antibodies
7.2. Chemical Agents
7.3. Other Strategies
8. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | angiotensin converting enzyme |
| AVA | anti-vimentin autoantibodies |
| BALF | bronchoaveolar lavage fluid |
| CHO | Chinese hamster ovary |
| CSV | cell surface vimentin |
| DENV | dengue virus |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| HMEC | human microvascular endothelial cells |
| HUVEC | human umbilical endothelial cells |
| IAV | influenza A virus |
| ICAM-1 | intercellular adhesion molecule 1 |
| IGF-1R | insulin-like growth factor receptor 1 |
| LAMP1 | lysosomal-associated membrane protein 1 |
| LPS | bacterial lipopolycaccharide |
| mAB | monoclonal antibody |
| N-AcGln | N-acetylglucosamine |
| NRLP3 | nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 |
| NS4A | non-structural protein 4A |
| PAI-1 | plasminogen activator inhibitor 1 |
| PSLG-1 | P-selectin glycoprotein ligand 1 |
| PTM | posttranslational modification |
| PRR | pattern recognition receptor |
| RA | rheumatoid arthritis |
| RIG-I | retinoic acid-inducible gene I |
| ROS | reactive oxygen species |
| SARS-CoV | severe acute respiratory syndrome-related coronavirus |
| TMPRSS2 | transmembrane protease, serine 2 |
| ULF | unit-length filament |
| VCAM-1 | vascular cell adhesion molecule 1 |
| vRNP | viral ribonucleoprotein |
| VWF | Von Willebrandt Factor |
| WFA | withaferin A |
References
- Gan, Z.; Ding, L.; Burckhardt, C.J.; Lowery, J.; Zaritsky, A.; Sitterley, K.; Mota, A.; Costigliola, N.; Starker, C.G.; Voytas, D.F.; et al. Vimentin Intermediate Filaments Template Microtubule Networks to Enhance Persistence in Cell Polarity and Directed Migration. Cell Syst. 2016, 3, 252–263 e258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, 1796. [Google Scholar] [CrossRef] [Green Version]
- Etienne-Manneville, S. Cytoplasmic Intermediate Filaments in Cell Biology. Annu Rev. Cell Dev. Biol 2018, 34, 1–28. [Google Scholar] [CrossRef]
- Duarte, S.; Viedma-Poyatos, A.; Navarro-Carrasco, E.; Martinez, A.E.; Pajares, M.A.; Perez-Sala, D. Vimentin filaments interact with the actin cortex in mitosis allowing normal cell division. Nat. Commun. 2019, 10, 4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Styers, M.L.; Salazar, G.; Love, R.; Peden, A.A.; Kowalczyk, A.P.; Faundez, V. The endo-lysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol. Biol. Cell 2004, 15, 5369–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sala, D.; Oeste, C.L.; Martínez, A.E.; Garzón, B.; Carrasco, M.J.; Cañada, F.J. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015, 6, 7287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernoivanenko, I.S.; Matveeva, E.A.; Gelfand, V.I.; Goldman, R.D.; Minin, A.A. Mitochondrial membrane potential is regulated by vimentin intermediate filaments. FASEB J. 2015, 29, 820–827. [Google Scholar] [CrossRef] [Green Version]
- Watabe, M.; Nakaki, T. Protein kinase CK2 regulates the formation and clearance of aggresomes in response to stress. J. Cell Sci. 2011, 124, 1519–1532. [Google Scholar] [CrossRef] [Green Version]
- Patteson, A.E.; Vahabikashi, A.; Pogoda, K.; Adam, S.A.; Mandal, K.; Kittisopikul, M.; Sivagurunathan, S.; Goldman, A.; Goldman, R.D.; Janmey, P.A. Vimentin protects cells against nuclear rupture and DNA damage during migration. J. Cell Biol. 2019, 218, 4079–4092. [Google Scholar] [CrossRef]
- Danielsson, F.; Peterson, M.K.; Caldeira Araujo, H.; Lautenschlager, F.; Gad, A.K.B. Vimentin Diversity in Health and Disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Eckes, B.; Colucci-Guyon, E.; Smola, H.; Nodder, S.; Babinet, C.; Krieg, T.; Martin, P. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 2000, 113 (Pt. 13), 2455–2462. [Google Scholar]
- Dos Santos, G.; Rogel, M.R.; Baker, M.A.; Troken, J.R.; Urich, D.; Morales-Nebreda, L.; Sennello, J.A.; Kutuzov, M.A.; Sitikov, A.; Davis, J.M.; et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat. Commun. 2015, 6, 6574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haversen, L.; Sundelin, J.P.; Mardinoglu, A.; Rutberg, M.; Stahlman, M.; Wilhelmsson, U.; Hulten, L.M.; Pekny, M.; Fogelstrand, P.; Bentzon, J.F.; et al. Vimentin deficiency in macrophages induces increased oxidative stress and vascular inflammation but attenuates atherosclerosis in mice. Sci. Rep. 2018, 8, 16973. [Google Scholar] [CrossRef] [PubMed]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Markovitz, D.M. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 2003, 5, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Divanyan, A.; Jourd’heuil, F.L.; Goldman, R.D.; Ridge, K.M.; Jourd’heuil, D.; Lopez-Soler, R.I. Vimentin expression is required for the development of EMT-related renal fibrosis following unilateral ureteral obstruction in mice. Am. J. Physiol. Ren. Physiol. 2018, 315, F769–F780. [Google Scholar] [CrossRef] [PubMed]
- Surolia, R.; Li, F.J.; Wang, Z.; Li, H.; Dsouza, K.; Thomas, V.; Mirov, S.; Perez-Sala, D.; Athar, M.; Thannickal, V.J.; et al. Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.Y.; Lin, H.H.; Tang, M.J.; Wang, Y.K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.M.; Havel, L.S.; Koyen, A.E.; Konen, J.M.; Shupe, J.; Wiles, W.G.T.; Martin, W.D.; Grossniklaus, H.E.; Sica, G.; Gilbert-Ross, M.; et al. Vimentin Is Required for Lung Adenocarcinoma Metastasis via Heterotypic Tumor Cell-Cancer-Associated Fibroblast Interactions during Collective Invasion. Clin. Cancer Res. 2018, 24, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Vossenaar, E.R.; Despres, N.; Lapointe, E.; van der Heijden, A.; Lora, M.; Senshu, T.; van Venrooij, W.J.; Menard, H.A. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. 2004, 6, R142–R150. [Google Scholar] [CrossRef] [Green Version]
- Du, N.; Cong, H.; Tian, H.; Zhang, H.; Zhang, W.; Song, L.; Tien, P. Cell surface vimentin is an attachment receptor for enterovirus 71. J. Virol. 2014, 88, 5816–5833. [Google Scholar] [CrossRef] [Green Version]
- Musaelyan, A.; Lapin, S.; Nazarov, V.; Tkachenko, O.; Gilburd, B.; Mazing, A.; Mikhailova, L.; Shoenfeld, Y. Vimentin as antigenic target in autoimmunity: A comprehensive review. Autoimmun. Rev. 2018, 17, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.N.; Bruggemann, H. Vimentin in Bacterial Infections. Cells 2016, 5, 18. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Structure and Assembly. Cold Spring Harb. Perspect. Biol. 2016, 8, a018242. [Google Scholar] [CrossRef]
