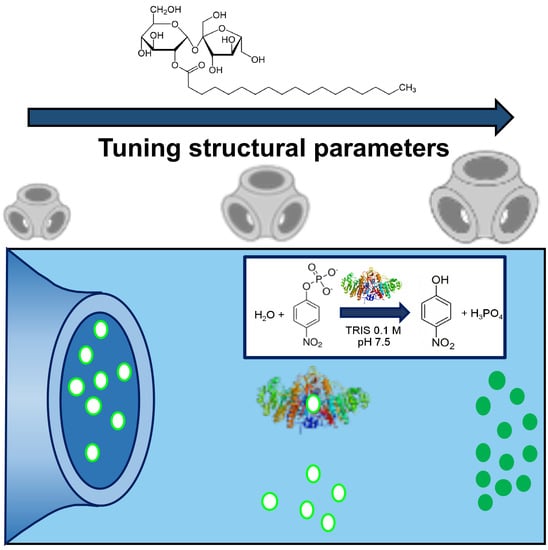
Controlling the Kinetics of an Enzymatic Reaction through Enzyme or Substrate Confinement into Lipid Mesophases with Tunable Structural Parameters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure of Phytantriol/Sucrose Stearate Lipid Mesophases
2.2. Alkaline Phosphatase Enzymatic Reaction in Aqueous Solution
2.3. Alkaline Phosphatase Substrate Inclusion in Lipid Mesophases
2.4. Alkaline Phosphatase Inclusion in Lipid Mesophases
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.2.1. Small Angle X-ray Scattering
3.2.2. UV–Vis Spectrophotometer:
3.3. Samples Preparation
3.3.1. SAXS Bulk Cubic Phases
3.3.2. UV–Vis Bulk Cubic Phases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP | Alkaline Phosphatase |
| Phyt | Phytantriol |
| SS | Sucrose Stearate |
| GMO | Glycerol monooleate |
| IPMS | Infinite Periodical Minimal Surfaces |
| SAXS | Small-Angle X-ray Scattering |
References
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef] [PubMed]
- Bulpett, J.M.; Snow, T.; Quignon, B.; Beddoes, C.M.; Tang, T.-Y.D.; Mann, S.; Shebanova, O.; Pizzey, C.L.; Terrill, N.J.; Davis, S.A.; et al. Hydrophobic nanoparticles promote lamellar to inverted hexagonal transition in phospholipid mesophases. Soft Matter 2015, 11, 8789–8800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, T.; Hasan, M.; Islam, M.Z.; Moniruzzaman, M.; Yamazaki, M. Low-pH-Induced Lamellar to Bicontinuous Primitive Cubic Phase Transition in Dioleoylphosphatidylserine/Monoolein Membranes. Langmuir 2017, 33, 12487–12496. [Google Scholar] [CrossRef]
- Oka, T.; Tsuboi, T.A.; Saiki, T.; Takahashi, T.; Alam, J.M.; Yamazaki, M. Initial step of pH-jump-induced lamellar to bicontinuous cubic phase transition in dioleoylphosphatidylserine/monoolein. Langmuir 2014, 30, 8131–8140. [Google Scholar] [CrossRef]
- Oka, T.; Saiki, T.; Alam, J.M.; Yamazaki, M. Activation Energy of the Low-pH-Induced Lamellar to Bicontinuous Cubic Phase Transition in Dioleoylphosphatidylserine/Monoolein. Langmuir 2016, 32, 1327–1337. [Google Scholar] [CrossRef]
- Yaghmur, A.; Laggner, P.; Zhang, S.; Rappolt, M. Tuning curvature and stability of monoolein bilayers by designer lipid-like peptide surfactants. PLoS ONE 2007, 2, e479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearman, G.C.; Ces, O.; Templer, R.H.; Seddon, J.M. Inverse lyotropic phases of lipids and membrane curvature. J. Phys. Condens. Matter 2006, 18, S1105–S1124. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. Diffusion, Molecular Separation, and Drug Delivery from Lipid Mesophases with Tunable Water Channels. Langmuir 2012, 28, 16455–16462. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Cubic Lipid−Water Phase Dispersed into Submicron Particles. Langmuir 1996, 12, 4611–4613. [Google Scholar] [CrossRef]
- Van’T Hag, L.; De Campo, L.; Garvey, C.J.; Feast, G.C.; Leung, A.E.; Yepuri, N.R.; Knott, R.; Greaves, T.L.; Tran, N.; Gras, S.L.; et al. Using SANS with Contrast-Matched Lipid Bicontinuous Cubic Phases to Determine the Location of Encapsulated Peptides, Proteins, and Other Biomolecules. J. Phys. Chem. Lett. 2016, 7, 2862–2866. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, Y.D.; Boyd, B.J. Selective Sequence for the Peptide-Triggered Phase Transition of Lyotropic Liquid-Crystalline Structures. Langmuir 2016, 32, 5155–5161. [Google Scholar] [CrossRef] [PubMed]
