Cyclic AMP: A Polyhedral Signalling Molecule in Plants
Abstract
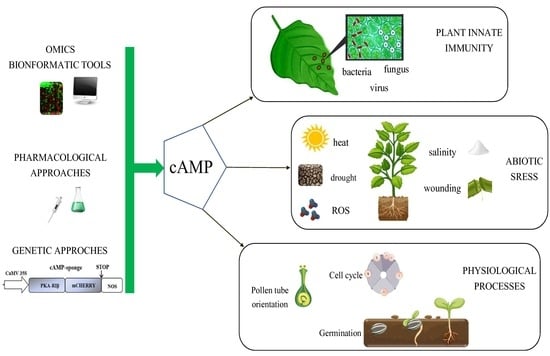
:1. Introduction
2. The cAMP-Sponge, a New Genetic Tool to Unravel cAMP Functions in Plants
3. cAMP in Plant Physiological Processes
4. cAMP Involvement in Plant Response to Abiotic Stress
5. Role of cAMP in Plant Innate Immunity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rall, T.W.; Sutherland, E.W.; Berthet, J. The relation of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J. Biol. Chem. 1957, 224, 1987–1995. [Google Scholar]
- Shabb, J.B.; Corbin, J.D. Cyclic nucleotide-binding domains in proteins having diverse functions. J. Biol. Chem. 1992, 267, 5723–57236. [Google Scholar] [PubMed]
- Newton, R.P.; Smith, C.J. Cyclic nucleotides. Phytochemistry 2004, 65, 2423–2437. [Google Scholar] [CrossRef]
- Gancedo, J.M. Biological roles of cAMP: Variations on a theme in the different kingdoms of life. Biol. Rev. Camb. Philos. Soc. 2013, 88, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Sinha, C.; Zhang, W.; Ren, A.; Moon, C.S.; Yarlagadda, S.; Naren, A.P. Compartmentalization of cyclic nucleotide signaling: A question of when, where, and why? Pflugers Arch. 2013, 465, 1397–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheepala, S.; Hulot, J.S.; Morgan, J.A.; Sassi, Y.; Zhang, W.; Naren, A.P.; Schuetz, J.D. Cyclic nucleotide compartmentalization: Contributions of phosphodiesterases and ATP-binding cassette transporters. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Stork, P.J.S.; Schmitt, J.M. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002, 12, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Amrhein, N. The current status of cyclic AMP in higher plants. Ann. Rev. Plant Physiol. 1977, 28, 123–132. [Google Scholar] [CrossRef]
- Brown, E.G.; Newton, R.P. Cyclic AMP and higher plants. Phytochemistry 1981, 20, 2453–2463. [Google Scholar] [CrossRef]
- Witters, E.; Roef, L.; Newton, R.P.; VanDongen, W.; Esmans, E.L.; VanOnckelen, H.A. Quantitation of cyclic nucleotides in biological samples by negative electrospray tandem mass spectrometry coupled to ion suppression liquid chromatography. Rapid Commun. Mass Spectrom. 1996, 10, 225–231. [Google Scholar] [CrossRef]
- Witters, E.; Vanhoutte, K.; Dewitte, W.; Machackova, I.; Benkova, E.; Van Dongen, W.; Esmans, E.L.; Van Onckelen, H.A. Analysis of cyclic nucleotides and cytokinins in minute plant samples using phase-system switching capillary electrospray-liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 1999, 10, 143–151. [Google Scholar] [CrossRef]
- Beavo, J.A.; Brunton, L.L. Cyclic nucleotide research—Still expanding after half a century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Craven, K.B.; Zagotta, W.N. CNG and HCN channels: Two peas, one pod. Annu. Rev. Physiol. 2006, 68, 375–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moutinho, A.; Hussey, P.J.; Trewavas, A.J.; Malho, R. cAMP acts as a second messenger in pollen tube growth and reorientation. Proc. Natl. Acad. Sci. USA 2001, 98, 10481–10486. [Google Scholar] [CrossRef] [Green Version]
- Gehring, C. Adenyl cyclases and cAMP in plant signaling—Past and present. Cell Commun. Signal. 2010, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, C.; Turek, I.S. Cyclic Nucleotide monophosphates and their cyclases in plant signaling. Front. Plant Sci. 2017, 8, 1704. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.; Tian, X.C.; Gehring, C.; Marondedze, C. Discovery of novel functional centers with rationally designed amino acid motifs. Comput. Struct. Biotechnol. J. 2018, 16, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ruzvidzo, O.; Gehring, C.; Wong, A. New perspectives on plant adenylyl cyclases. Front. Mol. Biosci. 2019, 6, 136. [Google Scholar] [CrossRef] [Green Version]
