Gastrointestinal Disorders and Metabolic Syndrome: Dysbiosis as a Key Link and Common Bioactive Dietary Components Useful for their Treatment
Abstract
:1. Introduction
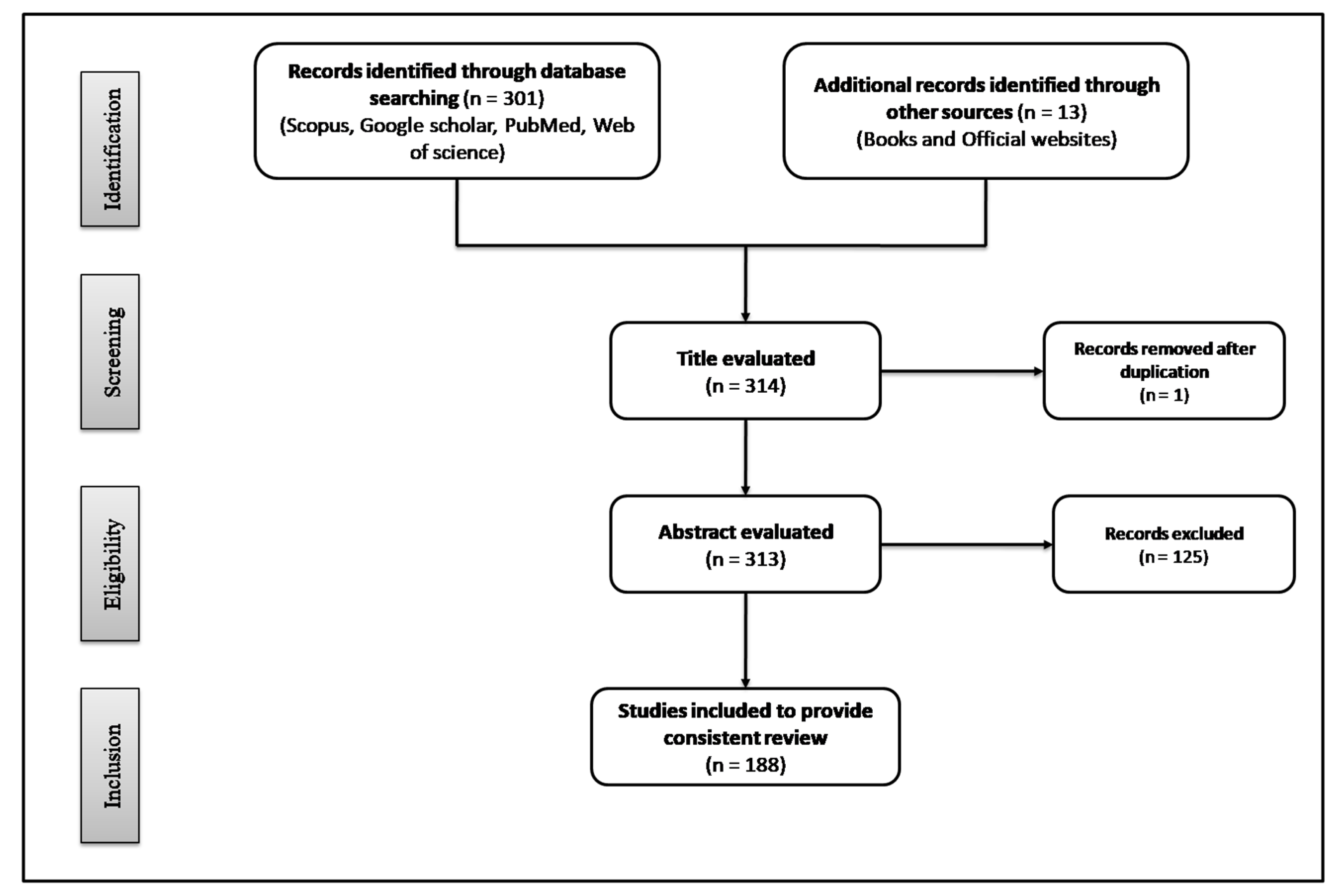
2. Methodology
3. Bioactive Dietary Components and Gastrointestinal Disorders
3.1. Dietary Fibers
Prebiotics
3.2. Probiotics
3.3. Polyphenols
3.4. Spices
4. Bioactive Dietary Components and Metabolic Syndrome
4.1. High Fiber Diet
Prebiotics
4.2. Probiotics
4.3. Fatty Acids
4.4. Polyphenols
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| ANGPTL4 | Angiopoietin-like protein-4 |
| BMI | Body mass index |
| CD | Crohn’s disease |
| CD8 | Cluster of differentiation 8 |
| CFU | Colony forming units |
| COX-2 | Cyclooxygenase-2 |
| CYP7A1 | Cholesterol 7a-hydroxylase |
| DSS | Dextran sulfate sodium |
| EGCG | Epigallocatechin-3- gallate |
| FDA | Food and Drug Administration |
| FGF15 | Fibroblast growth factor 15 |
| FGF21 | Fibroblast growth factor 21 |
| FFA | Free fatty acids |
| FOS | Fructo-oligosaccharide |
| FXR | Farnesoid X receptor |
| GABA | Gamma-aminobutyric acid |
| GERD | Gastrointestinal reflux disease |
| GIT | Gastrointestinal tract |
| GLP-1 | Glucagon like peptide-1 |
| GLUT4 | Glucose transporter type 4 |
| GOS | Galacto-oligosaccharide |
| GPR 40 | G-protein coupled receptor 40 |
| GSPE | Grape seed proanthocyanidin extract |
| HDL | High density lipoprotein |
| HOMA-β | Homeostatic model assessment for β cell function |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| HSHF | High-sucrose high-fat diet |
| IBD | Inflammatory bowel diseases |
| IBS | Irritable bowel syndrome |
| IDF | International Diabetes Federation |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| JNK | Jun N-terminal kinase |
| KYN | Kynurenine |
| LDL | Low density lipoprotein |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen activated protein kinase |
| MCP-1 | Monocyte chemotactic protein-1 |
| MIC | Minimum inhibitory concentration |
| MS | Metabolic syndrome |
| NAFLD | Nonalcoholic fatty liver disease |
| NF-kβ | Nuclear factor-kappa Beta |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| NSP | Non-starch polysaccharides |
| PGE2 | Prostaglandin E2 |
| PPARα | Peroxisome proliferator activated receptor alpha |
| PUFAs | Polyunsaturated fatty acids |
| RAAS | Renin-angiotensin-aldosterone system |
| SCFAs | Short-chain fatty acids |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TG | Triglycerides |
| TLR | Toll-like receptor |
| TMAO | Trimethylamine-N-oxide |
| TNF-α | Tumor necrosis factor alpha |
| TRP | Tryptophan |
| UC | Ulcerative colitis |
| WHO | World Health Organization |
References
- Oshima, T.; Miwa, H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J. Neurogastroenterol. Motil. 2015, 21, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG clinical guideline: Management of Crohn’s disease in adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiorino, G.; Peyrin-Biroulet, L. Positioning therapies in ulcerative colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Scaioli, E.; Barbaro, M.R.; Biagi, E.; Laghi, L.; Cremon, C.; Marasco, G.; Colecchia, A.; Picone, G.; Salfi, N. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut 2017, 66, 1252–1261. [Google Scholar] [CrossRef]
