A Dipeptidyl Peptidase-4 Inhibitor Inhibits Foam Cell Formation of Macrophages in Type 1 Diabetes via Suppression of CD36 and ACAT-1 Expression
Abstract
:1. Introduction
2. Results
2.1. Characteristics and Laboratory Data of Mice and Humans
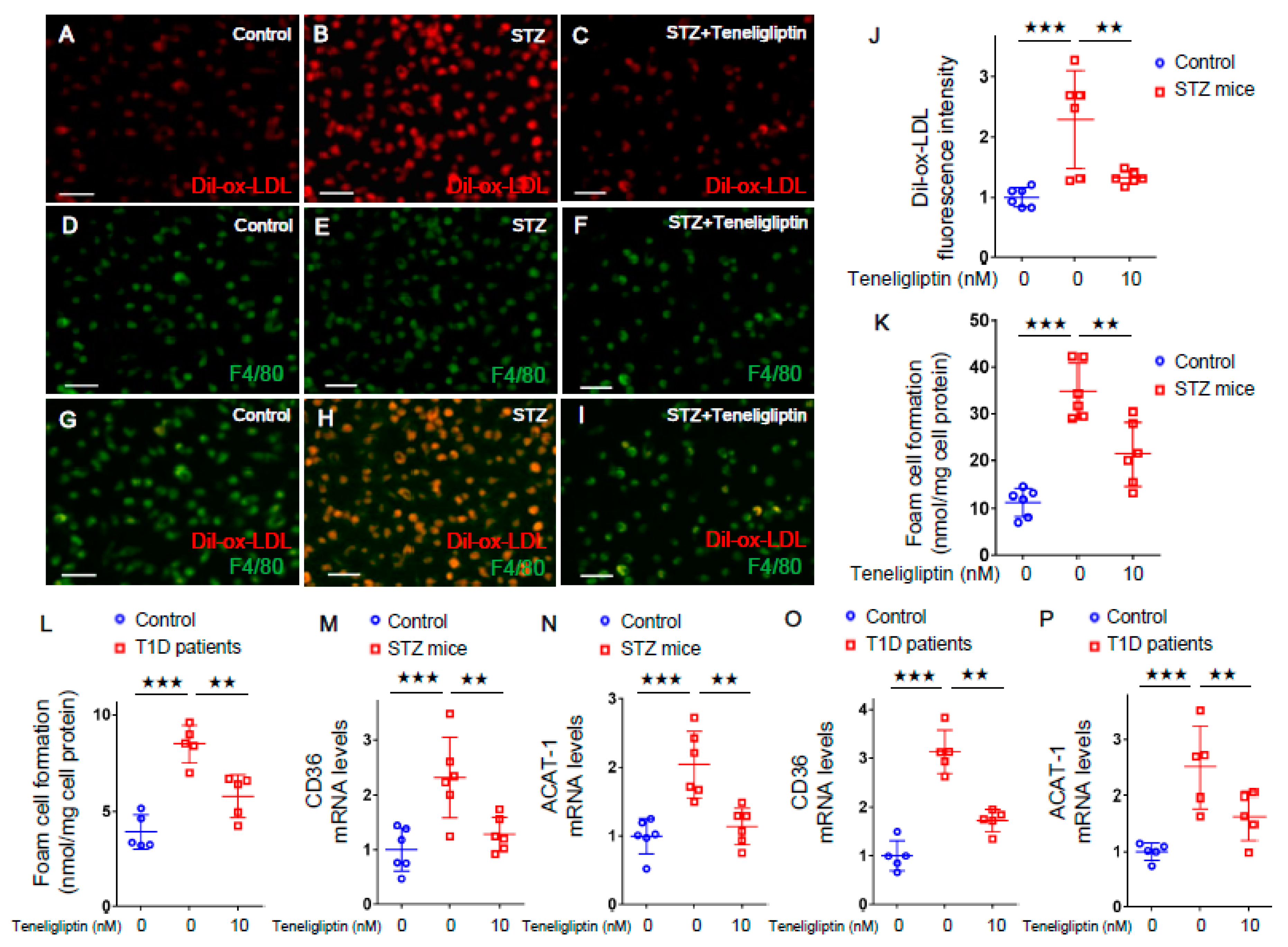
2.2. Teneligliptin Suppressed Foam Cell Formation of Macrophages Isolated from T1D Mice and T1D Patients
2.3. Teneligliptin Inhibited ox-LDL Uptake in AGE-exposed Mouse Macrophages and Human THP-1 Macrophages
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Measurements of Laboratory Parameters in Mice
4.3. Experiments of Human Macrophages
4.4. Measurements of Clinical Parameters in Humans
4.5. Preparation of AGE-BSA
4.6. Differentiation of THP-1 Macrophages
4.7. Immunofluorescent Staining of Mouse Macrophages and THP-1 Macrophages
4.8. Cholesterol Esterification Assay in Macrophages Isolated from Mice and Humans
4.9. Gene Expression Levels in Macrophages Isolated from T1D Mice and T1D Patients and in AGE-Exposed Cells
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclosure
Data Availability Statement
References
- Rao Kondapally Seshasai, S.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar] [PubMed] [Green Version]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Kilhovd, B.K.; Juutilainen, A.; Lehto, S.; Rönnemaa, T.; Torjesen, P.A.; Hanssen, K.F.; Laakso, M. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: A population-based 18 year follow-up study. Diabetologia 2007, 50, 1409–1417. [Google Scholar] [CrossRef] [Green Version]
- Kilhovd, B.K.; Juutilainen, A.; Lehto, S.; Rönnemaa, T.; Torjesen, P.A.; Hanssen, K.F.; Laakso, M. Increased serum levels of methylglyoxal-derived hydroimidazolone-AGE are associated with increased cardiovascular disease mortality in nondiabetic women. Atherosclerosis 2009, 205, 590–594. [Google Scholar] [CrossRef]
- Kume, S.; Takeya, M.; Mori, T.; Araki, N.; Suzuki, H.; Horiuchi, S.; Kodama, T.; Miyauchi, Y.; Takahashi, K. Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am. J. Pathol. 1995, 147, 654–667. [Google Scholar]
- Tahara, N.; Yamagishi, S.; Takeuchi, M.; Honda, A.; Tahara, A.; Nitta, Y.; Kodama, N.; Mizoguchi, M.; Kaida, H.; Ishibashi, M.; et al. Positive association between serum level of glyceraldehyde-derived advanced glycation end products and vascular inflammation evaluated by [(18)F]fluorodeoxyglucose positron emission tomography. Diabetes Care 2012, 35, 2618–2625. [Google Scholar] [CrossRef] [Green Version]
- Kajikawa, M.; Nakashima, A.; Fujimura, N.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Matsumoto, T.; Oda, N.; Hidaka, T.; Kihara, Y.; et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care 2015, 38, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Nin, J.W.; Jorsal, A.; Ferreira, I.; Schalkwijk, C.G.; Prins, M.H.; Parving, H.H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12-year follow-up study. Diabetes Care 2011, 34, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, N.M.; Beulens, J.W.; van Dieren, S.; Scheijen, J.L.; Daphne van der, A.; Spijkerman, A.M.; van der Schouw, Y.T.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: A case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes 2015, 64, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Tahara, N.; Yamagishi, S.; Matsui, T.; Nishino, Y.; Honda, A.; Tahara, A.; Igata, S.; Fukumoto, Y. Serum levels of pigment epithelium-derived factor (PEDF) are inversely associated with circulating levels of dipeptidyl peptidase-4 (DPP-4) in humans. Int. J. Cardiol. 