- Irving, H.R.; Cahill, D.M.; Gehring, C. Moonlighting proteins and their role in the control of signaling microenvironments, as exemplified by cGMP and Phytosulfokine Receptor 1 (PSKR1). Front. Plant Sci. 2018, 9, 415. [Google Scholar] [CrossRef]
- Al-Younis, I.; Wong, A.; Gehring, C. The Arabidopsis thaliana K+-uptake permease 7 (AtKUP7) contains a functional cytosolic adenylate cyclase catalytic centre. FEBS Lett. 2015, 589, 3848–3852. [Google Scholar] [CrossRef] [Green Version]
- Al-Younis, I.; Wong, A.; Lemtiri-Chlieh, F.; Schrnockel, S.; Tester, M.; Gehring, C.; Donaldson, L. The Arabidopsis thaliana K+-uptake permease 5 (AtKUP5) contains a functional cytosolic adenylate cyclase essential for K+ transport. Front. Plant Sci. 2018, 9, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchet, C.; Wong, A.; Quaglia, M.; Alqurashi, M.; Gehring, C.; Ntoukakis, V.; Pasqualini, S. An Arabidopsis thaliana leucine-rich repeat protein harbors an adenylyl cyclase catalytic center and affects responses to pathogens. J. Plant Physiol. 2019, 232, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiezawska, B.; Duszyn, M.; Jaworski, K.; Szmidt-Jaworska, A. Downstream targets of cyclic nucleotides in plants. Front. Plant Sci. 2018, 9, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasahara, M.; Suetsugu, N.; Urano, Y.; Yamamoto, C.; Ohmori, M.; Takada, Y.; Okuda, S.; Nishiyama, T.; Sakayama, H.; Kohchi, T.; et al. An adenylyl cyclase with a phosphodiesterase domain in basal plants with a motile sperm system. Sci. Rep. 2016, 6, 39232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; He, K.; Berkowitz, G.A. Editorial: From structure to signalsomes: New perspectives about membrane receptors and channels. Front. Plant Sci. 2019, 10, 682. [Google Scholar] [CrossRef]
- Martinez-Atienza, J.; Van Ingelgem, C.; Roef, L.; Maathuis, F.J. Plant cyclic nucleotide signalling: Facts and fiction. Plant Signal. Behav. 2007, 2, 540–543. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Smigel, A.; Walker, R.K.; Moeder, W.; Yoshioka, K.; Berkowitz, G.A. Leaf senescence signaling: The Ca2+-conducting Arabidopsis cyclic nucleotide gated channel2 acts through nitric oxide to repress senescence programming. Plant Physiol. 2010, 154, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Berkowitz, G.A. Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytol. 2011, 190, 566–572. [Google Scholar] [CrossRef]
- Jha, S.K.; Sharma, M.; Pandey, G.K. Role of Cyclic nucleotide gated channels in stress management in plants. Curr. Genomics 2016, 17, 315–329. [Google Scholar] [CrossRef]
- Duszyn, M.; Swiezawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Cyclic nucleotide gated channels (CNGCs) in plant signalling-Current knowledge and perspectives. J. Plant Physiol. 2019, 241, 153035. [Google Scholar] [CrossRef]
- Lefkimmiatis, K.; Moyer, M.P.; Curci, S.; Hofer, A.M. “cAMP Sponge”: A buffer for cyclic adenosine 3′,5′-monophosphate. PLoS ONE 2009, 4, e7649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabetta, W.; Vannini, C.; Sgobba, A.; Marsoni, M.; Paradiso, A.; Ortolani, F.; Bracale, M.; Viggiano, L.; Blanco, E.; de Pinto, M.C. Cyclic AMP deficiency negatively affects cell growth and enhances stress-related responses in tobacco Bright Yellow-2 cells. Plant Mol. Biol. 2016, 90, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Sabetta, W.; Vandelle, E.; Locato, V.; Costa, A.; Cimini, S.; Bittencourt Moura, A.; Luoni, L.; Graf, A.; Viggiano, L.; De Gara, L.; et al. Genetic buffering of cyclic AMP in Arabidopsis thaliana compromises the plant immune response triggered by an avirulent strain of Pseudomonas syringae pv. tomato. Plant J. 2019, 98, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Huprikar, S.S.; Kochian, L.V.; Lucas, W.J.; Gaber, R.F. Functional expression of a probable Arabidopsis-thaliana potassium channel in Saccharomyces-cerevisiae. Proc. Natl. Acad. Sci. USA 1992, 89, 3736–3740. [Google Scholar] [CrossRef] [Green Version]