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of functional gastrointestinal disorders: A holistic overview. Dig. Dis. 2017, 35, 5–13. [Google Scholar] [CrossRef]
- Philpott, H.; Nandurkar, S.; Lubel, J.; Gibson, P.R. Republished: Drug-induced gastrointestinal disorders. Postgrad. Med. J. 2014, 90, 411–419. [Google Scholar] [CrossRef]
- Francino, M. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [Green Version]
- Scarborough, P.; Bhatnagar, P.; Wickramasinghe, K.K.; Allender, S.; Foster, C.; Rayner, M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: An update to 2006–07 NHS costs. J. Public Health 2011, 33, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Sachs, G.; Scott, D.R. Helicobacter pylori: Eradication or preservation. F1000 Med. Rep. 2012, 4, 4. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Xavier, R.J. Gastrointestinal diseases. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 16–26. [Google Scholar]
- McQuaid, K.R. Drugs used in the treatment of gastrointestinal diseases. In Basic and Clinical Pharmacology, 12th ed.; Katzung, B.G., Ed.; McGraw-Hill Education/Medical: New York, NY, USA, 2012; pp. 1081–1114. [Google Scholar]
- Gangarosa, L.M.; Seibert, D.G. Drugs used in gastrointestinal disorders. In Modern Pharmacology with Clinical Applications, 5th ed.; Craig, C.R., Stitzel, R.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; pp. 470–483. [Google Scholar]
- Hindryckx, P.; Novak, G.; Bonovas, S.; Peyrin-Biroulet, L.; Danese, S. Infection risk with biologic therapy in patients with inflammatory bowel disease. Clin. Pharmacol. Ther. 2017, 102, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, J.R.; Driman, D.K. Pathological effects of drugs on the gastrointestinal tract: A review. Hum. Pathol. 2007, 38, 527–536. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Marwaha, A.; Sood, R.; Moayyedi, P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: A meta-analysis. Gut 2015, 64, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States—Unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Scarpignato, C.; Mueller-Lissner, S.; Kamm, M.; Hinkel, U.; Helfrich, I.; Schuijt, C.; Mandel, K. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment. Pharmacol. Ther. 2008, 28, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Nyrop, K.; Palsson, O.; Levy, R.; Korff, M.V.; Feld, A.; Turner, M.; Whitehead, W. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment. Pharmacol. Ther. 2007, 26, 237–248. [Google Scholar] [CrossRef]
- Oświęcimska, J.; Szymlak, A.; Roczniak, W.; Girczys-Połedniok, K.; Kwiecień, J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv. Med. Sci. 2017, 62, 17–30. [Google Scholar] [CrossRef]
- Aguirre, J.E.; Winston, J.; Sarna, S.K. Neonatal immune challenge followed by adult immune challenge induces epigenetic-susceptibility to aggravated visceral hypersensitivity. Neurogastroenterol. Motil. 2017, 29, e13081. [Google Scholar] [CrossRef]
- Toro-Martín, D.; Arsenault, B.J.; Després, J.-P.; Vohl, M.-C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Obesity and Lipotoxicity; Springer: Cham, Switzerland, 2017; pp. 1–17. [Google Scholar]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Foster, M.C.; Anderson, C.A.; Burke, G.L.; Haq, N.; Kalyani, R.R.; Ouyang, P.; Sibley, C.T.; Tracy, R.; Woodward, M. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2018, 71, 1857–1865. [Google Scholar] [CrossRef]