- Strelkov, S.V.; Herrmann, H.; Geisler, N.; Wedig, T.; Zimbelmann, R.; Aebi, U.; Burkhard, P. Conserved segments 1A and 2B of the intermediate filament dimer: Their atomic structures and role in filament assembly. EMBO J. 2002, 21, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernyatina, A.A.; Nicolet, S.; Aebi, U.; Herrmann, H.; Strelkov, S.V. Atomic structure of the vimentin central alpha-helical domain and its implications for intermediate filament assembly. Proc. Natl. Acad. Sci. USA 2012, 109, 13620–13625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolova, A.V.; Kreplak, L.; Wedig, T.; Mucke, N.; Svergun, D.I.; Herrmann, H.; Aebi, U.; Strelkov, S.V. Monitoring intermediate filament assembly by small-angle x-ray scattering reveals the molecular architecture of assembly intermediates. Proc. Natl. Acad. Sci. USA 2006, 103, 16206–16211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirmse, R.; Bouchet-Marquis, C.; Page, C.; Hoenger, A. Three-dimensional cryo-electron microscopy on intermediate filaments. Methods Cell Biol. 2010, 96, 565–589. [Google Scholar] [CrossRef]
- Chernyatina, A.A.; Guzenko, D.; Strelkov, S.V. Intermediate filament structure: The bottom-up approach. Curr. Opin. Cell Biol. 2015, 32, 65–72. [Google Scholar] [CrossRef]
- Kornreich, M.; Avinery, R.; Malka-Gibor, E.; Laser-Azogui, A.; Beck, R. Order and disorder in intermediate filament proteins. FEBS Lett. 2015, 589, 2464–2476. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, J.E.; He, T.; Trejo-Skalli, A.V.; Härmälä-Braskén, A.-S.; Hellman, J.; Chou, Y.-H.; Goldman, R.D. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci. 2004, 117, 919–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Goldman, R.D. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 2004, 5, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Snider, N.T.; Omary, M.B. Post-translational modifications of intermediate filament proteins: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 163–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viedma-Poyatos, A.; Pajares, M.A.; Pérez-Sala, D. Type III intermediate filaments as targets and effectors of electrophiles and oxidants. Redox Biol. 2020, 101582. [Google Scholar] [CrossRef]
- Chavez, J.; Chung, W.G.; Miranda, C.L.; Singhal, M.; Stevens, J.F.; Maier, C.S. Site-specific protein adducts of 4-hydroxy-2(E)-nonenal in human THP-1 monocytic cells: Protein carbonylation is diminished by ascorbic acid. Chem. Res. Toxicol. 2010, 23, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mónico, A.; Duarte, S.; Pajares, M.A.; Pérez-Sala, D. Vimentin disruption by lipoxidation and electrophiles: Role of the cysteine residue and filament dynamics. Redox Biol. 2019, 23, 101098. [Google Scholar] [CrossRef]
- Tarbet, H.J.; Dolat, L.; Smith, T.J.; Condon, B.M.; O’Brien, E.T., 3rd; Valdivia, R.H.; Boyce, M. Site-specific glycosylation regulates the form and function of the intermediate filament cytoskeleton. eLife 2018, 7, e31807. [Google Scholar] [CrossRef]
- Byun, Y.; Chen, F.; Chang, R.; Trivedi, M.; Green, K.J.; Cryns, V.L. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001, 8, 443–450. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, P.; Hu, F.; Yan, R.; He, M.; Li, W.; Xu, J.; Xu, K. Hypotonic Stress Induces Fast, Reversible Degradation of the Vimentin Cytoskeleton via Intracellular Calcium Release. Adv. Sci. (Weinh) 2019, 6, 1900865. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Arlinghaus, R.B. Vimentin phosphorylation by p37mos protein kinase in vitro and generation of a 50-kDa cleavage product in v-mos-transformed cells. Virology 1989, 173, 144–156. [Google Scholar] [CrossRef]
- Shoeman, R.L.; Honer, B.; Stoller, T.J.; Kesselmeier, C.; Miedel, M.C.; Traub, P.; Graves, M.C. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc. Natl. Acad. Sci. USA 1990, 87, 6336–6340. [Google Scholar] [CrossRef] [Green Version]
- Frescas, D.; Roux, C.M.; Aygun-Sunar, S.; Gleiberman, A.S.; Krasnov, P.; Kurnasov, O.V.; Strom, E.; Virtuoso, L.P.; Wrobel, M.; Osterman, A.L.; et al. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc. Natl. Acad. Sci. USA 2017, 114, E1668–E1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janciauskiene, S.; Tumpara, S.; Wiese, M.; Wrenger, S.; Vijayan, V.; Gueler, F.; Chen, R.; Madyaningrana, K.; Mahadeva, R.; Welte, T.; et al. Alpha1-antitrypsin binds hemin and prevents oxidative activation of human neutrophils: Putative pathophysiological significance. J. Leukoc Biol. 2017, 102, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; He, L.; Huang, S.H. Identification of a surface protein on human brain microvascular endothelial cells as vimentin interacting with Escherichia coli invasion protein IbeA. Biochem. Biophys. Res. Commun. 2006, 351, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Pall, T.; Pink, A.; Kasak, L.; Turkina, M.; Anderson, W.; Valkna, A.; Kogerman, P. Soluble CD44 interacts with intermediate filament protein vimentin on endothelial cell surface. PLoS ONE 2011, 6, e29305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da, Q.; Behymer, M.; Correa, J.I.; Vijayan, K.V.; Cruz, M.A. Platelet adhesion involves a novel interaction between vimentin and von Willebrand factor under high shear stress. Blood 2014, 123, 2715–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; deWaal, R.M.; Mor-Vaknin, N.; Hibbard, C.; Markovitz, D.M.; Kahn, M.L. The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol. Cell Biol. 2004, 24, 9198–9206. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Cho, W.; Kim, I.; Lee, S.H.; Oh, G.T.; Park, Y.M. Oxidized LDL induces vimentin secretion by macrophages and contributes to atherosclerotic inflammation. J. Mol. Med. 2020. [Google Scholar] [CrossRef]
- Avram, D.; Romijn, E.P.; Pap, E.H.; Heck, A.J.; Wirtz, K.W. Identification of proteins in activated human neutrophils susceptible to tyrosyl radical attack. A proteomic study using a tyrosylating fluorophore. Proteomics 2004, 4, 2397–2407. [Google Scholar] [CrossRef]
- Komura, K.; Ise, H.; Akaike, T. Dynamic behaviors of vimentin induced by interaction with GlcNAc molecules. Glycobiology 2012, 22, 1741–1759. [Google Scholar] [CrossRef] [Green Version]
- Laragione, T.; Gianazza, E.; Tonelli, R.; Bigini, P.; Mennini, T.; Casoni, F.; Massignan, T.; Bonetto, V.; Ghezzi, P. Regulation of redox-sensitive exofacial protein thiols in CHO cells. Biol. Chem. 2006, 387, 1371–1376. [Google Scholar] [CrossRef]
- Checconi, P.; Salzano, S.; Bowler, L.; Mullen, L.; Mengozzi, M.; Hanschmann, E.M.; Lillig, C.H.; Sgarbanti, R.; Panella, S.; Nencioni, L.; et al. Redox proteomics of the inflammatory secretome identifies a common set of redoxins and other glutathionylated proteins released in inflammation, influenza virus infection and oxidative stress. PLoS ONE 2015, 10, e0127086. [Google Scholar] [CrossRef] [Green Version]