- Bolwell, G.P. Cyclic-AMP, the reluctant messenger in plants. Trends Biochem. Sci. 1995, 20, 492–495. [Google Scholar] [CrossRef]
- Trewavas, A.J. Plant cyclic AMP comes in from the cold. Nature 1997, 390, 657–658. [Google Scholar] [CrossRef]
- Ehsan, H.; Reichheld, J.P.; Roef, L.; Witters, E.; Lardon, F.; Van Bockstaele, D.; Van Montagu, M.; Inze, D.; Van Onckelen, H. Effect of indomethacin on cell cycle dependent cyclic AMP fluxes in tobacco BY-2 cells. FEBS Lett. 1998, 422, 165–169. [Google Scholar] [CrossRef]
- Ehsan, H.; Roef, L.; Witters, E.; Reichheld, J.P.; Van Bockstaele, D.; Inze, D.; Van Onckelen, H. Indomethacin-induced G1/S phase arrest of the plant cell cycle. FEBS Lett. 1999, 458, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Curvetto, N. Effect of two cAMP analogs on stomatal opening in Vicia faba: Possible relationship with cytosolic calcium concentration. Plant Physiol. Biochem. 1994, 32, 365–372. [Google Scholar]
- Jin, X.C.; Wu, W.H. Involvement of cyclic AMP in ABA- and Ca2+-mediated signal transduction of stomatal regulation in Vicia faba. Plant Cell Physiol. 1999, 40, 1127–1133. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Marondedze, C.; Ederli, L.; Pasqualini, S.; Gehring, C. Proteomic signatures implicate cAMP in light and temperature responses in Arabidopsis thaliana. J. Proteom. 2013, 83, 47–59. [Google Scholar] [CrossRef]
- Alqurashi, M.; Gehring, C.; Marondedze, C. Changes in the Arabidopsis thaliana proteome implicate cAMP in biotic and abiotic stress responses and changes in energy metabolism. Int. J. Mol. Sci. 2016, 17, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, L.; Meier, S.; Gehring, C. The arabidopsis cyclic nucleotide interactome. Cell Commun. Signal. 2016, 14, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurosaki, F.; Tsurusawa, Y.; Nishi, A. The elicitation of phytoalexins by Ca2+ and cyclic AMP in carrot cells. Phytochemistry 1987, 26, 1919–1923. [Google Scholar] [CrossRef]
- Li, W.W.; Luan, S.; Schreiber, S.L.; Assmann, S.M. Cyclic-AMP stimulates K+ channel activity in mesophyll-cells of Vicia-faba-K. Plant Physiol. 1994, 106, 957–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, T.; Yoshikawa, F.; Hishida, A.; Furuichi, T.; Mikoshiba, K. A novel recombinant hyperaffinity inositol 1,4,5-trisphosphate (IP3) absorbent traps IP3, resulting in specific inhibition of IP3-mediated calcium signaling. J. Biol. Chem. 2002, 277, 8106–8113. [Google Scholar] [CrossRef] [Green Version]
- Saraswat, L.D.; Ringheim, G.E.; Bubis, J.; Taylor, S.S. Deletion mutants as probes for localizing regions of subunit interaction in cAMP-dependent protein-kinase. J. Biol. Chem. 1988, 263, 18241–18246. [Google Scholar]
- Volotovski, I.D.; Sokolovsky, S.G.; Molchan, O.V.; Knight, M.R. Second messengers mediate increases in cytosolic calcium in tobacco protoplasts. Plant Physiol. 1998, 117, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Lemtiri-Chlieh, F.; Berkowitz, G.A. Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. J. Biol. Chem. 2004, 279, 35306–35312. [Google Scholar] [CrossRef] [Green Version]
