Rapid Regulation of Human Multidrug and Extrusion Transporters hMATE1 and hMATE2K
Abstract
:1. Introduction
2. Results
Rapid Regulation of Efflux
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASP+ | 4-(4-(dimethylamino)styryl)-N-methylpyridinium |
| a.u. | arbitrary units |
| BCECF-AM | 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester |
| BSA | bovine serum albumin |
| CaM | calmodulin |
| CKII | casein-kinase II |
| DAPI | 2-(4-amidinophenyl)-1H-indole-6-carboxamidine |
| DOG | 1,2-dioctanoyl-sn-glycerol |
| HEK | human embryonic kidney |
| hMATE | human multidrug and toxins extrusion protein |
| hOCT | human organic cation transporter |
| IL | interleukin |
| MDCK | Madin–Darby canine kidney |
| NHE1 | Na+/H+ exchanger 1 |
| OCs | organic cations |
| PBS | phosphate-buffered saline |
| PDGF | platelet-derived growth factor |
| PFA | paraformaldehyde |
| PI3K | phosphatidylinositol 3-kinase |
| PKA | protein kinase A |
| PKC | protein kinase C |
| p56lck | p56lck tyrosine kinase |
| SDS | sodium dodecyl sulfate |
| SEM | standard error of the mean |
| TBBz | 4,5,6,7-tetrabromo-1H-benzimidazole |
| TNF | tumor necrosis factor |
References
- Koepsell, H. Polyspecific organic cation transporters: Their functions and interactions with drugs. Trends Pharmacol. Sci. 2004, 25, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.H. Role of organic cation transporters in the renal handling of therapeutic agents and xenobiotics. Toxicol. Appl. Pharmacol. 2005, 204, 309–319. [Google Scholar] [CrossRef]
- Wright, S.H.; Dantzler, W.H. Molecular and cellular physiology of renal organic cation and anion transport. Physiol. Rev. 2004, 84, 987–1049. [Google Scholar] [CrossRef]