- Gronwall, C.; Amara, K.; Hardt, U.; Krishnamurthy, A.; Steen, J.; Engstrom, M.; Sun, M.; Ytterberg, A.J.; Zubarev, R.A.; Scheel-Toellner, D.; et al. Autoreactivity to malondialdehyde-modifications in rheumatoid arthritis is linked to disease activity and synovial pathogenesis. J. Autoimmun. 2017, 84, 29–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moisan, E.; Girard, D. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J. Leukoc Biol. 2006, 79, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.; Egerer, K.; Gauliard, A.; Luthke, K.; Rudolph, P.E.; Fredenhagen, G.; Berg, W.; Feist, E.; Burmester, G.R. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum 2007, 56, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, E.; Kato, M.; Kudo, Y.; Lee, W.; Hisada, R.; Fujieda, Y.; Oku, K.; Bohgaki, T.; Amengual, O.; Yasuda, S.; et al. Autophagy promotes citrullination of VIM (vimentin) and its interaction with major histocompatibility complex class II in synovial fibroblasts. Autophagy 2020, 16, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Toth, E.; Tarcsa, E.; Falus, A.; Buzas, E.I. Citrullination: A posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef] [PubMed]
- Brentville, V.A.; Metheringham, R.L.; Gunn, B.; Symonds, P.; Daniels, I.; Gijon, M.; Cook, K.; Xue, W.; Durrant, L.G. Citrullinated vimentin presented on MHC-II in tumor cells is a target for CD4+ T cell-mediated antitumor immunity. Cancer Res. 2016, 76, 548–570. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Spencer, B.L.; Holmes, J.A.; Mu, R.; Rego, S.; Weston, T.A.; Hu, Y.; Sanches, G.F.; Yoon, S.; Park, N.; et al. The Group B Streptococcal surface antigen I/II protein, BspC, interacts with host vimentin to promote adherence to brain endothelium and inflammation during the pathogenesis of meningitis. PLoS Pathog. 2019, 15, e1007848. [Google Scholar] [CrossRef] [Green Version]
- Hwang, B.; Ise, H. Multimeric conformation of type III intermediate filaments but not the filamentous conformation exhibits high affinity to lipid bilayers. Genes Cells 2020, 25, 413–426. [Google Scholar] [CrossRef]
- Fasipe, T.A.; Hong, S.H.; Da, Q.; Valladolid, C.; Lahey, M.T.; Richards, L.M.; Dunn, A.K.; Cruz, M.A.; Marrelli, S.P. Extracellular Vimentin/VWF (von Willebrand Factor) Interaction Contributes to VWF String Formation and Stroke Pathology. Stroke 2018, 49, 2536–2540. [Google Scholar] [CrossRef]
- Shigyo, M.; Kuboyama, T.; Sawai, Y.; Tada-Umezaki, M.; Tohda, C. Extracellular vimentin interacts with insulin-like growth factor 1 receptor to promote axonal growth. Sci. Rep. 2015, 5, 12055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiagarajan, P.S.; Yakubenko, V.P.; Elsori, D.H.; Yadav, S.P.; Willard, B.; Tan, C.D.; Rodriguez, E.R.; Febbraio, M.; Cathcart, M.K. Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc. Res. 2013, 99, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ise, H.; Kobayashi, S.; Goto, M.; Sato, T.; Kawakubo, M.; Takahashi, M.; Ikeda, U.; Akaike, T. Vimentin and desmin possess GlcNAc-binding lectin-like properties on cell surfaces. Glycobiology 2010, 20, 843–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ise, H.; Matsunaga, K.; Shinohara, M.; Sakai, Y. Improved Isolation of Mesenchymal Stem Cells Based on Interactions between N-Acetylglucosamine-Bearing Polymers and Cell-Surface Vimentin. Stem Cells Int. 2019, 2019, 4341286. [Google Scholar] [CrossRef]
- Bryant, A.E.; Bayer, C.R.; Huntington, J.D.; Stevens, D.L. Group A streptococcal myonecrosis: Increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J. Infect. Dis. 2006, 193, 1685–1692. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.T.; Chien, S.C.; Chen, I.Y.; Lai, C.T.; Tsay, Y.G.; Chang, S.C.; Chang, M.F. Surface vimentin is critical for the cell entry of SARS-CoV. J. Biomed. Sci. 2016, 23, 14. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Eriksson, J.E. Intermediate Filaments and the Regulation of Cell Motility during Regeneration and Wound Healing. Cold Spring Harb. Perspect. Biol. 2017, 9, a022046. [Google Scholar] [CrossRef]
- Walker, J.L.; Bleaken, B.M.; Romisher, A.R.; Alnwibit, A.A.; Menko, A.S. In wound repair vimentin mediates the transition of mesenchymal leader cells to a myofibroblast phenotype. Mol. Biol. Cell 2018, 29, 1555–1570. [Google Scholar] [CrossRef]
- Shigyo, M.; Tohda, C. Extracellular vimentin is a novel axonal growth facilitator for functional recovery in spinal cord-injured mice. Sci. Rep. 2016, 6, 28293. [Google Scholar] [CrossRef]
- Lam, F.W.; Da, Q.; Guillory, B.; Cruz, M.A. Recombinant Human Vimentin Binds to P-Selectin and Blocks Neutrophil Capture and Rolling on Platelets and Endothelium. J. Immunol. 2018, 200, 1718–1726. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.H.; Dai, Y.; Chen, S.; Wang, X.Q.; Yan, X.X.; Shen, Y.; Liu, J.; Yang, Z.K.; Hu, J.; Yu, L.J.; et al. Secretory vimentin is associated with coronary artery disease in patients and induces atherogenesis in ApoE(-/-) mice. Int. J. Cardiol. 2019, 283, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, A.; Barnes, P.F.; Porgador, A.; Roy, S.; Wu, S.; Nanda, J.S.; Griffith, D.E.; Girard, W.M.; Rawal, N.; Shetty, S.; et al. Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J. Immunol. 2006, 177, 6192–6198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linder, E.; Helin, H.; Chang, C.M.; Edgington, T.S. Complement-mediated binding of monocytes to intermediate filaments in vitro. Am. J. Pathol. 1983, 112, 267–277. [Google Scholar] [PubMed]
- Linder, E. Binding of C1q and complement activation by vascular endothelium. J. Immunol 1981, 126, 648–658. [Google Scholar]
- Hansson, G.K.; Starkebaum, G.A.; Benditt, E.P.; Schwartz, S.M. Fc-mediated binding of IgG to vimentin-type intermediate filaments in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1984, 81, 3103–3107. [Google Scholar] [CrossRef] [Green Version]
- Ise, H.; Goto, M.; Komura, K.; Akaike, T. Engulfment and clearance of apoptotic cells based on a GlcNAc-binding lectin-like property of surface vimentin. Glycobiology 2012, 22, 788–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teder, P.; Vandivier, R.W.; Jiang, D.; Liang, J.; Cohn, L.; Pure, E.; Henson, P.M.; Noble, P.W. Resolution of lung inflammation by CD44. Science 2002, 296, 155–158. [Google Scholar] [CrossRef]
- Podor, T.J.; Singh, D.; Chindemi, P.; Foulon, D.M.; McKelvie, R.; Weitz, J.I.; Austin, R.; Boudreau, G.; Davies, R. Vimentin exposed on activated platelets and platelet microparticles localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J. Biol. Chem. 2002, 277, 7529–7539. [Google Scholar] [CrossRef] [Green Version]