- Talke, I.N.; Blaudez, D.; Maathuis, F.J.M.; Sanders, D. CNGCs: Prime targets of plant cyclic nucleotide signalling? Trends Plant Sci. 2003, 8, 286–293. [Google Scholar] [CrossRef]
- Ali, R.; Ma, W.; Lemtiri-Chlieh, F.; Tsaltas, D.; Leng, Q.; von Bodman, S.; Berkowitz, G.A. Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 2007, 19, 1081–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munemasa, S.; Hossain, M.A.; Nakamura, Y.; Mori, I.C.; Murata, Y. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol. 2011, 155, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Zhang, Y.Y.; Tang, S.K.; Pan, J.B.; Yu, Y.K.; Han, J.; Li, Y.Y.; Du, X.H.; Nan, Z.J.; Sun, Q.P. AtCNGC2 is involved in jasmonic acid-induced calcium mobilization. J. Exp. Bot. 2016, 67, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malho, R.; Camacho, L.; Moutinho, A. Signalling pathways in pollen tube growth and reorientation. Ann. Bot. 2000, 85, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Qu, H.Y.; Jin, C.; Shang, Z.L.; Wu, J.; Xu, G.H.; Gao, Y.B.; Zhang, S.L. cAMP activates hyperpolarization-activated Ca2+ channels in the pollen of Pyrus pyrifolia. Plant Cell Rep. 2011, 30, 1193–1200. [Google Scholar] [CrossRef]
- Tezuka, T.; Hiratsuka, S.; Takahashi, S.Y. Promotion of the growth of self-incompatible pollen tubes in lily by cAMP. Plant Cell Physiol. 1993, 34, 955–958. [Google Scholar] [CrossRef]
- Tsuruhara, A.; Tezuka, T. Relationship between the self-incompatibility and cAMP level in Lilium longiflorum. Plant Cell Physiol. 2001, 42, 1234–1238. [Google Scholar] [CrossRef] [Green Version]
- Tezuka, T.; Akita, I.; Yoshino, N. Self-incompatibility involved in the level of acetylcholine and cAMP. Plant Signal. Behav. 2007, 2, 475–476. [Google Scholar] [CrossRef] [Green Version]
- Duffus, C.M.; Duffus, J.H. A possible role for cyclic AMP in gibberellic acid triggered release of alpha-amylase in barley endosperm slices. Experientia 1969, 25, 581. [Google Scholar] [CrossRef]
- Hall, K.A.; Galsky, A.G. The action of cyclic-AMP on GA3 controlled responses IV. Characteristics of the promotion of seed germination in Lactuca sative variety ‘Spartan Lake’ by gibberellic acid and cyclic 3,5′-adenosine monophosphate. Plant Cell Physiol. 1973, 14, 565–571. [Google Scholar] [CrossRef]
- Holm, R.; Miller, M. Hormonal control of weed seed germination. Weed Sci. 1972, 20, 209–212. [Google Scholar] [CrossRef]
- Uematsu, K.; Nakajima, M.; Yamaguchi, I.; Yoneyama, K.; Fukui, Y. Role of cAMP in gibberellin promotion of seed germination in Orobanche minor smith. J. Plant Growth Regul. 2007, 26, 245–254. [Google Scholar] [CrossRef]
- Uematsu, K.; Fukui, Y. Role and regulation of cAMP in seed germination of Phacelia tanacetifolia. Plant Physiol. Biochem. 2008, 46, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Domanska, A.; Godlewski, M.; Aniol, P. Db-cAMP, forskolin or 2′-d3′-AMP influence on proliferation of Raphanus sativus root meristem cells. Caryologia 2009, 62, 267–275. [Google Scholar] [CrossRef]
- Moulding, D.A.; Blundell, M.P.; Spiller, D.G.; White, M.R.; Cory, G.O.; Calle, Y.; Kempski, H.; Sinclair, J.; Ancliff, P.J.; Kinnon, C.; et al. Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J. Exp. Med. 2007, 204, 2213–2224. [Google Scholar] [CrossRef]
- Terakado, J.; Okamura, M.; Fujihara, S.; Ohmori, M.; Yoneyama, T. Cyclic AMP in rhizobia and symbiotic nodules. Ann. Bot-Lond. 1997, 80, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Terakado, J.; Fujihara, S.; Yoneyama, T. Changes in cyclic nucleotides during nodule formation. Soil Sci. Plant Nutr. 2003, 49, 459–462. [Google Scholar] [CrossRef] [Green Version]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [Green Version]