- Åberg, F.; Helenius-Hietala, J.; Puukka, P.; Färkkilä, M.; Jula, A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology 2018, 67, 2141–2149. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed] [Green Version]
- Sadeghi, M. The metabolic syndrome. Arya Atheroscler. 2010, 2, 1–2. [Google Scholar]
- Vague, J. Sexual differentiation, a factor affecting the forms of obesity. La Presse Médicale 1947, 30, 339–340. [Google Scholar]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443. [Google Scholar]
- Zimmet, P.; Alberti, K.; Shaw, J. International Diabetes Federation: The IDF consensus worldwide definition of the metabolic syndrome. Diabetes Voice 2005, 50, 31–33. [Google Scholar]
- Lipińska, A.; Koczaj-Bremer, M.; Jankowski, K.; Kaźmierczak, A.; Ciurzyński, M.; Ou-Pokrzewińska, A.; Mikocka, E.; Lewandowski, Z.; Demkow, U.; Pruszczyk, P. Does family history of metabolic syndrome affect the metabolic profile phenotype in young healthy individuals? Diabetol. Metab. Syndr. 2014, 6, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Liu, J.; Ning, G. Active smoking and risk of metabolic syndrome: A meta-analysis of prospective studies. PLoS ONE 2012, 7, e47791. [Google Scholar] [CrossRef] [Green Version]
- Fan, A.Z.; Russell, M.; Naimi, T.; Li, Y.; Liao, Y.; Jiles, R.; Mokdad, A.H. Patterns of alcohol consumption and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 3833–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.-W.; Zhu, S.; Palaniappan, L.; Heshka, S.; Carnethon, M.R.; Heymsfield, S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2003, 163, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Gennuso, K.P.; Gangnon, R.E.; Thraen-Borowski, K.M.; Colbert, L.H. Dose–response relationships between sedentary behaviour and the metabolic syndrome and its components. Diabetologia 2015, 58, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamberti, J.S.; Olson, D.; Crilly, J.F.; Olivares, T.; Williams, G.C.; Tu, X.; Tang, W.; Wiener, K.; Dvorin, S.; Dietz, M.B. Prevalence of the metabolic syndrome among patients receiving clozapine. Am. J. Psychiatry 2006, 163, 1273–1276. [Google Scholar] [CrossRef]
- Lim, S.; Eckel, R.H. Pharmacological treatment and therapeutic perspectives of metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 329–341. [Google Scholar] [CrossRef]
- Rask Larsen, J.; Dima, L.; Correll, C.U.; Manu, P. The pharmacological management of metabolic syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 397–410. [Google Scholar] [CrossRef]
- Prasad, H.; Ryan, D.A.; Celzo, M.F.; Stapleton, D. Metabolic syndrome: Definition and therapeutic implications. Postgrad. Med. 2012, 124, 21–30. [Google Scholar] [CrossRef]
- Stern, M.P.; Williams, K.; González-Villalpando, C.; Hunt, K.J.; Haffner, S.M. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 2004, 27, 2676–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 March 2020).
- WHO. Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 2 March 2020).
- WHO. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 2 March 2020).