- Wagner, D.J.; Hu, T.; Wang, J. Polyspecific organic cation transporters and their impact on drug intracellular levels and pharmacodynamics. Pharmacol. Res. 2016, 111, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.E.; Jay, C.E.; Sweet, D.H. Organic solute carrier 22 (SLC22) family: Potential for interactions with food, herbal/dietary supplements, endogenous compounds, and drugs. J. Food Drug Anal. 2018, 26, S45–S60. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, H.; Sakurai, Y.; Saito, H.; Masuda, S.; Urakami, Y.; Goto, M.; Fukatsu, A.; Ogawa, O.; Inui, K.K. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 2002, 13, 866–874. [Google Scholar]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. USA 2005, 102, 17923–17928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terada, T.; Inui, K. Physiological and pharmacokinetic roles of H+/organic cation antiporters (MATE/SLC47A). Biochem. Pharmacol. 2008, 75, 1689–1696. [Google Scholar] [CrossRef]
- Masuda, S.; Terada, T.; Yonezawa, A.; Tanihara, Y.; Kishimoto, K.; Katsura, T.; Ogawa, O.; Inui, K. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol. 2006, 17, 2127–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, T.; Hiasa, M.; Miyaji, T.; Kanamoto, T.; Matsumoto, T.; Otsuka, M.; Moriyama, Y.; Omote, H. Characterization of the human MATE2 proton-coupled polyspecific organic cation exporter. Int. J. Biochem. Cell Biol. 2011, 43, 913–918. [Google Scholar] [CrossRef]
- Yonezawa, A.; Inui, K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br. J. Pharmacol. 2011, 164, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wright, S.H. MATE1 has an external COOH terminus, consistent with a 13-helix topology. Am. J. Physiol Renal Physiol. 2009, 297, F263–F271. [Google Scholar] [CrossRef] [Green Version]