- Kosova, K.; Vitamvas, P.; Prasil, I.T.; Renaut, J. Plant proteome changes under abiotic stress-contribution of proteomics studies to understanding plant stress response. J. Proteomics 2011, 74, 1301–1322. [Google Scholar] [CrossRef]
- Lamers, J.; van der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [Green Version]
- Maathuis, F.J.; Sanders, D. Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol. 2001, 127, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, N.M.; Marondedze, C.; Thomas, L.; Pasqualini, S.; Shabala, L.; Shabala, S.; Gehring, C. Cyclic mononucleotides modulate potassium and calcium flux responses to H2O2 in Arabidopsis roots. FEBS Lett. 2014, 588, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Ryan, P.R.; Tyerman, S.D. Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiol. 2001, 125, 1459–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Han, X.W.; Wu, J.H.; Zheng, S.Z.; Shang, Z.L.; Sun, D.Y.; Zhou, R.G.; Li, B. A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—Is involved in heat shock responses. Plant J. 2012, 70, 1056–1069. [Google Scholar] [CrossRef]
- Maksyutova, N.N.; Viktorova, L.V. A comparative study of the effect of abscisic acid and cAMP on protein synthesis in wheat caryopses under drought conditions. Biochemistry (Mosc) 2003, 68, 424–428. [Google Scholar] [CrossRef]
- Swieiawska, B.; Jaworski, K.; Pawelek, A.; Grzegorzewska, W.; Szewczuk, P.; Szmidt-Jaworska, A. Molecular cloning and characterization of a novel adenylyl cyclase gene, HpAC1, involved in stress signaling in Hippeastrum x hybridum. Plant Physiol. Biochem. 2014, 80, 41–52. [Google Scholar] [CrossRef]
- Ahn, S.J.; Shin, R.; Schachtman, D.P. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 2004, 134, 1135–1145. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Wu, W.; Wu, W.H.; Wang, Y. Potassium transporter KUP7 is involved in K(+) acquisition and translocation in Arabidopsis root under K(+)-limited conditions. Mol. Plant 2016, 9, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Kohler, C.; Merkle, T.; Neuhaus, G. Characterisation of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis thaliana. Plant J. 1999, 18, 97–104. [Google Scholar] [CrossRef]
- Arazi, T.; Kaplan, B.; Fromm, H. A high-affinity calmodulin-binding site in a tobacco plasma-membrane channel protein coincides with a characteristic element of cyclic nucleotide-binding domains. Plant Mol. Biol. 2000, 42, 591–601. [Google Scholar] [CrossRef]
- Hua, B.G.; Mercier, R.W.; Zielinski, R.E.; Berkowitz, G.A. Functional interaction of calmodulin with a plant cyclic nucleotide gated cation channel. Plant Physiol. Biochem. 2003, 41, 945–954. [Google Scholar] [CrossRef]
- Leng, Q.; Mercier, R.W.; Yao, W.; Berkowitz, G.A. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999, 121, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugler, A.; Kohler, B.; Palme, K.; Wolff, P.; Dietrich, P. Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol. 2009, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, K.M.; Babourina, O.; Christopher, D.A.; Borsics, T.; Rengel, Z. The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol. Plant 2008, 134, 499–507. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Tang, C.; Ishka, M.R.; Brown, E.; Groves, N.R.; Myers, C.T.; Rato, C.; Poulsen, L.R.; McDowell, S.; Miller, G.; et al. A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 2013, 161, 1010–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finka, A.; Cuendet, A.F.; Maathuis, F.J.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef] [Green Version]