- Lazar, M.H.; Christensen, P.J.; Du, M.; Yu, B.; Subbotina, N.M.; Hanson, K.E.; Hansen, J.M.; White, E.S.; Simon, R.H.; Sisson, T.H. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am. J. Respir. Cell Mol. Biol. 2004, 31, 672–678. [Google Scholar] [CrossRef]
- Courey, A.J.; Horowitz, J.C.; Kim, K.K.; Koh, T.J.; Novak, M.L.; Subbotina, N.; Warnock, M.; Xue, B.; Cunningham, A.K.; Lin, Y.; et al. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood 2011, 118, 2313–2321. [Google Scholar] [CrossRef] [Green Version]
- Schuliga, M.; Grainge, C.; Westall, G.; Knight, D. The fibrogenic actions of the coagulant and plasminogen activation systems in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2018, 97, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Reales-Calderon, J.A.; Aguilera-Montilla, N.; Corbi, A.L.; Molero, G.; Gil, C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics 2014, 14, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, F.R.; Craig, J.M.; Singer, B.D.; Files, D.C.; Mock, J.R.; Garibaldi, B.T.; Fallica, J.; Tripathi, A.; Mandke, P.; Gans, J.H.; et al. Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L733–L746. [Google Scholar] [CrossRef] [PubMed]
- Gindele, J.A.; Mang, S.; Pairet, N.; Christ, I.; Gantner, F.; Schymeinsky, J.; Lamb, D.J. Opposing effects of in vitro differentiated macrophages sub-type on epithelial wound healing. PLoS ONE 2017, 12, e0184386. [Google Scholar] [CrossRef] [Green Version]
- Rogel, M.R.; Soni, P.N.; Troken, J.R.; Sitikov, A.; Trejo, H.E.; Ridge, K.M. Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells. FASEB J. 2011, 25, 3873–3883. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Ramos, I.; Fernandez-Sesma, A. Modulating the Innate Immune Response to Influenza A Virus: Potential Therapeutic Use of Anti-Inflammatory Drugs. Front. Immunol. 2015, 6, 361. [Google Scholar] [CrossRef] [Green Version]
- Stevens, C.; Henderson, P.; Nimmo, E.R.; Soares, D.C.; Dogan, B.; Simpson, K.W.; Barrett, J.C.; Wilson, D.C.; Satsangi, J. The intermediate filament protein, vimentin, is a regulator of NOD2 activity. Gut 2013, 62, 695–707. [Google Scholar] [CrossRef]
- De Rivero Vaccari, J.P.; Minkiewicz, J.; Wang, X.; De Rivero Vaccari, J.C.; German, R.; Marcillo, A.E.; Dietrich, W.D.; Keane, R.W. Astrogliosis involves activation of retinoic acid-inducible gene-like signaling in the innate immune response after spinal cord injury. Glia 2012, 60, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Ramos, I.; Bernal-Rubio, D.; Durham, N.; Belicha-Villanueva, A.; Lowen, A.C.; Steel, J.; Fernandez-Sesma, A. Effects of receptor binding specificity of avian influenza virus on the human innate immune response. J. Virol. 2011, 85, 4421–4431. [Google Scholar] [CrossRef] [Green Version]
- Ramos, I.; Fernandez-Sesma, A. Cell receptors for influenza a viruses and the innate immune response. Front. Microbiol. 2012, 3, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandakumar, V.; Hebrink, D.; Jenson, P.; Kottom, T.; Limper, A.H. Differential Macrophage Polarization from Pneumocystis in Immunocompetent and Immunosuppressed Hosts: Potential Adjunctive Therapy during Pneumonia. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.B.; Guerra, J.; Firek, A.; Langridge, W.H.R. Extracellular vimentin modulates human dendritic cell activation. Mol. Immunol. 2018, 104, 37–46. [Google Scholar] [CrossRef] [PubMed]
- McDonald-Hyman, C.; Muller, J.T.; Loschi, M.; Thangavelu, G.; Saha, A.; Kumari, S.; Reichenbach, D.K.; Smith, M.J.; Zhang, G.; Koehn, B.H.; et al. The vimentin intermediate filament network restrains regulatory T cell suppression of graft-versus-host disease. J. Clin. Investig. 2018, 128, 4604–4621. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.; Henttinen, T.; Merinen, M.; Marttila-Ichihara, F.; Eriksson, J.E.; Jalkanen, S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 2006, 8, 156–162. [Google Scholar] [CrossRef]
- Tsui, C.; Maldonado, P.; Montaner, B.; Borroto, A.; Alarcon, B.; Bruckbauer, A.; Martinez-Martin, N.; Batista, F.D. Dynamic reorganisation of intermediate filaments coordinates early B-cell activation. Life Sci. Alliance 2018, 1, e201800060. [Google Scholar] [CrossRef]
- Mayet, W.J.; Wandel, E.; Hermann, E.; Dumann, H.; Kohler, H. Antibodies to cytoskeletal components in patients undergoing long-term hemodialysis detected by a sensitive enzyme-linked immunosorbent assay (ELISA). Clin. Nephrol. 1990, 33, 272–278. [Google Scholar]
- Divanyan, T.; Acosta, E.; Patel, D.; Constantino, D.; Lopez-Soler, R.I. Anti-vimentin antibodies in transplant and disease. Hum. Immunol. 2019, 80, 602–607. [Google Scholar] [CrossRef]
- Tilleman, K.; Van Steendam, K.; Cantaert, T.; De Keyser, F.; Elewaut, D.; Deforce, D. Synovial detection and autoantibody reactivity of processed citrullinated isoforms of vimentin in inflammatory arthritides. Rheumatology 2008, 47, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Ytterberg, A.J.; Joshua, V.; Reynisdottir, G.; Tarasova, N.K.; Rutishauser, D.; Ossipova, E.; Haj Hensvold, A.; Eklund, A.; Skold, C.M.; Grunewald, J.; et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: Identification and validation. Ann. Rheum. Dis. 2015, 74, 1772–1777. [Google Scholar] [CrossRef] [Green Version]
- Bay-Jensen, A.C.; Karsdal, M.A.; Vassiliadis, E.; Wichuk, S.; Marcher-Mikkelsen, K.; Lories, R.; Christiansen, C.; Maksymowych, W.P. Circulating citrullinated vimentin fragments reflect disease burden in ankylosing spondylitis and have prognostic capacity for radiographic progression. Arthritis Rheum 2013, 65, 972–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, J.H.; Godskesen, L.E.; Jensen, M.D.; Van Haaften, W.T.; Klinge, L.G.; Olinga, P.; Dijkstra, G.; Kjeldsen, J.; Karsdal, M.A.; Bay-Jensen, A.C.; et al. Fragments of Citrullinated and MMP-degraded Vimentin and MMP-degraded Type III Collagen Are Novel Serological Biomarkers to Differentiate Crohn’s Disease from Ulcerative Colitis. J. Crohn’s Colitis 2015, 9, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.H.; van Haaften, W.T.; Karsdal, M.A.; Bay-Jensen, A.C.; Olinga, P.; Gronbaek, H.; Hvas, C.L.; Manon-Jensen, T.; Dijkstra, G.; Dige, A. The Citrullinated and MMP-degraded Vimentin Biomarker (VICM) Predicts Early Response to Anti-TNFalpha Treatment in Crohn’s Disease. J. Clin. Gastroenterol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.J.; Chang, A.; Ko, K.; Henry Dunand, C.J.; Henderson, S.; Maienschein-Cline, M.; Kaverina, N.; Rovin, B.H.; Salgado Ferrer, M.; Wolfgeher, D.; et al. Vimentin is a dominant target of in situ humoral immunity in human lupus tubulointerstitial nephritis. Arthritis Rheumatol. 2014, 66, 3359–3370. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Cascino, M.D.; Dai, J.; Bermea, R.S.; Ko, K.; Vesselits, M.; Dragone, L.L.; Mor Vaknin, N.; Legendre, M.; Markovitz, D.M.; et al. Anti-vimentin antibodies: A unique antibody class associated with therapy-resistant lupus nephritis. Lupus 2020, 29, 569–577. [Google Scholar] [CrossRef]