- König, J.; Zolk, O.; Singer, K.; Hoffmann, C.; Fromm, M.F. Double-transfected MDCK cells expressing human OCT1/MATE1 or OCT2/MATE1: Determinants of uptake and transcellular translocation of organic cations. Br. J. Pharmacol. 2011, 163, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inui, K.I.; Masuda, S.; Saito, H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 2000, 58, 944–958. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Masuda, S.; Yonezawa, A.; Tanihara, Y.; Katsura, T.; Inui, K. Transcellular transport of organic cations in double-transfected MDCK cells expressing human organic cation transporters hOCT1/hMATE1 and hOCT2/hMATE1. Biochem. Pharmacol. 2008, 76, 894–903. [Google Scholar] [CrossRef]
- Yonezawa, A.; Inui, K. Organic cation transporter OCT/SLC22A and H+/organic cation antiporter MATE/SLC47A are key molecules for nephrotoxicity of platinum agents. Biochem. Pharmacol. 2011, 81, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, S.; Yonezawa, A.; Masuda, S.; Fukatsu, A.; Katsura, T.; Inui, K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem. Pharmacol. 2007, 74, 477–487. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Schlatter, E. Regulation of organic cation transport. Pflügers Arch. 2005, 449, 423–441. [Google Scholar] [CrossRef]
- Matsushima, S.; Maeda, K.; Inoue, K.; Ohta, K.Y.; Yuasa, H.; Kondo, T.; Nakayama, H.; Horita, S.; Kusuhara, H.; Sugiyama, Y. The inhibition of human multidrug and toxin extrusion 1 is involved in the drug-drug interaction caused by cimetidine. Drug Metab. Dispos. 2009, 37, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Massmann, V.; Edemir, B.; Schlatter, E.; Al-Monajjed, R.; Harrach, S.; Klassen, P.; Holle, S.K.; Sindic, A.; Dobrivojevic, M.; Pavenstadt, H.; et al. The organic cation transporter 3 (OCT3) as molecular target of psychotropic drugs: Transport characteristics and acute regulation of cloned murine OCT3. Pflügers Arch. 2014, 466, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, E.; Klassen, P.; Massmann, V.; Holle, S.K.; Guckel, D.; Edemir, B.; Pavenstädt, H.; Ciarimboli, G. Mouse organic cation transporter 1 determines