- Witters, E.; Valcke, R.; van Onckelen, H. Cytoenzymological analysis of adenylyl cyclase activity and 3′:5′-cAMP immunolocalization in chloroplasts of Nicotiana tabacum. New Phytol. 2005, 168, 99–107. [Google Scholar] [CrossRef]
- Molchan, O.V.; Sokolovsky, S.G.; Volotovsky, I.D. The phytochrome control of the cAMP endogenous level in oat seedlings. Russ. J. Plant Physl. 2000, 47, 463–467. [Google Scholar]
- Pietrowska-Borek, M.; Nuc, K. Both cyclic-AMP and cyclic-GMP can act as regulators of the phenylpropanoid pathway in Arabidopsis thaliana seedlings. Plant Physiol. Biochem. 2013, 70, 142–149. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007, 144, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- de Pinto, M.C.; Locato, V.; Paradiso, A.; De Gara, L. Role of redox homeostasis in thermo-tolerance under a climate change scenario. Ann. Bot. 2015, 116, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol. 2013, 163, 471–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Pottosin, I.; Velarde-Buendia, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: Implications for plant adaptive responses. J. Exp. Bot. 2014, 65, 1271–1283. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhou, J.M. Receptor-like kinases in plant innate immunity. J. Integr. Plant Biol. 2013, 55, 1271–1286. [Google Scholar] [CrossRef]
- Tang, D.Z.; Wang, G.X.; Zhou, J.M. Receptor kinases in plant-pathogen interactions: More than pattern recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef] [Green Version]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Cui, H.T.; Tsuda, K.; Parker, J.E. Effector-Triggered Immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, B.M.; Zhou, J.M.; Tang, D.Z. Plant immune signaling: Advancing on two frontiers. J. Integr. Plant Biol. 2020, 62, 2–24. [Google Scholar] [CrossRef] [Green Version]
- Kurosaki, F.; Nishi, A. Stimulation of calcium influx and calcium cascade by cyclic AMP in cultured carrot cells. Arch. Biochem. Biophys. 1993, 302, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.J.; Smith, C.J.; Walton, T.J.; Newton, R.P. Evidence that cyclic-AMP is involved in the hypersensitive response of Medicago-sativa to a fungal elicitor. Phytochemistry 1994, 35, 889–895. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Y.Q.; Fujita, K.; Sakai, K. Involvement of cAMP signaling in elicitor-induced phytoalexin accumulation in Cupressus lusitanica cell cultures. New Phytol. 2004, 161, 723–733. [Google Scholar] [CrossRef]
- Jiang, J.; Fan, L.W.; Wu, W.H. Evidences for involvement of endogenous cAMP in Arabidopsis defense responses to Verticillium toxins. Cell Res. 2005, 15, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Qi, Z.; Smigel, A.; Walker, R.K.; Verma, R.; Berkowitz, G.A. Ca2+, cAMP, and transduction of non-self perception during plant immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 20995–21000. [Google Scholar] [CrossRef] [Green Version]
- Lomovatskaya, L.A.; Kuzakova, O.V.; Romanenko, A.S.; Goncharova, A.M. Activities of adenylate cyclase and changes in cAMP concentration in root cells of pea seedlings infected with mutualists and phytopathogens. Russ. J. Plant Physiol. 2018, 65, 588–597. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Slusarenko, A.J. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 1996, 8, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shine, M.B.; Yang, J.W.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.Q.; Navarre, D.; Ghabrial, S.; Kachroo, P.; Kachroo, A. Cooperative functioning between phenylalanine ammonia lyase and isochorismate synthase activities contributes to salicylic acid biosynthesis in soybean. New Phytol. 2016, 212, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.A. ROS in biotic interactions. Physiol. Plant 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Qi, J.S.; Wang, J.L.; Gong, Z.Z.; Zhou, J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef]