- Li, Y.; Jia, R.; Liu, Y.; Tang, S.; Ma, X.; Shi, L.; Zhao, J.; Hu, F.; Li, Z. Antibodies against carbamylated vimentin exist in systemic lupus erythematosus and correlate with disease activity. Lupus 2020, 29, 239–247. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Kaiser, Y.; Wolfgeher, D.; Ai, J.; Eklund, A.; Clark, M.R.; Grunewald, J. In Situ Humoral Immunity to Vimentin in HLA-DRB1*03(+) Patients With Pulmonary Sarcoidosis. Front. Immunol. 2018, 9, 1516. [Google Scholar] [CrossRef]
- Li, F.J.; Surolia, R.; Li, H.; Wang, Z.; Kulkarni, T.; Liu, G.; de Andrade, J.A.; Kass, D.J.; Thannickal, V.J.; Duncan, S.R.; et al. Autoimmunity to Vimentin Is Associated with Outcomes of Patients with Idiopathic Pulmonary Fibrosis. J. Immunol. 2017, 199, 1596–1605. [Google Scholar] [CrossRef] [Green Version]
- Ortona, E.; Capozzi, A.; Colasanti, T.; Conti, F.; Alessandri, C.; Longo, A.; Garofalo, T.; Margutti, P.; Misasi, R.; Khamashta, M.A.; et al. Vimentin/cardiolipin complex as a new antigenic target of the antiphospholipid syndrome. Blood 2010, 116, 2960–2967. [Google Scholar] [CrossRef] [Green Version]
- Oldstone, M.B. Molecular mimicry: Its evolution from concept to mechanism as a cause of autoimmune diseases. Monoclon. Antibodies Immunodiagn. Immunother. 2014, 33, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, W.; Seyer, J.M.; Beachey, E.H. Vimentin-cross-reactive epitope of type 12 streptococcal M protein. Infect. Immun. 1989, 57, 2457–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujinami, R.S.; Oldstone, M.B.; Wroblewska, Z.; Frankel, M.E.; Koprowski, H. Molecular mimicry in virus infection: Crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc. Natl. Acad. Sci. USA 1983, 80, 2346–2350. [Google Scholar] [CrossRef] [Green Version]
- Dales, S.; Fujinami, R.S.; Oldstone, M.B. Infection with vaccinia favors the selection of hybridomas synthesizing autoantibodies against intermediate filaments, one of them cross-reacting with the virus hemagglutinin. J. Immunol. 1983, 131, 1546–1553. [Google Scholar] [PubMed]
- Su, L.; Pan, P.; Yan, P.; Long, Y.; Zhou, X.; Wang, X.; Zhou, R.; Wen, B.; Xie, L.; Liu, D. Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis. Sci. Rep. 2019, 9, 5747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, Y.; Valdivia, R.H. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe 2008, 4, 159–169. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Kong, L.; Zhou, L.; Xia, J.; Wei, H.; Liu, M.; Peng, H. Host Cell Vimentin Restrains Toxoplasma gondii Invasion and Phosphorylation of Vimentin is Partially Regulated by Interaction with TgROP18. Int. J. Biol. Sci. 2017, 13, 1126–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoa, R.R.; Calderita, G.; Arranz, R.; Fontana, J.; Granzow, H.; Risco, C. Virus factories: Associations of cell organelles for viral replication and morphogenesis. Biol. Cell 2005, 97, 147–172. [Google Scholar] [CrossRef]
- Babrak, L.; Danelishvili, L.; Rose, S.J.; Kornberg, T.; Bermudez, L.E. The environment of “Mycobacterium avium subsp. hominissuis” microaggregates induces synthesis of small proteins associated with efficient infection of respiratory epithelial cells. Infect. Immun. 2015, 83, 625–636. [Google Scholar] [CrossRef] [Green Version]
- Mak, T.N.; Fischer, N.; Laube, B.; Brinkmann, V.; Metruccio, M.M.; Sfanos, K.S.; Mollenkopf, H.J.; Meyer, T.F.; Bruggemann, H. Propionibacterium acnes host cell tropism contributes to vimentin-mediated invasion and induction of inflammation. Cell Microbiol. 2012, 14, 1720–1733. [Google Scholar] [CrossRef]
- Broers, J.L.; de Leij, L.; Rot, M.K.; ter Haar, A.; Lane, E.B.; Leigh, I.M.; Wagenaar, S.S.; Vooijs, G.P.; Ramaekers, F.C. Expression of intermediate filament proteins in fetal and adult human lung tissues. Differ. Res. Biol. Divers. 1989, 40, 119–128. [Google Scholar] [CrossRef]
- Yang, Y.; Riccio, P.; Schotsaert, M.; Mori, M.; Lu, J.; Lee, D.K.; Garcia-Sastre, A.; Xu, J.; Cardoso, W.V. Spatial-Temporal Lineage Restrictions of Embryonic p63(+) Progenitors Establish Distinct Stem Cell Pools in Adult Airways. Dev. Cell 2018, 44, 752–761 e754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastounis, E.E.; Yeh, Y.T.; Theriot, J.A. Matrix stiffness modulates infection of endothelial cells by Listeria monocytogenes via expression of cell surface vimentin. Mol. Biol. Cell 2018, 29, 1571–1589. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Halvorsen, E.M.; Ammendolia, D.A.; Mor-Vaknin, N.; O’Riordan, M.X.D.; Brumell, J.H.; Markovitz, D.M.; Higgins, D.E. Invasion of the Brain by Listeria monocytogenes Is Mediated by InlF and Host Cell Vimentin. mBio 2018, 9, e00160-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.H.; Chi, F.; Peng, L.; Bo, T.; Zhang, B.; Liu, L.Q.; Wu, X.; Mor-Vaknin, N.; Markovitz, D.M.; Cao, H.; et al. Vimentin, a Novel NF-kappaB Regulator, Is Required for Meningitic Escherichia coli K1-Induced Pathogen Invasion and PMN Transmigration across the Blood-Brain Barrier. PLoS ONE 2016, 11, e0162641. [Google Scholar] [CrossRef]
- Guignot, J.; Servin, A.L. Maintenance of the Salmonella-containing vacuole in the juxtanuclear area: A role for intermediate filaments. Microb. Pathog. 2008, 45, 415–422. [Google Scholar] [CrossRef]
- Icenogle, L.M.; Hengel, S.M.; Coye, L.H.; Streifel, A.; Collins, C.M.; Goodlett, D.R.; Moseley, S.L. Molecular and biological characterization of Streptococcal SpyA-mediated ADP-ribosylation of intermediate filament protein vimentin. J. Biol. Chem. 2012, 287, 21481–21491. [Google Scholar] [CrossRef] [Green Version]
- Rohrbeck, A.; Schroder, A.; Hagemann, S.; Pich, A.; Holtje, M.; Ahnert-Hilger, G.; Just, I. Vimentin mediates uptake of C3 exoenzyme. PLoS ONE 2014, 9, e101071. [Google Scholar] [CrossRef] [Green Version]
- Rohrbeck, A.; Holtje, M.; Adolf, A.; Oms, E.; Hagemann, S.; Ahnert-Hilger, G.; Just, I. The Rho ADP-ribosylating C3 exoenzyme binds cells via an Arg-Gly-Asp motif. J. Biol. Chem. 2017, 292, 17668–17680. [Google Scholar] [CrossRef] [Green Version]