- Speziale, C.; Zabara, A.F.; Drummond, C.J.; Mezzenga, R. Active Gating, Molecular Pumping, and Turnover Determination in Biomimetic Lipidic Cubic Mesophases with Reconstituted Membrane Proteins. ACS Nano 2017, 11, 11687–11693. [Google Scholar] [CrossRef]
- Azmi, I.D.M.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Murgia, S.; Bonacchi, S.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Talmon, Y.; et al. Drug-loaded fluorescent cubosomes: Versatile nanoparticles for potential theranostic applications. Langmuir 2013, 29, 6673–6679. [Google Scholar] [CrossRef] [PubMed]
- Murgia, S.; Falchi, A.M.; Meli, V.; Schillén, K.; Lippolis, V.; Monduzzi, M.; Rosa, A.; Schmidt, J.; Talmon, Y.; Bizzarri, R.; et al. Cubosome formulations stabilized by a dansyl-conjugated block copolymer for possible nanomedicine applications. Colloids Surf. B Biointerfaces 2015, 129, 87–94. [Google Scholar] [CrossRef]
- Caltagirone, C.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Sgarzi, M.; Talmon, Y.; et al. Cancer-cell-targeted theranostic cubosomes. Langmuir 2014, 30, 6228–6236. [Google Scholar] [CrossRef]
- Bazylińska, U.; Kulbacka, J.; Schmidt, J.; Talmon, Y.; Murgia, S. Polymer-free cubosomes for simultaneous bioimaging and photodynamic action of photosensitizers in melanoma skin cancer cells. J. Colloid Interface Sci. 2018, 522, 163–173. [Google Scholar] [CrossRef]
- Van’T Hag, L.; De Campo, L.; Tran, N.; Sokolova, A.; Trenker, R.; Call, M.E.; Call, M.J.; Garvey, C.J.; Leung, A.E.; Darwish, T.A.; et al. Protein-Eye View of the in Meso Crystallization Mechanism. Langmuir 2019, 35, 8344–8356. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Assenza, S.; Mezzenga, R. Spatiotemporal Control of Enzyme-Induced Crystallization Under Lyotropic Liquid Crystal Nanoconfinement. Angew. Chem. Int. Ed. 2019, 58, 7289–7293. [Google Scholar] [CrossRef]
- Montis, C.; Castroflorio, B.; Mendozza, M.; Salvatore, A.; Berti, D.; Baglioni, P. Magnetocubosomes for the delivery and controlled release of therapeutics. J. Colloid Interface Sci. 2015, 449, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mendozza, M.; Montis, C.; Baglioni, P.; Berti, D. On the thermotropic and magnetotropic phase behavior of lipid liquid crystals containing magnetic nanoparticles. Nanoscale 2018, 10, 3480–3488. [Google Scholar] [CrossRef]
- Tran, N.; Hawley, A.M.; Zhai, J.; Muir, B.W.; Fong, C.; Drummond, C.J.; Mulet, X. High-Throughput Screening of Saturated Fatty Acid Influence on Nanostructure of Lyotropic Liquid Crystalline Lipid Nanoparticles. Langmuir 2016, 32, 4509–4520. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. pH-responsive lyotropic liquid crystals for controlled drug delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef] [PubMed]
- Tyler, A.I.I.; Barriga, H.M.G.; Parsons, E.S.; McCarthy, N.L.C.; Ces, O.; Law, R.V.; Seddon, J.M.; Brooks, N.J. Electrostatic swelling of bicontinuous cubic lipid phases. Soft Matter 2015, 11, 3279–3286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barriga, H.M.G.; Tyler, A.I.I.; McCarthy, N.L.C.; Parsons, E.S.; Ces, O.; Law, R.V.; Seddon, J.M.; Brooks, N.J. Temperature and pressure tuneable swollen bicontinuous cubic phases approaching nature’s length scales. Soft Matter 2015, 11, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Tangso, K.J.; Fong, W.K.; Darwish, T.; Kirby, N.; Boyd, B.J.; Hanley, T.L. Novel spiropyran amphiphiles and their application as light-responsive liquid crystalline components. J. Phys. Chem. B 2013, 117, 10203–10210. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Du, J.D.; Hawley, A.; Fong, W.K.; Graham, B.; Boyd, B.J. Investigation of Donor-Acceptor Stenhouse Adducts as New Visible Wavelength-Responsive Switching Elements for Lipid-Based Liquid Crystalline Systems. Langmuir 2017, 33, 2215–2221. [Google Scholar] [CrossRef]