2015, 184, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Pannu, P.S.; Francis, G.A. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc. Res. 2012, 95, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasaki, M.; Hiromura, M.; Mori, Y.; Kohashi, K.; Kushima, H.; Koshibu, M.; Saito, T.; Yashima, H.; Watanabe, T.; Hirano, T. A dipeptidyl peptidase-4 inhibitor suppresses macrophage foam cell formation in diabetic db/db mice and type 2 diabetes patients. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuhara-Takaki, K.; Sakai, M.; Sakamoto, Y.; Takeya, M.; Horiuchi, S. Expression of class A scavenger receptor is enhanced by high glucose in vitro and under diabetic conditions in vivo: One mechanism for an increased rate of atherosclerosis in diabetes. J. Biol. Chem. 2005, 280, 3355–3364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Sawamura, T.; Renier, G. Glucose enhances human macrophage LOX-1 expression: Role for LOX-1 in glucose-induced macrophage foam cell formation. Circ. Res. 2004, 94, 892–901. [Google Scholar] [CrossRef]
- Terasaki, M.; Nagashima, M.; Nohtomi, K.; Kohashi, K.; Tomoyasu, M.; Sinmura, K.; Nogi, Y.; Katayama, Y.; Sato, K.; Itoh, F.; et al. Preventive effect of dipeptidyl peptidase-4 inhibitor on atherosclerosis is mainly attributable to incretin's actions in nondiabetic and diabetic apolipoprotein E-null mice. PLoS ONE 2013, 8, e70933. [Google Scholar] [CrossRef]
- Yamagishi, S.; Fukami, K.; Matsui, T. Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc. Diabetol. 2015, 14, 2. [Google Scholar] [CrossRef] [Green Version]
- Ta, N.N.; Schuyler, C.A.; Li, Y.; Lopes-Virella, M.F.; Huang, Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 2011, 58, 157–166. [Google Scholar] [CrossRef]
- Yang, T.Y.; Liaw, Y.P.; Huang, J.Y.; Chang, H.R.; Chang, K.W.; Ueng, K.C. Association of Sitagliptin with cardiovascular outcome in diabetic patients: A nationwide cohort study. Acta Diabetol. 2016, 53, 461–468. [Google Scholar] [CrossRef]
- Mita, T.; Katakami, N.; Shiraiwa, T.; Yoshii, H.; Onuma, T.; Kuribayashi, N.; Osonoi, T.; Kaneto, H.; Kosugi, K.; Umayahara, Y.; et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: The Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE): A randomized controlled trial. Diabetes Care 2016, 39, 455–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mita, T.; Katakami, N.; Yoshii, H.; Onuma, T.; Kaneto, H.; Osonoi, T.; Shiraiwa, T.; Kosugi, K.; Umayahara, Y.; Yamamoto, T.; et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: The Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A). Diabetes Care 2016, 39, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Nishino, Y.; Takeuchi, M.; Yamagishi, S. Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol. Res. 2011, 63, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Maeda, S.; Higashimoto, Y.; Yamagishi, S. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc. Diabetol. 2013, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Nakashima, S.; Nishino, Y.; Ojima, A.; Nakamura, N.; Arima, K.; Fukami, K.; Okuda, S.; Yamagishi, S. Dipeptidyl peptidase-4 deficiency protects against experimental diabetic nephropathy partly by blocking the advanced glycation end products-receptor axis. Lab. Invest. 