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Tang, T.W.; Chen, H.-C.; Chen, C.-Y.; Yen, C.Y.; Lin, C.-J.; Prajnamitra, R.P.; Chen, L.-L.; Ruan, S.-C.; Lin, J.-H.; Lin, P.-J. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 2019, 139, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Hur, K.Y.; Lee, M.-S. Gut microbiota and metabolic disorders. Diabetes Metab. J. 2015, 39, 198–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrapon, N.; Henrissat, B. How do gut microbes break down dietary fiber? Trends Biochem. Sci. 2014, 39, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Bondy, S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100170. [Google Scholar] [CrossRef]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Santos, J.; Vanner, S.J.; Vergnolle, N.; Zoetendal, E.G.; Quigley, E.M. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318. [Google Scholar] [CrossRef] [Green Version]
- Kannampalli, P.; Shaker, R.; Sengupta, J.N. Colonic butyrate-algesic or analgesic? Neurogastroenterol. Motil. 2011, 23, 975–979. [Google Scholar] [CrossRef] [Green Version]
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 2010, 22, 512. [Google Scholar] [CrossRef]
- Talukder, J. Nutraceuticals in gastrointestinal conditions. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 467–479. [Google Scholar]
- Myasoedova, E.; Talley, N.J.; Manek, N.J.; Crowson, C.S. Prevalence and risk factors of gastrointestinal disorders in patients with rheumatoid arthritis: Results from a population-based survey in Olmsted County, Minnesota. Gastroenterol. Res. Pract. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chuah, K.-H.; Mahadeva, S. Cultural factors influencing functional gastrointestinal disorders in the east. J. Neurogastroenterol. Motil. 2018, 24, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribaldone, D.G.; Pellicano, R.; Actis, G.C. Inflammation in gastrointestinal disorders: Prevalent socioeconomic factors. Clin. Exp. Gastroenterol. 2019, 12, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, K.; Singh, A.; Jabeen, R. Nutraceuticals and functional foods: The foods for the future world. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome. Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhou, Q.; Dorfman, R.G.; Huang, X.; Fan, T.; Zhang, H.; Zhang, J.; Yu, C. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016, 16, 84. [Google Scholar] [CrossRef] [Green Version]
- Velazquez, O.C.; Lederer, H.M.; Rombeau, J.L. Butyrate and the colonocyte: Production, absorption, metabolism, and therapeutic implications. In Dietary Fiber in Health and Disease; HumanaPress: Wimberley, TX, USA, 1997; Volume 427, pp. 123–134. [Google Scholar]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Eslamparast, T.; Zamani, F.; Hekmatdoost, A.; Sharafkhah, M.; Eghtesad, S.; Malekzadeh, R.; Poustchi, H. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: A randomised, double-blind, placebo-controlled pilot study. Br. J. Nutr. 2014, 112, 438–445. [Google Scholar] [CrossRef]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Im, S.-H. Probiotics as an immune modulator. J. Nutr. Sci. Vitaminol. 2015, 61, S103–S105. [Google Scholar] [CrossRef] [Green Version]
- Srinarong, C.; Siramolpiwat, S.; Wongcha-um, A.; Mahachai, V.; Vilaichone, R.-K. Improved eradication rate of standard triple therapy by adding bismuth and probiotic supplement for Helicobacter pylori treatment in Thailand. Asian Pac. J. Cancer Prev. 2014, 15, 9909–9913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, C.C.; Lorena, S.L.S.; Pavan, C.R.; Akasaka, H.M.I.; Mesquita, M.A. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr. Clin. Pract. 2012, 27, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.A.J.; Selma, M.A.V.; Gonzalez-Sarrias, A.; Toti, S.; Cerón, J.J.N.; Tomas-Barberan, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.M.; Yeo, M.; Choue, J.S.; Jin, J.H.; Park, S.J.; Cheong, J.Y.; Lee, K.J.; Kim, J.H.; Hahm, K.B. Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling. Helicobacter 2004, 9, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botschuijver, S.; Welting, O.; Levin, E.; Maria-Ferreira, D.; Koch, E.; Montijn, R.; Seppen, J.; Hakvoort, T.; Schuren, F.; De Jonge, W. Reversal of visceral hypersensitivity in rat by Menthacarin®, a proprietary combination of essential oils from peppermint and caraway, coincides with mycobiome modulation. Neurogastroenterol. Motil. 2018, 30, e13299. [Google Scholar] [CrossRef] [Green Version]