- Schafer, G.; Graham, L.M.; Lang, D.M.; Blumenthal, M.J.; Bergant Marusic, M.; Katz, A.A. Vimentin Modulates Infectious Internalization of Human Papillomavirus 16 Pseudovirions. J. Virol. 2017, 91, e00307–e00317. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Pante, N. Vimentin plays a role in the release of the influenza A viral genome from endosomes. Virology 2016, 497, 41–52. [Google Scholar] [CrossRef]
- Huang, S.Y.; Huang, C.H.; Chen, C.J.; Chen, T.W.; Lin, C.Y.; Lin, Y.T.; Kuo, S.M.; Huang, C.G.; Lee, L.A.; Chen, Y.H.; et al. Novel Role for miR-1290 in Host Species Specificity of Influenza A Virus. Mol. Ther. Nucleic Acids 2019, 17, 10–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, C.S.; Chu, J.J. Cellular vimentin regulates construction of dengue virus replication complexes through interaction with NS4A protein. J. Virol. 2014, 88, 1897–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risco, C.; Rodriguez, J.R.; Lopez-Iglesias, C.; Carrascosa, J.L.; Esteban, M.; Rodriguez, D. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J. Virol. 2002, 76, 1839–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Muller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Zhang, B.; Xu, J.; Chang, Q.; McNutt, M.A.; Korteweg, C.; Gong, E.; Gu, J. Molecular pathology in the lungs of severe acute respiratory syndrome patients. Am. J. Pathol. 2007, 170, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Mossel, E.C.; Wang, J.; Jeffers, S.; Edeen, K.E.; Wang, S.; Cosgrove, G.P.; Funk, C.J.; Manzer, R.; Miura, T.A.; Pearson, L.D.; et al. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology 2008, 372, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; O’Meara, M.J.; Guo, J.Z.; Swaney, D.L.; Tummino, T.A.; Hüttenhain, R.; et al. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. BioRxiv 2020, 10.1101. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Shalek, A.K.; Ordovas-Montanes, J.; Network, H.L.B. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020, 181, 1016–1035. [Google Scholar] [CrossRef] [PubMed]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasper, M.; Stosiek, P. The expression of vimentin in epithelial cells from human nasal mucosa. Eur. Arch. Otorhinolaryngol. 1990, 248, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, C.B.; Jhanji, V.; Xu, C.; Yuan, X.L.; Liang, J.J.; Huang, Y.; Cen, L.P.; Ng, T.K. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye 2020, 34, 1212–1219. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904. [Google Scholar] [CrossRef]
- Yang, J.; Zou, L.; Yang, Y.; Yuan, J.; Hu, Z.; Liu, H.; Peng, H.; Shang, W.; Zhang, X.; Zhu, J.; et al. Superficial vimentin mediates DENV-2 infection of vascular endothelial cells. Sci. Rep. 2016, 6, 38372. [Google Scholar] [CrossRef]
- Liang, J.J.; Yu, C.Y.; Liao, C.L.; Lin, Y.L. Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell Microbiol. 2011, 13, 1358–1370. [Google Scholar] [CrossRef]
- Das, S.K.; Gupta, I.; Cho, Y.K.; Zhang, X.; Uehara, H.; Muddana, S.K.; Bernhisel, A.A.; Archer, B.; Ambati, B.K. Vimentin knockdown decreases corneal opacity. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4030–4040. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Fahad, A.M.; Shanmukhappa, K.; Kapil, S. Defining the cellular target(s) of porcine reproductive and respiratory syndrome virus blocking monoclonal antibody 7G10. J. Virol. 2006, 80, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koudelka, K.J.; Destito, G.; Plummer, E.M.; Trauger, S.A.; Siuzdak, G.; Manchester, M. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog. 2009, 5, e1000417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavathekar, V.K.; Dhanavade, M.J.; Sonawane, K.D.; Balakrishnan, A. Role of cell surface vimentin in Chandipura virus replication in Neuro-2a cells. Virus Res. 2020, 285, 198014. [Google Scholar] [CrossRef]
- Turkki, P.; Laajala, M.; Flodstrom-Tullberg, M.; Marjomaki, V. Human Enterovirus Group B Viruses Rely on Vimentin Dynamics for Efficient Processing of Viral Nonstructural Proteins. J. Virol. 2020, 94, e01393-01319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Ling, Y.; Li, P.; Sun, P.; Cao, Y.; Bai, X.; Li, K.; Fu, Y.; Zhang, J.; Li, D.; et al. Cellular Vimentin Interacts with Foot-and-Mouth Disease Virus Nonstructural Protein 3A and Negatively Modulates Viral Replication. J. Virol. 2020, JVI.00273-20. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, S.; Windsor, M.; Nagata, K.I.; Inagaki, M.; Wileman, T. Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II. J. Virol. 2005, 79, 11766–11775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitahara-Kasahara, Y.; Fukasawa, M.; Shinkai-Ouchi, F.; Sato, S.; Suzuki, T.; Murakami, K.; Wakita, T.; Hanada, K.; Miyamura, T.; Nishijima, M. Cellular vimentin content regulates the protein level of hepatitis C virus core protein and the hepatitis C virus production in cultured cells. Virology 2009, 383, 319–327. [Google Scholar] [CrossRef] [Green Version]
- DeBoer, J.; Wojtkiewicz, M.S.; Haverland, N.; Li, Y.; Harwood, E.; Leshen, E.; George, J.W.; Ciborowski, P.; Belshan, M. Proteomic profiling of HIV-infected T-cells by SWATH mass spectrometry. Virology 2018, 516, 246–257. [Google Scholar] [CrossRef]
- Fernandez-Ortega, C.; Ramirez, A.; Casillas, D.; Paneque, T.; Ubieta, R.; Dubed, M.; Navea, L.; Castellanos-Serra, L.; Duarte, C.; Falcon, V.; et al. Identification of Vimentin as a Potential Therapeutic Target against HIV Infection. Viruses 2016, 8, 98. [Google Scholar] [CrossRef]
- Snasel, J.; Shoeman, R.; Horejsi, M.; Hruskova-Heidingsfeldova, O.; Sedlacek, J.; Ruml, T.; Pichova, I. Cleavage of vimentin by different retroviral proteases. Arch. Biochem. Biophys. 2000, 377, 241–245. [Google Scholar] [CrossRef]
- Alldridge, L.C.; O’Farrell, M.K.; Dealtry, G.B. Interferon beta increases expression of vimentin at the messenger RNA and protein levels in differentiated embryonal carcinoma (PSMB) cells. Exp. Cell Res. 1989, 185, 387–393. [Google Scholar] [CrossRef]
- Lv, N.; Gao, Y.; Guan, H.; Wu, D.; Ding, S.; Teng, W.; Shan, Z. Inflammatory mediators, tumor necrosis factor-alpha and interferon-gamma, induce EMT in human PTC cell lines. Oncol. Lett. 2015, 10, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.; Leube, R.E. Epithelial Intermediate Filaments: Guardians against Microbial Infection? Cells 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denes, C.E.; Miranda-Saksena, M.; Cunningham, A.L.; Diefenbach, R.J. Cytoskeletons in the Closet-Subversion in Alphaherpesvirus Infections. Viruses 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridge, K.M.; Shumaker, D.; Robert, A.; Hookway, C.; Gelfand, V.I.; Janmey, P.A.; Lowery, J.; Guo, M.; Weitz, D.A.; Kuczmarski, E.; et al. Methods for Determining the Cellular Functions of Vimentin Intermediate Filaments. Methods Enzym. 2016, 568, 389–426. [Google Scholar] [CrossRef] [Green Version]