2015, 95, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Sitagliptin augments protective effects of GLP-1 against advanced glycation end product receptor axis in endothelial cells. Horm. Metab. Res. 2011, 43, 731–734. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. SAVOR-TIMI 53 steering committee and investigators. saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. TECOS study group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. CARMELINA investigators. Effect of linagliptin vs. placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA randomized clinical trial. J. Am. Med. Assoc. 2019, 321, 69–79. [Google Scholar] [CrossRef]
- Terasaki, M.; Nagashima, M.; Watanabe, T.; Nohtomi, K.; Mori, Y.; Miyazaki, A.; Hirano, T. Effects of PKF275-055, a dipeptidyl peptidase-4 inhibitor, on the development of atherosclerotic lesions in apolipoprotein E-null mice. Metabolism 2012, 61, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Takahashi, Y.; Tezuka, K.; Akimoto, H.; Nakayama, T.; Asai, S. Comparative effect of dipeptidyl-peptidase 4 inhibitors on laboratory parameters in patients with diabetes mellitus. BMC Pharmacol. Toxicol. 2020, 21, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, S.; Kinoshita, Y.; Matsumoto, T.; Maruhashi, T.; Kajikawa, M.; Matsui, S.; Hashimoto, H.; Takaeko, Y.; Kihara, Y.; Chayama, K.; et al. Effects of the dipeptidyl peptidase 4 inhibitor alogliptin on blood pressure in hypertensive patients with type 2 diabetes mellitus. Am. J. Hypertens. 2019, 32, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T. Pleiotropic effects of glucagon-like peptide-1 (GLP-1)-based therapies on vascular complications in diabetes. Curr. Pharm. Des. 2011, 17, 4379–4385. [Google Scholar] [CrossRef] [PubMed]
- Ojima, A.; Matsui, T.; Maeda, S.; Takeuchi, M.; Yamagishi, S. Glucose-dependent insulinotropic polypeptide (GIP) inhibits signaling pathways of advanced glycation end products (AGEs) in endothelial cells via its antioxidative properties. Horm. Metab. Res. 2012, 44, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Watanabe, T.; Terasaki, M.; Tomoyasu, M.; Nohtomi, K.; Kim-Kaneyama, J.; Miyazaki, A.; Hirano, T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011, 54, 2649–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogi, Y.; Nagashima, M.; Terasaki, M.; Nohtomi, K.; Watanabe, T.; Hirano, T. Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein E-null mice. PLoS ONE 2012, 7, e35683. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.; Ding, Z.; Dai, D.; Mehta, J.L. DPP-4 inhibitors repress foam cell formation by inhibiting scavenger receptors through protein kinase C pathway. Acta Diabetol. 2014, 51, 471–478. [Google Scholar] [CrossRef]
- Halabi, A.; Maatouk, H.; Siegler, K.E.; Faisst, N.; Lufft, V.; Klause, N. Pharmacokinetics of teneligliptin in subjects with renal impairment. Clin. Pharmacol. Drug Dev. 2013, 2, 246–254. [Google Scholar] [CrossRef]