- Li, A.-L.; Ni, W.-W.; Zhang, Q.-M.; Li, Y.; Zhang, X.; Wu, H.-Y.; Du, P.; Hou, J.-C.; Zhang, Y. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol. Immunol. 2020, 64, 23–32. [Google Scholar] [CrossRef]
- Longe, J.L. The Gale Encyclopedia of Diets: A Guide to Health and Nutrition; Two Volume Set; Gale: Farmington Hills, MI, USA, 2008. [Google Scholar]
- Rana, V.; Bachheti, R.K.; Chand, T.; Barman, A. Dietary fibre and human health. Int. J. Food Saf. Nutr. Public Health 2011, 4, 101–118. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Asp, N.G.; Johansson, C.G.; Hallmer, H.; Siljestroem, M. Rapid enzymic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem. 1983, 31, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I. Fiber: Physiological and functional effects. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 2, pp. 240–245. [Google Scholar]
- Camilleri, M. Management of the irritable bowel syndrome. Gastroenterology 2001, 120, 652–668. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M.; Heading, R.; Thompson, W. Consensus report: Clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2002, 16, 1407–1430. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Allan, E.; Winter, S.; Light, A.; Allan, A. Mucosal enzyme activity for butyrate oxidation; no defect in patients with ulcerative colitis. Gut 1996, 38, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Floch, M.H. The role of prebiotics and probiotics in gastrointestinal disease. Gastroenterol. Clin. 2018, 47, 179–191. [Google Scholar] [CrossRef]
- Hord, N.G. Eukaryotic-microbiota crosstalk: Potential mechanisms for health benefits of prebiotics and probiotics. Annu. Rev. Nutr. 2008, 28, 215–231. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verna, E.C.; Lucak, S. Use of probiotics in gastrointestinal disorders: What to recommend? Ther. Adv. Gastroenterol. 2010, 3, 307–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.S.; Behera, P.K.; Kar, B.; Ray, R.C. Advances in probiotics, prebiotics and nutraceuticals. In Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Cham, Switzerland, 2018; pp. 121–141. [Google Scholar]
- Suvarna, V.; Boby, V. Probiotics in human health: A current assessment. Curr. Sci. 2005, 88, 1744–1748. [Google Scholar]
- Chugh, B.; Kamal-Eldin, A. Bioactive compounds produced by probiotics in food products. Curr. Opin. Food Sci. 2020, 32, 76–82. [Google Scholar] [CrossRef]
- Delgado, S.; Sánchez, B.; Margolles, A.; Ruas-Madiedo, P.; Ruiz, L. Molecules produced by probiotics and intestinal microorganisms with immunomodulatory activity. Nutrients 2020, 12, 391. [Google Scholar] [CrossRef] [Green Version]
- Krishna Rao, R.; Samak, G. Protection and restitution of gut barrier by probiotics: Nutritional and clinical implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Sreeja, V.; Prajapati, J.B. Probiotic formulations: Application and status as pharmaceuticals—A review. Probiotics Antimicrob. Proteins 2013, 5, 81–91. [Google Scholar] [CrossRef]
- Jesus, A.L.T.; Fernandes, M.S.; Kamimura, B.A.; Prado-Silva, L.; Silva, R.; Esmerino, E.A.; Cruz, A.G.; Sant’Ana, A.S. Growth potential of Listeria monocytogenes in probiotic cottage cheese formulations with reduced sodium content. Food Res. Int. 2016, 81, 180–187. [Google Scholar] [CrossRef]
- Chen, C.-C.; Kong, M.-S.; Lai, M.-W.; Chao, H.-C.; Chang, K.-W.; Chen, S.-Y.; Huang, Y.-C.; Chiu, C.-H.; Li, W.-C.; Lin, P.-Y. Probiotics have clinical, microbiologic, and immunologic efficacy in acute infectious diarrhea. Pediatr. Infect. Dis. J. 2010, 29, 135–138. [Google Scholar] [CrossRef]
- Fooladi, A.A.I.; Hosseini, H.M.; Nourani, M.R.; Khani, S.; Alavian, S.M. Probiotic as a novel treatment strategy against liver disease. Zahedan J. Res. Med Sci. 2013, 13, e7521. [Google Scholar]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Filannino, A.; Cavallo, L.; Francavilla, R. Lactobacillus reuteri accelerates gastric emptying and improves regurgitation in infants. Eur. J. Clin. Investig. 2011, 41, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Cianci, R.; Bibbò, S.; Gasbarrini, A.; Currò, D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: Potential for therapy. Pharmacol. Ther. 2015, 149, 191–212. [Google Scholar] [CrossRef]