- Jia, S.; Tan, A.; Hawley, A.; Graham, B.; Boyd, B.J. Visible light-triggered cargo release from donor acceptor Stenhouse adduct (DASA)-doped lyotropic liquid crystalline nanoparticles. J. Colloid Interface Sci. 2019, 548, 151–159. [Google Scholar] [CrossRef]
- Mendozza, M.; Caselli, L.; Montis, C.; Orazzini, S.; Carretti, E.; Baglioni, P.; Berti, D. Inorganic nanoparticles modify the phase behavior and viscoelastic properties of non-lamellar lipid mesophases. J. Colloid Interface Sci. 2019, 541, 329–338. [Google Scholar] [CrossRef]
- Fong, W.K.; Hanley, T.L.; Thierry, B.; Tilley, A.; Kirby, N.; Waddington, L.J.; Boyd, B.J. Understanding the photothermal heating effect in non-lamellar liquid crystalline systems, and the design of new mixed lipid systems for photothermal on-demand drug delivery. Phys. Chem. Chem. Phys. 2014, 16, 24936–24953. [Google Scholar] [CrossRef] [PubMed]
- Wlodek, M.; Kolasinska-Sojka, M.; Szuwarzynski, M.; Kereïche, S.; Kovacik, L.; Zhou, L.; Islas, L.; Warszynski, P.; Briscoe, W.H. Supported lipid bilayers with encapsulated quantum dots (QDs): Via liposome fusion: Effect of QD size on bilayer formation and structure. Nanoscale 2018, 10, 17965–17974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendozza, M.; Caselli, L.; Salvatore, A.; Montis, C.; Berti, D. Nanoparticles and organized lipid assemblies: From interaction to design of hybrid soft devices. Soft Matter 2019, 15, 8951–8970. [Google Scholar] [CrossRef]
- Das, K.; Roy, B.; Satpathi, S.; Hazra, P. Impact of Topology on the Characteristics of Water inside Cubic Lyotropic Liquid Crystalline Systems. J. Phys. Chem. B 2019, 123, 4118–4128. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Vallooran, J.J.; Zabara, A.; Mezzenga, R. Controlling enzymatic activity and kinetics in swollen mesophases by physical nano-confinement. Nanoscale 2014, 6, 6853–6859. [Google Scholar] [CrossRef]
- Assenza, S.; Mezzenga, R. Curvature and bottlenecks control molecular transport in inverse bicontinuous cubic phases. J. Chem. Phys. 2018, 148, 054902. [Google Scholar] [CrossRef] [PubMed]
- Barauskas, J.; Landh, T. Phase Behavior of the Phytantriol/Water System. Langmuir 2003, 19, 9562–9565. [Google Scholar] [CrossRef]
- Marlow, J.B.; McCoy, T.M.; Ho, C.Q.; de Campo, L.; Knott, R.; Bell, T.D.M.; Tabor, R.F. Tuning the structure, thermal stability and rheological properties of liquid crystal phases via the addition of silica nanoparticles. Phys. Chem. Chem. Phys. 2019, 21, 25649–25657. [Google Scholar] [CrossRef]
- Dean, R.L. Kinetic studies with alkaline phosphatase in the presence and absence of inhibitors and divalent cations. Biochem. Mol. Biol. Educ. 2002, 30, 401–407. [Google Scholar] [CrossRef]
- Marlow, J.B.; Pottage, M.J.; McCoy, T.M.; De Campo, L.; Sokolova, A.; Bell, T.D.M.; Tabor, R.F. Structural and rheological changes of lamellar liquid crystals as a result of compositional changes and added silica nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 16592–16603. [Google Scholar] [CrossRef] [PubMed]