- Bolwell, G.P. Role of active oxygen species and NO in plant defence responses. Curr. Opin. Plant Biol. 1999, 2, 287–294. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Minibayeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early signalling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol. 2001, 151, 185–194. [Google Scholar] [CrossRef]
- Davies, D.R.; Bindschedler, L.V.; Strickland, T.S.; Bolwell, G.P. Production of reactive oxygen species in Arabidopsis thaliana cell suspension cultures in response to an elicitor from Fusarium oxysporum: Implications for basal resistance. J. Exp. Bot. 2006, 57, 1817–1827. [Google Scholar] [CrossRef] [Green Version]
- Clough, S.J.; Fengler, K.A.; Yu, I.C.; Lippok, B.; Smith, R.K.; Bent, A.F. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 2000, 97, 9323–9328. [Google Scholar] [CrossRef] [Green Version]
- Balague, C.; Lin, B.Q.; Alcon, C.; Flottes, G.; Malmstrom, S.; Kohler, C.; Neuhaus, G.; Pelletier, G.; Gaymard, F.; Roby, D. HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 2003, 15, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, K.; Moeder, W.; Kang, H.G.; Kachroo, P.; Masmoudi, K.; Berkowitz, G.; Klessig, D.F. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 2006, 18, 747–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. Death don’t have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 1996, 8, 1793–1807. [Google Scholar] [CrossRef]
- Grant, M.; Brown, I.; Adams, S.; Knight, M.; Ainslie, A.; Mansfield, J. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000, 23, 441–450. [Google Scholar] [CrossRef]
- Lecourieux, D.; Raneva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, O.; Courtois, C.; Dobrowolska, G.; Besson, A.; Pugin, A.; Wendehenne, D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic. Biol. Med. 2006, 40, 1369–1376. [Google Scholar] [CrossRef]
- Delledonne, M.; Xia, Y.J.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef]
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 10328–10333. [Google Scholar] [CrossRef] [Green Version]
- de Jong, C.F.; Laxalt, A.M.; Bargmann, B.O.R.; de Wit, P.J.G.M.; Joosten, M.H.A.J.; Munnik, T. Phosphatidic acid accumulation is an early response in the Cf-4/Avr4 interaction. Plant J. 2004, 39, 1–12. [Google Scholar] [CrossRef]
- Andersson, M.X.; Kourtchenko, O.; Dangl, J.L.; Mackey, D.; Ellerstrom, M. Phospholipase-dependent signalling during the AvrRpm1- and AvrRpt2-induced disease resistance responses in Arabidopsis thaliana. Plant J. 2006, 47, 947–959. [Google Scholar] [CrossRef]
- D’Ambrosio, J.M.; Couto, D.; Fabro, G.; Scuffi, D.; Lamattina, L.; Munnik, T.; Andersson, M.X.; Alvarez, M.E.; Zipfel, C.; Laxalt, A.M. Phospholipase C2 affects MAMP-triggered immunity by modulating ROS production. Plant Physiol. 2017, 175, 970–981. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.B.; Poovaiah, B.W. A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J. Biol. Chem. 2002, 277, 45049–45058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.Q.; Ali, G.S.; Simons, K.A.; Hou, J.G.; Yang, T.B.; Reddy, A.S.N.; Poovaiah, B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef]