- Waller, P.A.; Gopal, P.K.; Leyer, G.J.; Ouwehand, A.C.; Reifer, C.; Stewart, M.E.; Miller, L.E. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 2011, 46, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Hollman, P.C.H.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Andrew, R.; Izzo, A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 174, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.; Ullah, H.; Tundis, R.; Belwal, T.; Devkota, H.P.; Daglia, M.; Cetin, Z.; Saygili, E.I.; da Graça Campos, M.; Capanoglu, E. Dietary flavonoids in the management of huntington’s disease: Mechanism and clinical perspective. eFood 2020, 1, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.; Zarzuelo, A.; Martinez-Augustin, O.; Medina, F.S.D. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Saha, P.; San Yeoh, B.; Singh, R.; Chandrasekar, B.; Vemula, P.K.; Haribabu, B.; Vijay-Kumar, M.; Jala, V.R. Gut microbiota conversion of dietary ellagic acid into bioactive phytoceutical urolithin a inhibits heme peroxidases. PLoS ONE 2016, 11, e0156811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Zhang, S.; Al-Maghout, T.; Cao, I.; Pelzl, L.; Salker, M.; Veldhoen, M.; Cheng, A.; Lang, F. Gut bacterial metabolite Urolithin A (UA) mitigates Ca2+ entry in T cells by regulating miR-10a-5p. Front. Immunol. 2019, 10, 1737. [Google Scholar]
- Mena, P.; Dall’Asta, M.; Calani, L.; Brighenti, F.; Del Rio, D. Gastrointestinal stability of urolithins: An in vitro approach. Eur. J. Nutr. 2017, 56, 99–106. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; García-Villalba, R.; Mira, A.; Espín, J.C.; Tomás-Barberán, F.A. Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin a from ellagic acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human gut microbiota and gastrointestinal cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef]
- Allsopp, P.; Possemiers, S.; Campbell, D.; Gill, C.; Rowland, I. A comparison of the anticancer properties of isoxanthohumol and 8-prenylnaringenin using in vitro models of colon cancer. Biofactors 2013, 39, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M. Pharmacological properties and traditional therapeutic uses of important Indian spices: A review. Int. J. Food Prop. 2010, 13, 1092–1116. [Google Scholar] [CrossRef]
- Singh, V.K.; Yadav, P.; Tadigoppula, N. Recent advances in the synthesis, chemical transformations and pharmacological studies of some important dietary spice’s constituents. Chem. Biol. Interface 2014, 4, 66–99. [Google Scholar]
- Kochhar, K. Dietary spices in health and diseases: I. Indian J. Physiol. Pharmacol. 2008, 52, 106–122. [Google Scholar] [PubMed]
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Indian J. Med Res. 2004, 119, 167. [Google Scholar] [PubMed]
- Shen, L.; Ji, H.-F. Intestinal microbiota and metabolic diseases: Pharmacological Implications. Trends Pharmacol. Sci. 2016, 37, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-Y.; Zhao, C.-N.; Xu, X.-Y.; Tang, G.-Y.; Corke, H.; Gan, R.-Y.; Li, H.-B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Ríos-Hoyo, A.; Cortés, M.J.; Rios-Ontiveros, H.; Meaney, E.; Ceballos, G.; Gutierrez-Salmean, G. Obesity, metabolic syndrome, and dietary therapeutical approaches with a special focus on nutraceuticals (polyphenols): A mini-review. Int. J. Vitam. Nutr. Res. 2014, 84, 113–123. [Google Scholar] [CrossRef]
- Davì, G.; Santilli, F.; Patrono, C. Nutraceuticals in diabetes and metabolic syndrome. Cardiovasc. Ther. 2010, 28, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Das, L. Nutraceuticals and their role in human health: A review. In Nutraceuticals and Functional Foods in Human Health and Disease Prevention; Bagchi, D., Preuss, H.G., Swaroop, A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 61–72. [Google Scholar]
- Kjølbæk, L.; Benítez-Páez, A.; Del Pulgar, E.M.G.; Brahe, L.K.; Liebisch, G.; Matysik, S.; Rampelli, S.; Vermeiren, J.; Brigidi, P.; Larsen, L.H. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin. Nutr. 2020, 39, 67–79. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Mäkeläinen, H.; Saarinen, M.; Stowell, J.; Rautonen, N.; Ouwehand, A. Xylo-oligosaccharides and lactitol promote the growth of Bifidobacterium lactis and Lactobacillus species in pure cultures. Benef. Microbes 2010, 1, 139–148. [Google Scholar] [CrossRef] [PubMed]