properties and regulation of basolateral organic cation transport in renal proximal tubules. Pflügers Arch. 2014, 466, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Wilde, S.; Schlatter, E.; Koepsell, H.; Edemir, B.; Reuter, S.; Pavenstädt, H.; Neugebauer, U.; Schröter, R.; Brast, S.; Ciarimboli, G. Calmodulin-associated post-translational regulation of rat organic cation transporter 2 in the kidney is gender dependent. Cell. Mol. Life Sci. 2009, 66, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.; Lang, D.; Gorboulev, V.; Koepsell, H.; Sindic, A.; Schröter, R.; Zvirbliene, A.; Pavenstädt, H.; Schlatter, E.; Ciarimboli, G. Characterization of regulatory mechanisms and states of human organic cation transporter 2. Am. J. Physiol. Cell Physiol. 2006, 290, C1521–C1531. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Koepsell, H.; Iordanova, M.; Gorboulev, V.; Dürner, B.; Lang, D.; Edemir, B.; Schröter, R.; van Le, T.; Schlatter, E. Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. J. Am. Soc. Nephrol. 2005, 16, 1562–1570. [Google Scholar] [CrossRef] [Green Version]
- Ciarimboli, G.; Struwe, K.; Arndt, P.; Gorboulev, V.; Koepsell, H.; Schlatter, E.; Hirsch, J.R. Regulation of the human organic cation transporter hOCT1. J. Cell Physiol. 2004, 201, 420–428. [Google Scholar] [CrossRef]
- Cetinkaya, I.; Ciarimboli, G.; Yalcinkaya, G.; Mehrens, T.; Velic, A.; Hirsch, J.R.; Gorboulev, V.; Koepsell, H.; Schlatter, E. Regulation of human organic cation transporter hOCT2 by PKA, PI3K, and calmodulin-dependent kinases. Am. J. Physiol. Renal Physiol. 2003, 284, F293–F302. [Google Scholar] [CrossRef] [Green Version]
- Willoughby, D.; Masada, N.; Crossthwaite, A.J.; Ciruela, A.; Cooper, D.M. Localized Na+/H+ exchanger 1 expression protects Ca2+-regulated adenylyl cyclases from changes in intracellular pH. J. Biol. Chem. 2005, 280, 30864–30872. [Google Scholar] [CrossRef] [Green Version]