- Kaifu, K.; Ueda, S.; Nakamura, N.; Matsui, T.; Yamada-Obara, N.; Ando, R.; Kaida, Y.; Nakata, M.; Matsukuma-Toyonaga, M.; Higashimoto, Y.; et al. Advanced glycation end products evoke inflammatory reactions in proximal tubular cells via autocrine production of dipeptidyl peptidase. Microvasc. Res. 2018, 120, 90–93. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Wouters, K.; Huijberts, M.S.; Gijbels, M.J.; Sluimer, J.C.; Scheijen, J.L.; Heeneman, S.; Biessen, E.A.; Daemen, M.J.; Brownlee, M.; et al. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype. Eur. Heart J. 2014, 35, 1137–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Bandinelli, S.; Sun, K.; Guralnik, J.M.; Ferrucci, L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J. Am. Geriatr. Soc. 2009, 57, 1874–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Beck, J.; Dalal, M.; Varadhan, R.; Walston, J.; Guralnik, J.M.; Fried, L.P. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin. Exp. Res. 2009, 21, 182–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Waateringe, R.P.; Fokkens, B.T.; Slagter, S.N.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Smit, A.J.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Skin autofluorescence predicts incident type 2 diabetes, cardiovascular disease and mortality in the general population. Diabetologia 2019, 62, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S. Role of Advanced Glycation Endproduct (AGE)-Receptor for Advanced Glycation Endproduct (RAGE) axis in cardiovascular disease and its therapeutic intervention. Circ. J. 2019, 83, 1822–1828. [Google Scholar] [CrossRef] [Green Version]
- Sakata, K.; Hayakawa, M.; Yano, Y.; Tamaki, N.; Yokota, N.; Eto, T.; Watanabe, R.; Hirayama, N.; Matsuo, T.; Kuroki, K.; et al. Efficacy of alogliptin, a dipeptidyl peptidase-4 inhibitor, on glucose parameters, the activity of the advanced glycation end product (AGE)-Receptor for AGE (RAGE) axis and albuminuria in Japanese type 2 diabetes. Diabetes Metab. Res. Rev. 2013, 29, 624–630. [Google Scholar] [CrossRef]
- Yamagishi, S. Sex disparity in cardiovascular mortality rates associated with diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3059. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.R.; Li, P.C.; Feng, B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: Increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis. 2016, 15, 161. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Nakamura, N.; Suematsu, M.; Kaseda, K.; Matsui, T. Advanced glycation end products: A molecular target for vascular complications in diabetes. Mol. Med. 2015, 21, S32–S40. [Google Scholar] [CrossRef]
- Takeuchi, M.; Makita, Z.; Yanagisawa, K.; Kameda, K.; Koike, T. Detection of noncarboxymethyllysine and carboxymethyllysine advanced glycation end products (AGE) in serum of diabetic patients. Mol. Med. 1999, 5, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Joo, J.; Jeon, Y.; Lee, J.; In, J.; Kim, D.; Kang, E.; Kim, Y.; Lim, Y.; Kang, J.; et al. Advanced glycation end products downregulate glucokinase in mice. Diabetes Metab. Res. Rev. 2011, 27, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research; Division on Earth and Life Studies; National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Ohara, M.; Hiromura, M.; Nagaike, H.; Kohata, Y.; Fujikawa, T.; Goto, S.; Sato, N.; Kushima, H.; Terasaki, M.; Yamamoto, T.; et al. Relationship between glucose variability evaluated by continuous glucose monitoring and clinical factors, including glucagon-stimulated insulin secretion in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2019, 158, 107904. [Google Scholar] [CrossRef]