- de Cossío, L.F.; Fourrier, C.; Sauvant, J.; Everard, A.; Capuron, L.; Cani, P.D.; Layé, S.; Castanon, N. Impact of prebiotics on metabolic and behavioral alterations in a mouse model of metabolic syndrome. Brain Behav. Immun. 2017, 64, 33–49. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sroka-Oleksiak, A.; Młodzińska, A.; Bulanda, M.; Salamon, D.; Major, P.; Stanek, M.; Gosiewski, T. Metagenomic analysis of duodenal microbiota reveals a potential biomarker of dysbiosis in the course of obesity and type 2 diabetes: A pilot study. J. Clin. Med. 2020, 9, 369. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Liu, Y.; Li, F.; Gu, Z.; Liu, M.; Shao, T.; Zhang, L.; Zhou, G.; Pan, C.; He, L. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J. Nutr. Biochem. 2020, 75, 108256. [Google Scholar] [CrossRef]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.-H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef]
- Michael, D.; Jack, A.; Masetti, G.; Davies, T.; Loxley, K.; Kerry-Smith, J.; Plummer, J.; Marchesi, J.; Mullish, B.; McDonald, J. A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves well-being. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Marette, A.; Jobin, C. SCFAs take a toll en route to metabolic syndrome. Cell Metab. 2015, 22, 954–956. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Korecka, A.; de Wouters, T.; Cultrone, A.; Lapaque, N.; Pettersson, S.; Doré, J.; Blottière, H.M.; Arulampalam, V. ANGPTL4 expression induced by butyrate and rosiglitazone in human intestinal epithelial cells utilizes independent pathways. Am. J. Physiol. Liver Physiol. 2013, 304, G1025–G1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujico, J.R.; Baccan, G.C.; Gheorghe, A.; Díaz, L.E.; Marcos, A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br. J. Nutr. 2013, 110, 711–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kien, C.L.; Bunn, J.Y.; Poynter, M.E.; Stevens, R.; Bain, J.; Ikayeva, O.; Fukagawa, N.K.; Champagne, C.M.; Crain, K.I.; Koves, T.R. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes 2013, 62, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Molcan, E.; DeCoffe, D.; Dai, C.; Gibson, D.L. Diets rich in n-6 PUFA induce intestinal microbial dysbiosis in aged mice. Br. J. Nutr. 2013, 110, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-N.; Zhu, J.; Pan, W.-S.; Shen, S.-R.; Shan, W.-G.; Das, U.N. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch. Med. Res. 2014, 45, 195–202. [Google Scholar] [CrossRef]
- Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Francini-Pesenti, F.; Spinella, P.; Calò, L.A. Potential role of phytochemicals in metabolic syndrome prevention and therapy. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1987. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.-D.; Zhang, Q.-Y.; Mi, M.-T. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef]
- Papathanasopoulos, A.; Camilleri, M. Dietary fiber supplements: Effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology 2010, 138, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Sohrab, G.; Hosseini-Esfahani, F.; Azizi, F. Inverse association between fruit, legume, and cereal fiber and the risk of metabolic syndrome: Tehran lipid and glucose study. Diabetes Res. Clin. Pract. 2011, 94, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Galisteo, M.; Duarte, J.; Zarzuelo, A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008, 19, 71–84. [Google Scholar] [CrossRef]
- Aleixandre, A.; Miguel, M. Dietary fiber in the prevention and treatment of metabolic syndrome: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 905–912. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Muccioli, G.G.; Deldicque, L.; Bindels, L.B.; Pachikian, B.D.; Sohet, F.M. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 712–722. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Possemiers, S.; Druart, C.; Van de Wiele, T.; De Backer, F.; Cani, P.D.; Larondelle, Y.; Delzenne, N.M. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 2011, 6, e20944. [Google Scholar] [CrossRef] [Green Version]