- Zhou, W.B.; Freed, C.R. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J. Biol. Chem. 2005, 280, 43150–43158. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.R.; Persson, S.; Mehta, T.; Srinivasasainagendra, V.; Chen, L.; Page, G.P.; Somerville, C.; Loraine, A. Transcriptional coordination of the metabolic network in Arabidopsis. Plant Physiol. 2006, 142, 762–774. [Google Scholar] [CrossRef] [Green Version]
- Shirasu, K. The HSP90-SGT1 Chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 2009, 60, 139–164. [Google Scholar] [CrossRef] [Green Version]
- Yadeta, K.A.; Elmore, J.M.; Creer, A.Y.; Feng, B.M.; Franco, J.Y.; Rufian, J.S.; He, P.; Phinney, B.; Coaker, G. A Cysteine-rich protein kinase associates with a membrane immune complex and the cysteine residues are required for cell death. Plant Physiol. 2017, 173, 771–787. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Takahashi, H.; Sawasaki, T.; Ohnishi, K.; Hikichi, Y.; Kiba, A. Novel type of adenylyl cyclase participates in tabtoxinine-beta-lactam-induced cell death and occurrence of wildfire disease in Nicotiana benthamiana. Plant Signal. Behav. 2014, 9, 27420. [Google Scholar] [CrossRef] [Green Version]
- Rich, T.C.; Webb, K.J.; Leavesley, S.J. Can we decipher the information content contained within cyclic nucleotide signals? J. Gen. Physiol. 2014, 143, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, J.I.; Freihat, L.; Irving, H.R. A cyclic nucleotide sensitive promoter reporter system suitable for bacteria and plant cells. BMC Biotechnol. 2013, 13, 97. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.Y.; Falcone, J.L.; Curci, S.; Hofer, A.M. Interrogating cyclic AMP signaling using optical approaches. Cell Calcium 2017, 64, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Klausen, C.; Kaiser, F.; Stuven, B.; Hansen, J.N.; Wachten, D. Elucidating cyclic AMP signaling in subcellular domains with optogenetic tools and fluorescent biosensors. Biochem. Soc. Trans. 2019, 47, 1733–1747. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Buck, J.; Levin, L.R. pH sensing via bicarbonate-regulated “soluble” adenylyl cyclase (sAC). Front. Physiol. 2013, 4, 343. [Google Scholar] [CrossRef] [Green Version]
- Paramonov, V.M.; Mamaeva, V.; Sahlgren, C.; Rivero-Muller, A. Genetically-encoded tools for cAMP probing and modulation in living systems. Front. Pharmacol. 2015, 6, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Stress | Mechanisms | Molecular Players | References |
|---|---|---|---|
| Salinity | Limitation of Na+ influx | VICs; CNGCs | [71,72] |
| Aluminium | K+ current permitting malate outflux | Cation channels | [73] |
| K+ deficiency | K+ homeostasis regulation | AtKUP5; AtKUP7; CNGCs. | [20,21] |
| Heat | Ca2+ influx and HSPs expression | CNGCs; HSPs. | [74] |
| Drought | Synthesis of protective polypeptides | ABA signalling | [75] |
| Wounding | Regulations of the phenylpropanoid pathway | PAL; 4CL; CHS. | [76] |
| ROS | Reduction of Ca2+ influx and K+ efflux | CNGCs | [72] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, E.; Fortunato, S.; Viggiano, L.; de Pinto, M.C. Cyclic AMP: A Polyhedral Signalling Molecule in Plants. Int. J. Mol. Sci. 2020, 21, 4862. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144862
Blanco E, Fortunato S, Viggiano L, de Pinto MC. Cyclic AMP: A Polyhedral Signalling Molecule in Plants. International Journal of Molecular Sciences. 2020; 21(14):4862. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144862
Chicago/Turabian StyleBlanco, Emanuela, Stefania Fortunato, Luigi Viggiano, and Maria Concetta de Pinto. 2020. "Cyclic AMP: A Polyhedral Signalling Molecule in Plants" International Journal of Molecular Sciences 21, no. 14: 4862. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144862