- Vallooran, J.J.; Negrini, R.; Mezzenga, R. Controlling anisotropic drug diffusion in lipid-Fe3O4 nanoparticle hybrid mesophases by magnetic alignment. Langmuir 2013, 29, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Vallooran, J.J.; Mezzenga, R. Enzyme Kinetics in Liquid Crystalline Mesophases: Size Matters, But Also Topology. Langmuir 2015, 31, 4558–4565. [Google Scholar] [CrossRef] [PubMed]
- Caritá, A.C.; Mattei, B.; Domingues, C.C.; De Paula, E.; Riske, K.A. Effect of Triton X-100 on Raft-Like Lipid Mixtures: Phase Separation and Selective Solubilization. Langmuir 2017, 33, 7312–7321. [Google Scholar] [CrossRef] [PubMed]
- Pizzirusso, A.; De Nicola, A.; Sevink, G.J.A.; Correa, A.; Cascella, M.; Kawakatsu, T.; Rocco, M.; Zhao, Y.; Celino, M.; Milano, G. Biomembrane solubilization mechanism by Triton X-100: A computational study of the three stage model. Phys. Chem. Chem. Phys. 2017, 19, 29780–29794. [Google Scholar] [CrossRef] [PubMed]
- De Backer, M.; McSweeney, S.; Rasmussen, H.B.; Riise, B.W.; Lindley, P.; Hough, E. The 1.9 Å crystal structure of heat-labile shrimp alkaline phosphatase. J. Mol. Biol. 2002, 318, 1265–1274. [Google Scholar] [CrossRef]





| 0% SS | 5% SS | 10% SS | 15% SS | |||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | d (nm) 4 | dw (nm) | d (nm) | dw (nm) | d (nm) | dw (nm) | d (nm) | dw (nm) |
| 25 | 6.6 1 ± 0.1 | 2.3 ± 0.1 | 7.2 1 ± 0.1 | 2.8 ± 0.1 | 7.5 1 ± 0.1 | 3.0 ± 0.1 | 10.5 3 ± 0.2 | 3.6 ± 0.1 |
| 30 | 6.5 1 ± 0.1 | 2.2 ± 0.1 | 7.0 1 ± 0.1 | 2.6 ± 0.1 | 7.2 1 ± 0.2 | 2.8 ± 0.1 | 10.3 3 ± 0.1 | 3.4 ± 0.1 |
| 35 | 6.4 1 ± 0.1 | 2.2 ± 0.1 | 6.7 1 ± 0.1 | 2.4 ± 0.1 | 7.1 1 ± 0.2 | 2.7 ± 0.1 | 9.9 3 ± 0.2 | 3.2 ± 0.1 |
| 40 | 6.3 1 ± 0.1 | 2.0 ± 0.1 | 6.6 1 ± 0.1 | 2.3 ± 0.1 | 6.9 1 ± 0.1 | 2.6 ± 0.1 | 7.4 1 ± 0.2 | 2.9 ± 0.1 |
| 45 | 4.8 2 ± 0.2 | 1.9 ± 0.1 | 6.6 1 ± 0.2 | 2.3 ± 0.1 | 6.7 1 ± 0.2 | 2.4 ± 0.1 | 7.1 1 ± 0.1 | 2.7 ± 0.1 |
| 50 | 4.0 2 ± 0.1 | 1.2 ± 0.1 | 6.4 1 ± 0.2 | 2.2 ± 0.1 | 6.6 1 ± 0.1 | 2.3 ± 0.1 | 7.0 1 ± 0.1 | 2.6 ± 0.1 |
| Sample | Rate (mmol/min) Confined Substrate |
|---|---|
| No mesophase | 1.18 × 10−2 (±6 × 10−5) |
| Phyt | 3.78 × 10−4 (±3 × 10−6) |
| Phyt/5% SS | 5.30 × 10−4 (±1 × 10−5) |
| Phyt/10% SS | 5.5 × 10−4 (±1 × 10−5) |
| Phyt/15% SS | 8.56 × 10−4 (±3 × 10−6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendozza, M.; Balestri, A.; Montis, C.; Berti, D. Controlling the Kinetics of an Enzymatic Reaction through Enzyme or Substrate Confinement into Lipid Mesophases with Tunable Structural Parameters. Int. J. Mol. Sci. 2020, 21, 5116. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21145116
Mendozza M, Balestri A, Montis C, Berti D. Controlling the Kinetics of an Enzymatic Reaction through Enzyme or Substrate Confinement into Lipid Mesophases with Tunable Structural Parameters. International Journal of Molecular Sciences. 2020; 21(14):5116. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21145116
Chicago/Turabian StyleMendozza, Marco, Arianna Balestri, Costanza Montis, and Debora Berti. 2020. "Controlling the Kinetics of an Enzymatic Reaction through Enzyme or Substrate Confinement into Lipid Mesophases with Tunable Structural Parameters" International Journal of Molecular Sciences 21, no. 14: 5116. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21145116