- Dhein, S.; Salameh, A. Na+/H+-exchange inhibition by cariporide (Hoe 642): A new principle in cardiovascular medicin. Cardiovasc. Drug Rev. 1999, 17, 134–146. [Google Scholar] [CrossRef]
- Berkhin, E.B.; Humphreys, M.H. Regulation of renal tubular secretion of organic compounds. Kidney Int 2001, 59, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Czuba, L.C.; Hillgren, K.M.; Swaan, P.W. Post-translational modifications of transporters. Pharmacol. Ther. 2018, 192, 88–99. [Google Scholar] [CrossRef]
- Xu, D.; You, G. Loops and layers of post-translational modifications of drug transporters. Adv. Drug Deliv. Rev. 2017, 116, 37–44. [Google Scholar] [CrossRef]
- Keller, T.; Egenberger, B.; Gorboulev, V.; Bernhard, F.; Uzelac, Z.; Gorbunov, D.; Wirth, C.; Koppatz, S.; Dotsch, V.; Hunte, C.; et al. The large extracellular loop of organic cation transporter 1 influences substrate affinity and is pivotal for oligomerization. J. Biol. Chem. 2011, 286, 37874–37886. [Google Scholar] [CrossRef] [Green Version]
- Pelis, R.M.; Suhre, W.M.; Wright, S.H. Functional influence of N-glycosylation in OCT2-mediated tetraethylammonium transport. Am. J. Physiol. Renal. Physiol. 2006, 290, F1118–F1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelis, R.M.; Zhang, X.; Dangprapai, Y.; Wright, S.H. Cysteine accessibility in the hydrophilic cleft of the human organic cation transporter 2. J. Biol. Chem. 2006, 281, 35272–35280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brast, S.; Grabner, A.; Sucic, S.; Sitte, H.H.; Hermann, E.; Pavenstädt, H.; Schlatter, E.; Ciarimboli, G. The cysteines of the extracellular loop are crucial for trafficking of human organic cation transporter 2 to the plasma membrane and are involved in oligomerization. FASEB J. 2012, 26, 976–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nies, A.T.; Damme, K.; Kruck, S.; Schaeffeler, E.; Schwab, M. Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Arch. Toxicol. 2016, 90, 1555–1584. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lauber, C.; Harrach, S.; Pap, T.; Fischer, M.; Victor, M.; Heitzmann, M.; Hansen, U.; Fobker, M.; Brand, S.M.; Sindic, A.; et al. Transport mechanisms and their pathology-induced regulation govern tyrosine kinase inhibitor delivery in rheumatoid arthritis. PLoS ONE 2012, 7, e52247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrach, S.; Barz, V.; Pap, T.; Pavenstädt, H.; Schlatter, E.; Edemir, B.; Distler, J.; Ciarimboli, G.; Bertrand, J. Notch signaling activity determines uptake and biological effect of Imatinib in systemic sclerosis dermal fibroblasts. J. Investig. Dermatol. 2019, 139, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, S.; Muller, J.; Neugebauer, U.; Schroter, R.; Herrmann, E.; Pavenstadt, H.; Ciarimboli, G. Protein abundance of clinically relevant drug transporters in the human kidneys. Int. J. Mol. Sci. 2019, 20, 5303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Ren, J.; Gao, X.; Jin, C.; Wen, L.; Yao, X. GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol. Cell Proteomics 2008, 7, 1598–1608. [Google Scholar] [CrossRef] [Green Version]
- Claxton, D.P.; Jagessar, K.L.; Steed, P.R.; Stein, R.A.; Mchaourab, H.S. Sodium and proton coupling in the conformational cycle of a MATE antiporter from Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2018, 115, E6182–E6190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouw, M.; Michael, S.; Samano-Sanchez, H.; Kumar, M.; Zeke, A.; Lang, B.; Bely, B.; Chemes, L.B.; Davey, N.E.; Deng, Z.; et al. The eukaryotic linear motif resource—2018 update. Nucleic. Acids Res. 2018, 46, D428–D434. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, D.; Köppen, C.; Bauer, O.B.; Karst, U.; Schröter, R.; Tzvetkov, M.V.; Ciarimboli, G. Effects of single nucleotide polymorphism Ala270Ser (rs316019) on the function and regulation of hOCT2. Biomolecules 2019, 9, 578. [Google Scholar] [CrossRef] [Green Version]