- Farhangi, M.A.; Javid, A.Z.; Sarmadi, B.; Karimi, P.; Dehghan, P. A randomized controlled trial on the efficacy of resistant dextrin, as functional food, in women with type 2 diabetes: Targeting the hypothalamic-pituitary-adrenal axis and immune system. Clin. Nutr. 2018, 37, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, X.; Li, J.; Li, Z.; Hu, Q.; Li, L.; Hao, X.; Song, M.; Li, C. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 2020, 60, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Cerdó, T.; García-Santos, J.A.; G Bermúdez, M.; Campoy, C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehl, A.M.; Day, C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef]
- Alcock, J.; Lin, H.C. Fatty acids from diet and microbiota regulate energy metabolism. F1000Research 2015, 4, 738. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [Green Version]
- Holzer, R.G.; Park, E.-J.; Li, N.; Tran, H.; Chen, M.; Choi, C.; Solinas, G.; Karin, M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 2011, 147, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.; Magness, S.; Jobin, C.; Lund, P.K. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Furet, J.-P.; Debard, C.; Daira, P.; Loizon, E.; Géloën, A.; Soulage, C.O.; Simonet, C.; Lefils-Lacourtablaise, J.; Bernoud-Hubac, N. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E374–E386. [Google Scholar] [CrossRef] [Green Version]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrne, S.; Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 2013, 8, e80476. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Liu, J.; Jackson, M.I.; Zhao, F.-Q.; Yan, L.; Combs, G.F., Jr. Fatty liver accompanies an increase in lactobacillus species in the hind gut of C57BL/6 mice fed a high-fat diet. J. Nutr. 2013, 143, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.Y.; Leone, V.A.; Devkota, S.; Wang, Y.; Brady, M.J.; Chang, E.B. Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue. J. Parenter. Enter. Nutr. 2013, 37, 746–754. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Bhan, A.K.; Kang, J.X. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int. J. Obes. 2016, 40, 1039–1042. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bobe, G.; Revel, J.S.; Rodrigues, R.R.; Sharpton, T.J.; Fantacone, M.L.; Raslan, K.; Miranda, C.L.; Lowry, M.B.; Blakemore, P.R. Improvements in metabolic syndrome by xanthohumol derivatives are linked to altered gut microbiota and bile acid metabolism. Mol. Nutr. Food Res. 2020, 64, 1900789. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szilagyi, A. Relationship (s) between obesity and inflammatory bowel diseases: Possible intertwined pathogenic mechanisms. Clin. J. Gastroenterol. 2019, 13, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
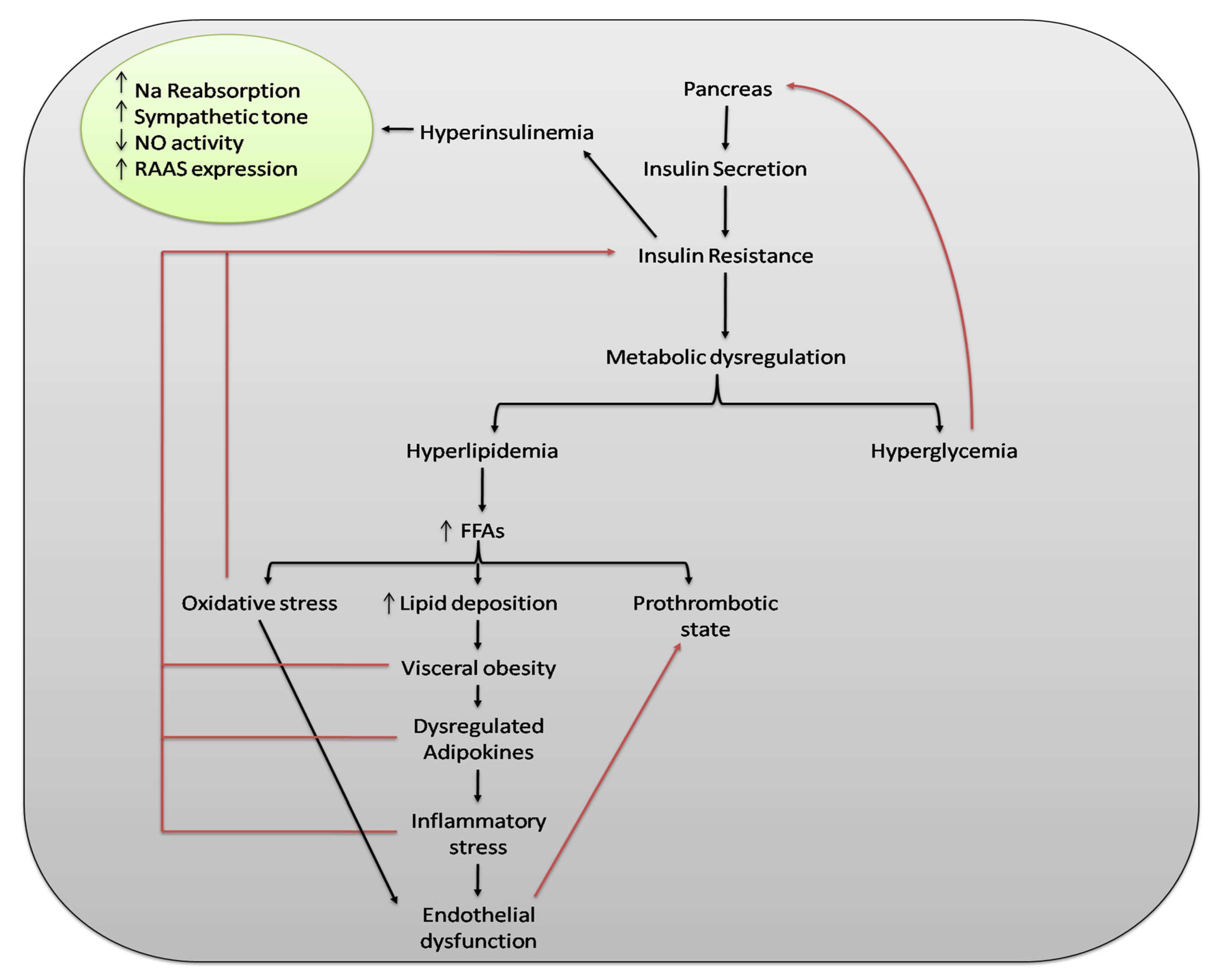

| Dietary Components | Potential Benefits in GI Health | References |
|---|---|---|
| Dietary fibers |
| [62,63,64,65,66] |
| Prebiotics |
| [67,68,69] |
| Probiotics |
| [70,71,72,73,74] |
| Polyphenols |
| [75,76,77] |
| Spices |
| [78,79,80] |
| Dietary Components | Potential Benefits in MS | References |
|---|---|---|
| Dietary fibers |
| [133,134] |
| Prebiotics |
| [135,136,137] |
| Probiotics |
| [138,139,140,141] |
| Short chain fatty acids |
| [142,143,144,145] |
| Monounsaturated fatty acids (oleic acid) |
| [146,147] |
| Polyunsaturated fatty acids (omega-3 fatty acids) |
| [148,149,150,151] |
| Polyphenols |
| [152,153,154,155,156] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Filippis, A.; Ullah, H.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Gastrointestinal Disorders and Metabolic Syndrome: Dysbiosis as a Key Link and Common Bioactive Dietary Components Useful for their Treatment. Int. J. Mol. Sci. 2020, 21, 4929. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144929
De Filippis A, Ullah H, Baldi A, Dacrema M, Esposito C, Garzarella EU, Santarcangelo C, Tantipongpiradet A, Daglia M. Gastrointestinal Disorders and Metabolic Syndrome: Dysbiosis as a Key Link and Common Bioactive Dietary Components Useful for their Treatment. International Journal of Molecular Sciences. 2020; 21(14):4929. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144929
Chicago/Turabian StyleDe Filippis, Anna, Hammad Ullah, Alessandra Baldi, Marco Dacrema, Cristina Esposito, Emanuele Ugo Garzarella, Cristina Santarcangelo, Ariyawan Tantipongpiradet, and Maria Daglia. 2020. "Gastrointestinal Disorders and Metabolic Syndrome: Dysbiosis as a Key Link and Common Bioactive Dietary Components Useful for their Treatment" International Journal of Molecular Sciences 21, no. 14: 4929. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21144929