- Strouhalova, K.; Prechova, M.; Gandalovicova, A.; Brabek, J.; Gregor, M.; Rosel, D. Vimentin Intermediate Filaments as Potential Target for Cancer Treatment. Cancers 2020, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Weidle, U.H.; Maisel, D.; Klostermann, S.; Schiller, C.; Weiss, E.H. Intracellular Proteins Displayed on the Surface of Tumor Cells as Targets for Therapeutic Intervention with Antibody-related Agents. J. Cell Sci. 2011, 8, 49–64. [Google Scholar]
- Noh, H.; Yan, J.; Hong, S.; Kong, L.Y.; Gabrusiewicz, K.; Xia, X.; Heimberger, A.B.; Li, S. Discovery of cell surface vimentin targeting mAb for direct disruption of GBM tumor initiating cells. Oncotarget 2016, 7, 72021–72032. [Google Scholar] [CrossRef] [Green Version]
- Babic, I.; Nurmemmedov, E.; Yenugonda, V.M.; Juarez, T.; Nomura, N.; Pingle, S.C.; Glassy, M.C.; Kesari, S. Pritumumab, the first therapeutic antibody for glioma patients. Hum. Antibodies 2018, 26, 95–101. [Google Scholar] [CrossRef]
- Hugwil, A.V. The meaning of the anti-cancer antibody CLN-IgG (Pritumumab) generated by human x human hybridoma technology against the cyto-skeletal protein, vimentin, in the course of the treatment of malignancy. Med. Hypotheses 2013, 81, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Huet, D.; Bagot, M.; Loyaux, D.; Capdevielle, J.; Conraux, L.; Ferrara, P.; Bensussan, A.; Marie-Cardine, A. SC5 mAb represents a unique tool for the detection of extracellular vimentin as a specific marker of Sezary cells. J. Immunol. 2006, 176, 652–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 2848–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.; Ha, Y.; Ha, S.K.; Han, D.U.; Kim, D.W.; Moon, W.K.; Chae, C. Antiviral effect of Saccharomyces cerevisiae beta-glucan to swine influenza virus by increased production of interferon-gamma and nitric oxide. J. Vet. Med. BInfect. Dis. Vet. Public Health 2004, 51, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Higashino-Kameda, M.; Yabe-Wada, T.; Matsuba, S.; Takeda, K.; Anzawa, K.; Mochizuki, T.; Makimura, K.; Saijo, S.; Iwakura, Y.; Toga, H.; et al. A critical role of Dectin-1 in hypersensitivity pneumonitis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2016, 65, 235–244. [Google Scholar] [CrossRef]
- Mohan, R.; Bargagna-Mohan, P. The Use of Withaferin A to Study Intermediate Filaments. Methods Enzym. 2016, 568, 187–218. [Google Scholar] [CrossRef]
- Kaschula, C.H.; Tuveri, R.; Ngarande, E.; Dzobo, K.; Barnett, C.; Kusza, D.A.; Graham, L.M.; Katz, A.A.; Rafudeen, M.S.; Parker, M.I.; et al. The garlic compound ajoene covalently binds vimentin, disrupts the vimentin network and exerts anti-metastatic activity in cancer cells. BMC Cancer 2019, 19, 248. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, S.; Choi, B.Y.; Choi, H.S.; Kang, B.S.; Bode, A.M.; Dong, Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 2005, 280, 16882–16890. [Google Scholar] [CrossRef] [Green Version]
- Miyai, S.; Yamaguchi, A.; Iwasaki, T.; Shamsa, F.; Ohtsuki, K. Biochemical characterization of epigallocatechin-3-gallate as an effective stimulator for the phosphorylation of its binding proteins by glycogen synthase kinase-3beta in vitro. Biol. Pharm. Bull. 2010, 33, 1932–1937. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S. Compounds Derived from Epigallocatechin-3-Gallate (EGCG) as a Novel Approach to the Prevention of Viral Infections. Inflamm. Allergy Drug Targets 2015, 14, 13–18. [Google Scholar] [CrossRef]
- Yue, Q.; Feng, L.; Cao, B.; Liu, M.; Zhang, D.; Wu, W.; Jiang, B.; Yang, M.; Liu, X.; Guo, D. Proteomic Analysis Revealed the Important Role of Vimentin in Human Cervical Carcinoma HeLa Cells Treated With Gambogic Acid. Mol. Cell Proteom. 2016, 15, 26–44. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Song, X.Y.; Yue, Q.X.; Cui, Y.J.; Liu, M.; Feng, L.X.; Wu, W.Y.; Jiang, B.H.; Yang, M.; Qu, X.B.; et al. Proteomic and bioinformatic analyses of possible target-related proteins of gambogic acid in human breast carcinoma MDA-MB-231 cells. Chin. J. Nat. Med. 2015, 13, 41–51. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, G.; Ji, Y.; Zhua, H.; Lv, C.; Jiang, W. Protective role of gambogic acid in experimental pulmonary fibrosis in vitro and in vivo. Phytomed.: Int. J. Phytother. Phytopharm. 2016, 23, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Trogden, K.P.; Battaglia, R.A.; Kabiraj, P.; Madden, V.J.; Herrmann, H.; Snider, N.T. An image-based small-molecule screen identifies vimentin as a pharmacologically relevant target of simvastatin in cancer cells. FASEB J. 2018, 32, 2841–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolova, M.; Tawab, A.; Marie-Cardine, A.; Bagot, M.; Boumsell, L.; Bensussan, A. Increased expression of a novel early activation surface membrane receptor in cutaneous T cell lymphoma cells. J. Investig. Derm. 2001, 116, 731–738. [Google Scholar] [CrossRef]
- Glassy, M.C.; Hagiwara, H. Summary analysis of the pre-clinical and clinical results of brain tumor patients treated with pritumumab. Hum. Antibodies 2009, 18, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Sager, P.R.; Matheson, D.W. Mechanisms of neurotoxicity related to selective disruption of microtubules and intermediate filaments. Toxicology 1988, 49, 479–492. [Google Scholar] [CrossRef]
- Arocena, M. Effect of acrylamide on the cytoskeleton and apoptosis of bovine lens epithelial cells. Cell Biol. Int. 2006, 30, 1007–1012. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Scruggs, J.L.; Herrington, J.; Welsh, M.J.; Carter-Su, C.; Housley, P.R.; Pratt, W.B. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol. Endocrinol. 1998, 12, 1903–1913. [Google Scholar] [CrossRef]