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Inagaki, Y.; Takenaka, K.; Jinnouchi, Y.; Yoshida, Y.; Matsuura, T.; Narama, I.; Motomiya, Y.; et al. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen speciesmediated vascular endothelial growth factor expression. J. Biol. Chem. 2006, 281, 20213–20220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashiba, S.; Van Dyke, T.E.; Amar, S.; Murayama, Y.; Soskolne, A.W.; Shapira, L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infect. Immun. 1999, 67, 5573–5578. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Wild-Type Mice | T1D Model Mice | p-Value | |
|---|---|---|---|
| Number | 6 | 6 | − |
| Final body weight (g) | 24.3 ± 1.6 | 20.3 ± 3.2 | P < 0.01 ★ |
| Food Intake (g/day) | 4.5 ± 0.4 | 4.8 ± 1.0 | 0.459 |
| SBP (mmHg) | 100 ± 16 | 102 ± 15 | 0.840 |
| DBP (mmHg) | 61 ± 6 | 65 ± 7 | 0.302 |
| Total-C (mg/dL) | 74 ± 9 | 86 ± 22 | 0.592 |
| HDL-C (mg/dL) | 40 ± 17 | 32 ± 15 | 0.378 |
| Triglycerides (mg/dL) | 60 ± 9 | 82 ± 39 | 0.205 |
| FBG (mg/dL) | 90 ± 8 | 164 ± 56 | p < 0.01 ★ |
| Insulin (ng/mL) | 0.27 ± 0.08 | 0.05 ± 0.02 | p < 0.001 ★ |
| HbA1c (%) | 4.3 ± 0.2 | 7.9 ± 0.5 | p < 0.001 ★ |
| OGTT-AUC of glucose(mg/dL x hour) | 608 ± 36 | 1150 ± 365 | p < 0.005 ★ |
| Controls | T1D Patients | p-Value | |
|---|---|---|---|
| Number (male/female) | 6 (5/1) | 5 (3/2) | 0.251 |
| Age (years) | 42 ± 10 | 58 ± 30 | 0.25 |
| Duration of diabetes (years) | − | 11 ± 10 | − |
| Body Weight (kg) | 67 ± 8 | 71 ± 20 | 0.725 |
| BMI (kg/m2) | 23.1 ± 1.3 | 21.3 ± 3.9 | 0.318 |
| SBP (mmHg) | 114 ± 5 | 114 ± 10 | 0.912 |
| DBP (mmHg) | 71 ± 7 | 68 ± 13 | 0.662 |
| Total-C (mg/dL) | 187 ± 7 | 167 ± 24 | 0.087 |
| LDL-C (mg/dL) | 110 ± 38 | 92 ± 28 | 0.11 |
| HDL-C (mg/dL) | 52 ± 14 | 57 ± 20 | 0.657 |
| Triglycerides (mg/dL) | 110 ± 38 | 90 ± 48 | 0.465 |
| FBG (mg/dL) | 93 ± 3 | 233 ± 78 | p < 0.005 ★ |
| HbA1c (%) | 5.2 ± 0.4 | 7.7 ± 0.4 | p < 0.001 ★ |
| Fasting C-peptide (ng/mL) | N.A. | 0.16 ± 0.09 | − |
| Stimulated C-peptide (ng/mL) | N.A. | 0.28 ± 0.25 | − |
| Retinopathy (NDR/SDR/PPDR/PDR) | N.A. | (4/1/0/0) | − |
| Nephropathy (1/2/3/4/5) | N.A. | (3/1/1/0/0) | − |
| PAD/none | N.A. | (1/4) | − |
| Total daily insulin dose (Unit) | − | 48 ± 36 | − |
| Lipid-lowering drugs (statins/none) | (1/5) | (2/3) | 0.251 |
| Anti-hypertensive drugs(ARBs/ARBs+CCBs/CCBs/none) | (0/0/0/6) | (1/1/1/2) | 0.176 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terasaki, M.; Yashima, H.; Mori, Y.; Saito, T.; Matsui, T.; Hiromura, M.; Kushima, H.; Osaka, N.; Ohara, M.; Fukui, T.; et al. A Dipeptidyl Peptidase-4 Inhibitor Inhibits Foam Cell Formation of Macrophages in Type 1 Diabetes via Suppression of CD36 and ACAT-1 Expression. Int. J. Mol. Sci. 2020, 21, 4811. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134811
Terasaki M, Yashima H, Mori Y, Saito T, Matsui T, Hiromura M, Kushima H, Osaka N, Ohara M, Fukui T, et al. A Dipeptidyl Peptidase-4 Inhibitor Inhibits Foam Cell Formation of Macrophages in Type 1 Diabetes via Suppression of CD36 and ACAT-1 Expression. International Journal of Molecular Sciences. 2020; 21(13):4811. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134811
Chicago/Turabian StyleTerasaki, Michishige, Hironori Yashima, Yusaku Mori, Tomomi Saito, Takanori Matsui, Munenori Hiromura, Hideki Kushima, Naoya Osaka, Makoto Ohara, Tomoyasu Fukui, and et al. 2020. "A Dipeptidyl Peptidase-4 Inhibitor Inhibits Foam Cell Formation of Macrophages in Type 1 Diabetes via Suppression of CD36 and ACAT-1 Expression" International Journal of Molecular Sciences 21, no. 13: 4811. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134811