- Stamatakis, K.; Sánchez-Gómez, F.J.; Pérez-Sala, D. Identification of novel protein targets for modification by 15-deoxy-Δ12,14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J. Am. Soc. Nephrol. 2006, 17, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.G. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 1997, 5, 276–281. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Hamza, A.; Kim, Y.E.; Khuan Abby Ho, Y.; Mor-Vaknin, N.; Wendschlag, N.; Liu, J.; Evans, R.M.; Markovitz, D.M.; Zhan, C.G.; et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 2007, 14, 623–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grin, B.; Mahammad, S.; Wedig, T.; Cleland, M.M.; Tsai, L.; Herrmann, H.; Goldman, R.D. Withaferin a alters intermediate filament organization, cell shape and behavior. PLoS ONE 2012, 7, e39065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yan, W.; Li, Y.; Niu, L.; Ye, H.; Chen, L. The Natural Compound Withaferin A Covalently Binds to Cys239 of beta-Tubulin to Promote Tubulin Degradation. Mol. Pharm. 2019, 96, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Bollong, M.J.; Pietila, M.; Pearson, A.D.; Sarkar, T.R.; Ahmad, I.; Soundararajan, R.; Lyssiotis, C.A.; Mani, S.A.; Schultz, P.G.; Lairson, L.L. A vimentin binding small molecule leads to mitotic disruption in mesenchymal cancers. Proc. Natl. Acad. Sci. USA 2017, 114, E9903–E9912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sala, D.; Mollinedo, F. Inhibition of isoprenoid biosynthesis induces apoptosis in human promyelocytic HL-60 cells. Biochem. Biophys. Res. Commun. 1994, 199, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sala, D.; Collado-Escobar, D.; Mollinedo, F. Intracellular Alkalinization Suppresses Lovastatin-induced Apoptosis in HL-60 Cells through the Inactivation of a pH-dependent Endonuclease. J. Biol. Chem. 1995, 270, 6235–6242. [Google Scholar] [CrossRef] [Green Version]
- Kanugula, A.K.; Dhople, V.M.; Volker, U.; Ummanni, R.; Kotamraju, S. Fluvastatin mediated breast cancer cell death: A proteomic approach to identify differentially regulated proteins in MDA-MB-231 cells. PLoS ONE 2014, 9, e108890. [Google Scholar] [CrossRef]
- Fedson, D.S.; Opal, S.M.; Rordam, O.M. Hiding in Plain Sight: An Approach to Treating Patients with Severe COVID-19 Infection. mBio 2020, 11, e00398-00320. [Google Scholar] [CrossRef] [Green Version]
- Esposito, A.M.; Cheung, P.; Swartz, T.H.; Li, H.; Tsibane, T.; Durham, N.D.; Basler, C.F.; Felsenfeld, D.P.; Chen, B.K. A high throughput Cre-lox activated viral membrane fusion assay identifies pharmacological inhibitors of HIV entry. Virology 2016, 490, 6–16. [Google Scholar] [CrossRef]
- Españo, E.; Nam, J.H.; Song, E.J.; Song, D.; Lee, C.K.; Kim, J.K. Lipophilic statins inhibit Zika virus production in Vero cells. Sci. Rep. 2019, 9, 11461. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Broedersz, C.P.; Rowat, A.C.; Wedig, T.; Herrmann, H.; Mackintosh, F.C.; Weitz, D.A. Divalent cations crosslink vimentin intermediate filament tail domains to regulate network mechanics. J. Mol. Biol. 2010, 399, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Monico, A.; Zorrilla, S.; Rivas, G.; Perez-Sala, D. Zinc Differentially Modulates the Assembly of Soluble and Polymerized Vimentin. Int. J. Mol. Sci. 2020, 21, 2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Cao, Y.; Su, W.; Huang, S.; Lu, W.; Zhou, Y.; Gao, J.; Zhao, W.; Zhang, B.; Wu, X. Enterovirus A71 VP1 Variation A289T Decreases the Central Nervous System Infectivity via Attenuation of Interactions between VP1 and Vimentin In Vitro and In Vivo. Viruses 2019, 11, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; He, S.; Waheed, A.A.; Dabbagh, D.; Zhou, Z.; Trinite, B.; Wang, Z.; Yu, J.; Wang, D.; Li, F.; et al. PSGL-1 restricts HIV-1 infectivity by blocking virus particle attachment to target cells. Proc. Natl. Acad. Sci. USA 2020, 117, 9537–9545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamay, T.N.; Kolovskaya, O.S.; Glazyrin, Y.E.; Zamay, G.S.; Kuznetsova, S.A.; Spivak, E.A.; Wehbe, M.; Savitskaya, A.G.; Zubkova, O.A.; Kadkina, A.; et al. DNA-aptamer targeting vimentin for tumor therapy in vivo. Nucleic Acid Ther. 2014, 24, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Jalalian, S.H.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Targeted co-delivery of epirubicin and NAS-24 aptamer to cancer cells using selenium nanoparticles for enhancing tumor response in vitro and in vivo. Cancer Lett. 2018, 416, 87–93. [Google Scholar] [CrossRef]
- Yoon, S.; Armstrong, B.; Habib, N.; Rossi, J.J. Blind SELEX Approach Identifies RNA Aptamers That Regulate EMT and Inhibit Metastasis. Mol. Cancer Res. MCR 2017, 15, 811–820. [Google Scholar] [CrossRef] [Green Version]


| Macromolecules | Clinical Use | Putative Effect | Specificity for Vimentin | References |
|---|---|---|---|---|
| Expression vectors, wt and mutants/fragments | Mimic/inhibit | Very high | [6] | |
| siRNAs | Inhibit expression | High | [20,129] | |
| Recombinant vimentin and fragments | Mimic/compete vimentin release or exposure | High | [44,66] | |
| Soluble CD44 | Compete for vimentin binding | High | [44] | |
| Pritumumab (anti-vimentin mAb) | Clinic, Phase II | Membrane vimentin binding | Very high | [169,170] |
| SC5 anti-vimentin mAb | Membrane vimentin binding | Very high | [171] | |
| Anti-Cell surface vimentin (CSV) 86C mAb | Membrane vimentin binding and internalization | Very high | [168] | |
| Anti-citrullinated Vimentin antibodies | Diagnostic | Biomarker | Very high | [19] |
| Hyaluronic acid (CTX-100) | Phase II | Compete with vimentin for CD44 | Moderate- Low | NCT00993707 * |
| PEGPH20 (Pegylated Hyaluronidase) | Phase I | Reduce hyaluronan levels | Moderate- Low | [172] |
| ß-glucans (Proglucamune) | Dietary supplement | Dectin-1 agonist | Low | [173,174] |
| Dectin-1 blocking antibodies | Block Dectin-1 signals | High | [62] | |
| Small Molecules | Clinical Use | Putative Effect | Specificity for Vimentin | References |
| Withaferin A | Withania Somnifera extract (WSE; Sensoril®) | Reduce vimentin levels, binds region of C328, phosphorylation | Moderate | [16,175] |
| Ajoene | Garlic oil & pure studies | Disrupt vimentin network and functions, bind C328 | Low | [176] |
| Epigallocathechin gallate | Dietary supplement trials | Inhibit vimentin phosphorylation | Low | [177,178,179] |
| Gambogic acid | Traditional Asian medicine | Vimentin cleavage | Low | [180,181,182] |
| Simvastatin | Clinic | Vimentin distribution; viral entry inhibition; anti-inflammatory | Low | [183] NCT04348695 * |
| Cantharidin | Phase I-IV | Vimentin distribution; antiviral | Low | [183] |
| Carvedilol | Phase I-IV | Vimentin distribution | Low | [183] |
| Ivermectin | Phase I-IV | Vimentin distribution; COVID-19 and Dengue treatment | Low | [183] NCT04343092 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134675
Ramos I, Stamatakis K, Oeste CL, Pérez-Sala D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. International Journal of Molecular Sciences. 2020; 21(13):4675. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134675
Chicago/Turabian StyleRamos, Irene, Konstantinos Stamatakis, Clara L. Oeste, and Dolores Pérez-Sala. 2020. "Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections" International Journal of Molecular Sciences 21, no. 13: 4675. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134675