- Hucke, A.; Park, G.Y.; Bauer, O.B.; Beyer, G.; Köppen, C.; Zeeh, D.; Wehe, C.A.; Sperling, M.; Schröter, R.; Kantauskaite, M.; et al. Interaction of the new monofunctional anticancer agent Phenanthriplatin with transporters for organic cations. Front. Chem. 2018, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Dukes, J.D.; Whitley, P.; Chalmers, A.D. The MDCK variety pack: Choosing the right strain. BMC Cell Biol. 2011, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Schulze, U.; Vollenbröker, B.; Braun, D.A.; Le, T.V.; Granado, D.; Kremerskothen, J.; Fränzel, B.; Klosowski, R.; Barth, J.; Fufezan, C.; et al. The Vac14-interaction network is linked to regulators of the endolysosomal and autophagic pathway. Mol. Cell Proteomics 2014, 13, 1397–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittwer, M.B.; Zur, A.A.; Khuri, N.; Kido, Y.; Kosaka, A.; Zhang, X.; Morrissey, K.M.; Sali, A.; Huang, Y.; Giacomini, K.M. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J. Med. Chem. 2013, 56, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Bright, G.R.; Fisher, G.W.; Rogowska, J.; Taylor, D.L. Fluorescence ratio imaging microscopy: Temporal and spatial measurements of cytoplasmic pH. J. Cell Biol. 1987, 104, 1019–1033. [Google Scholar] [CrossRef] [Green Version]
- Grant, R.L.; Acosta, D. Ratiometric measurement of intracellular pH of cultured cells with BCECF in a fluorescence multi-well plate reader. In Vitro Cell Dev. Biol. Anim. 1997, 33, 256–260. [Google Scholar] [CrossRef]
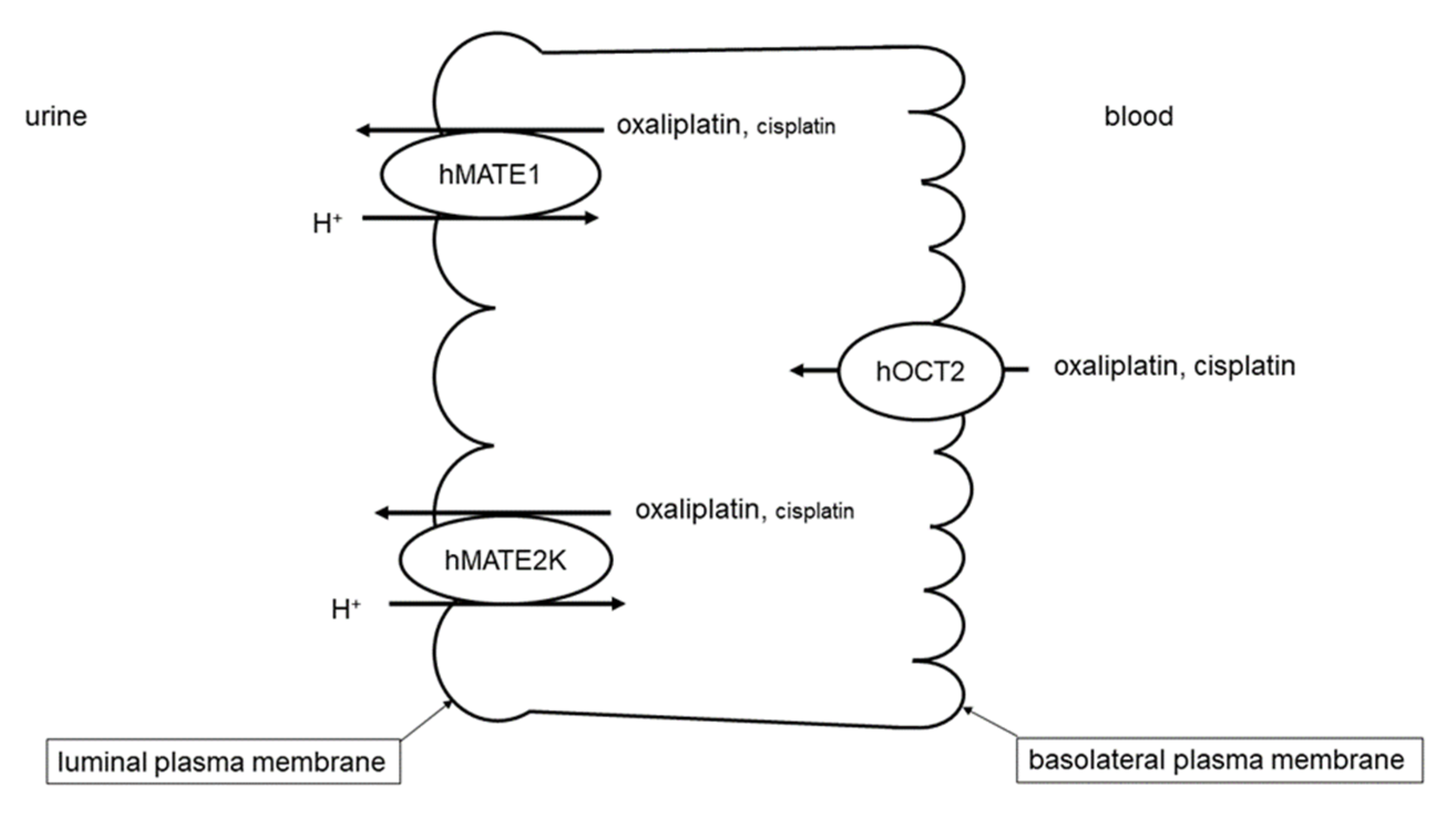

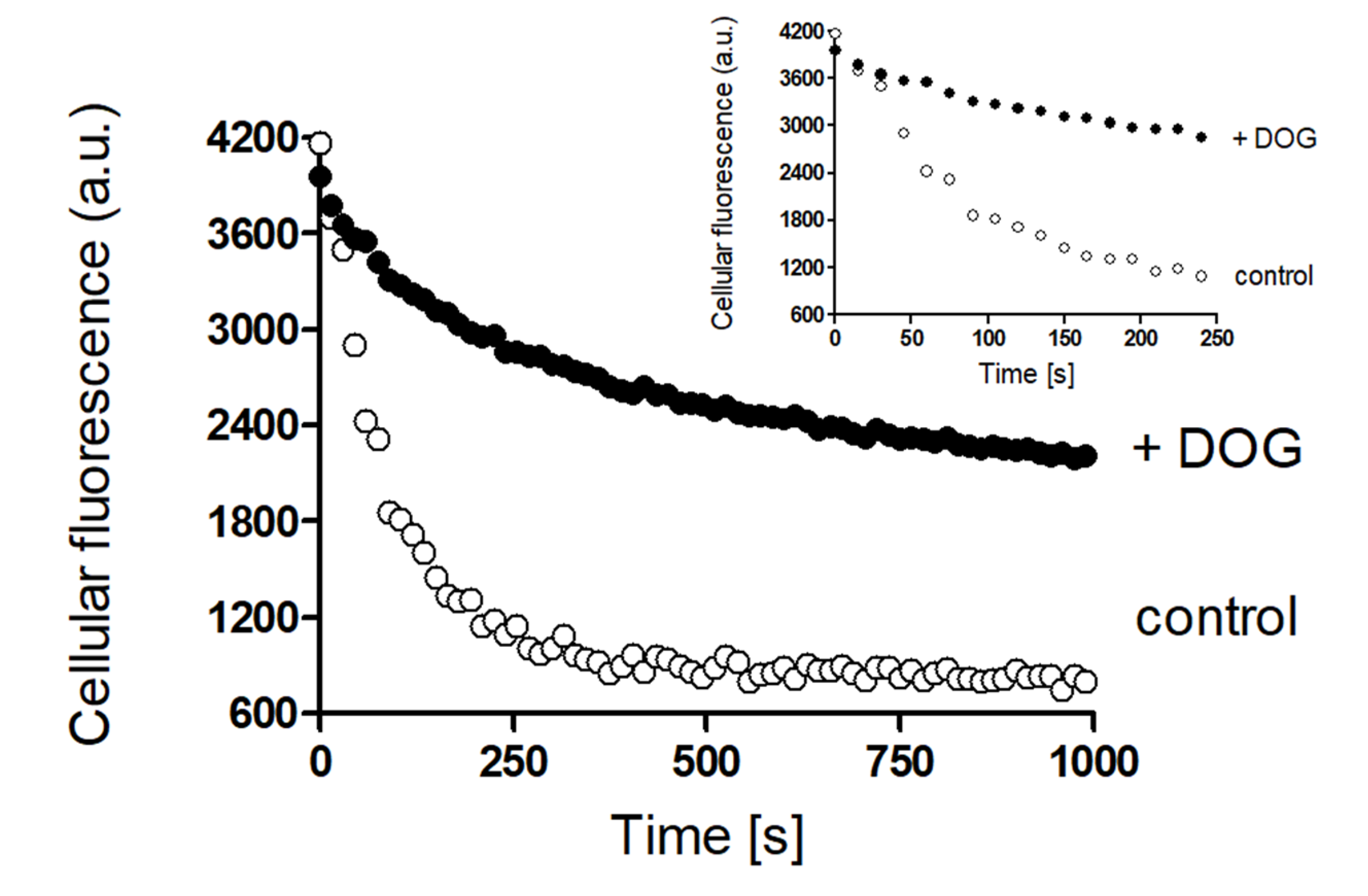
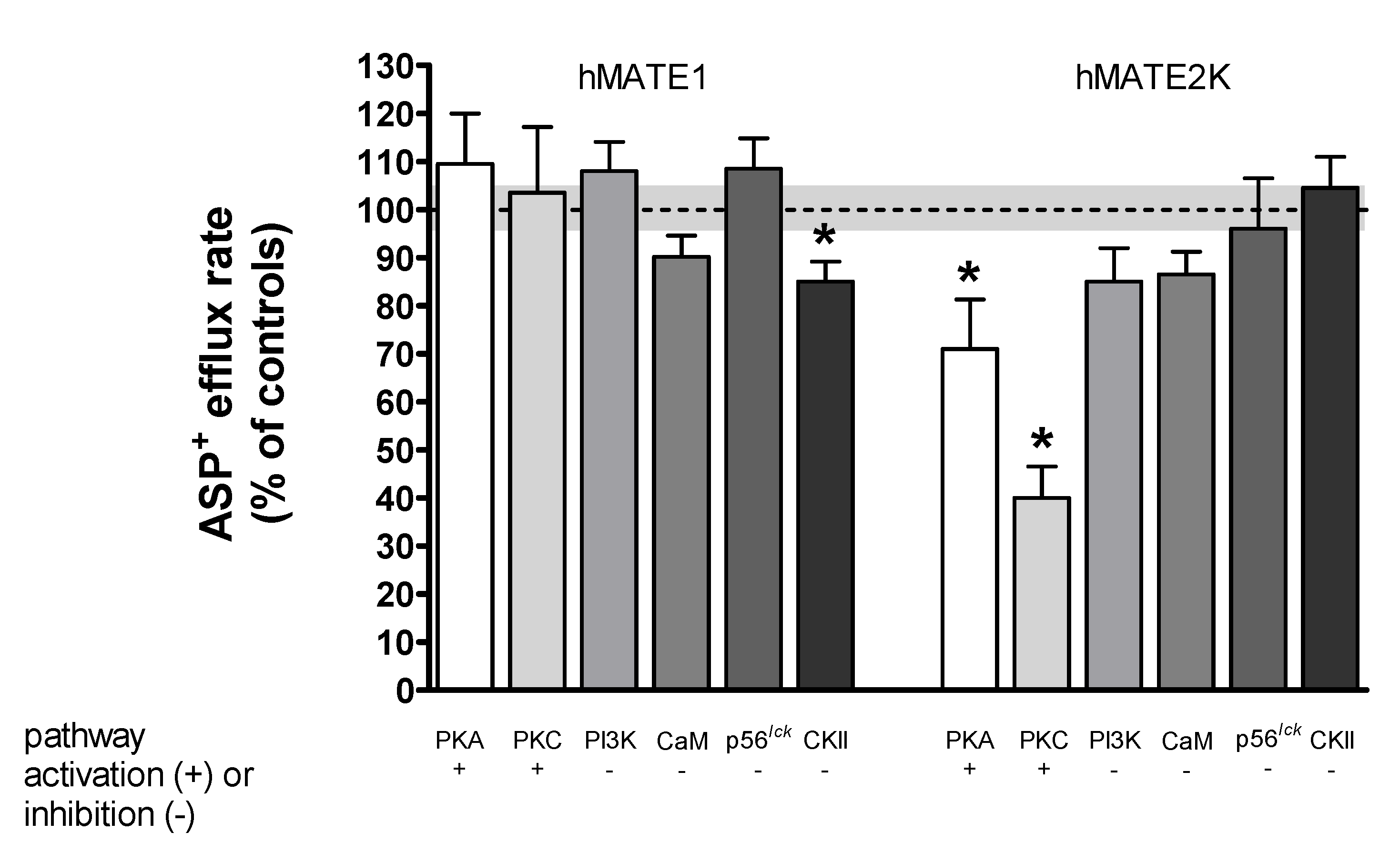
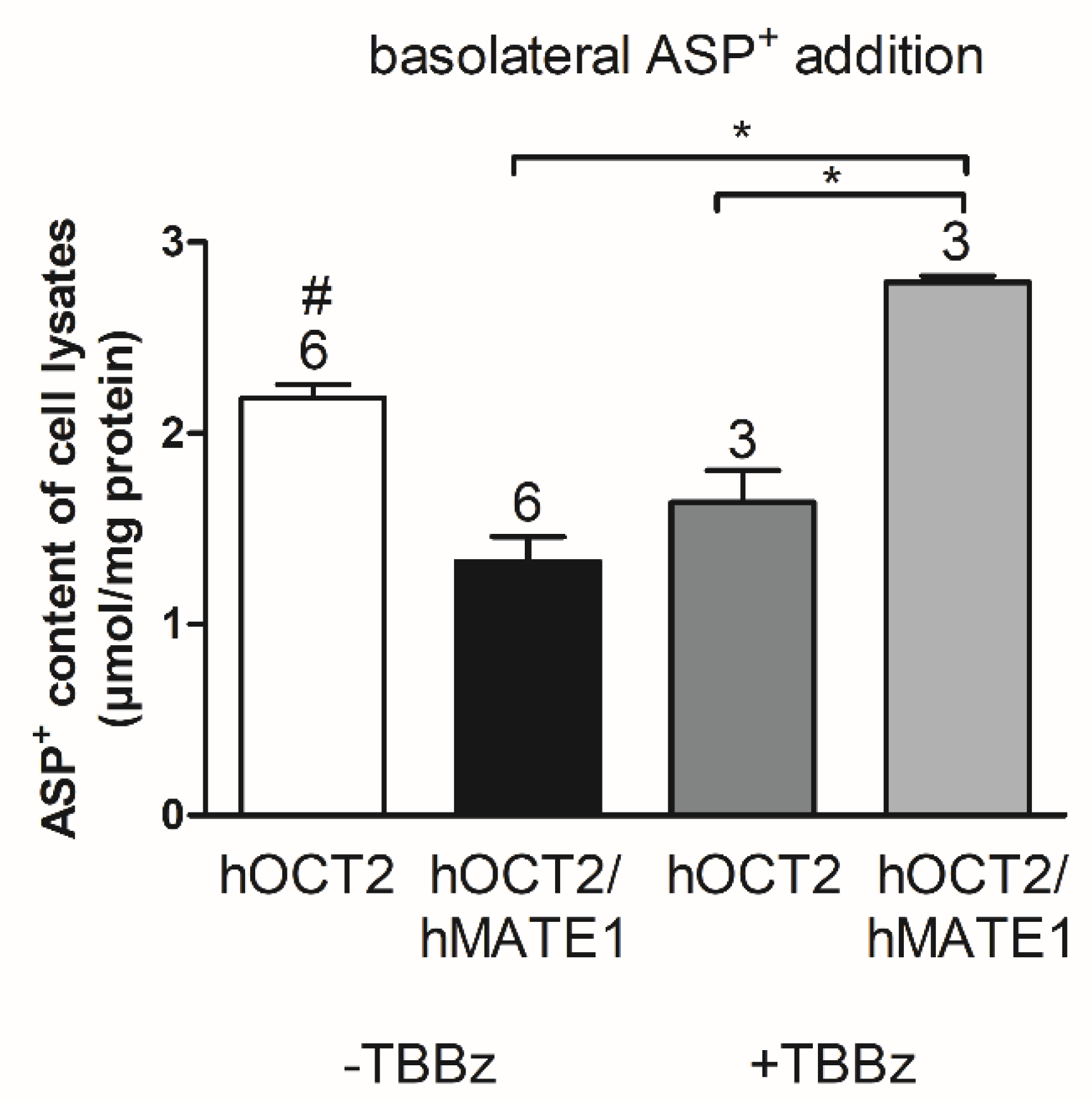
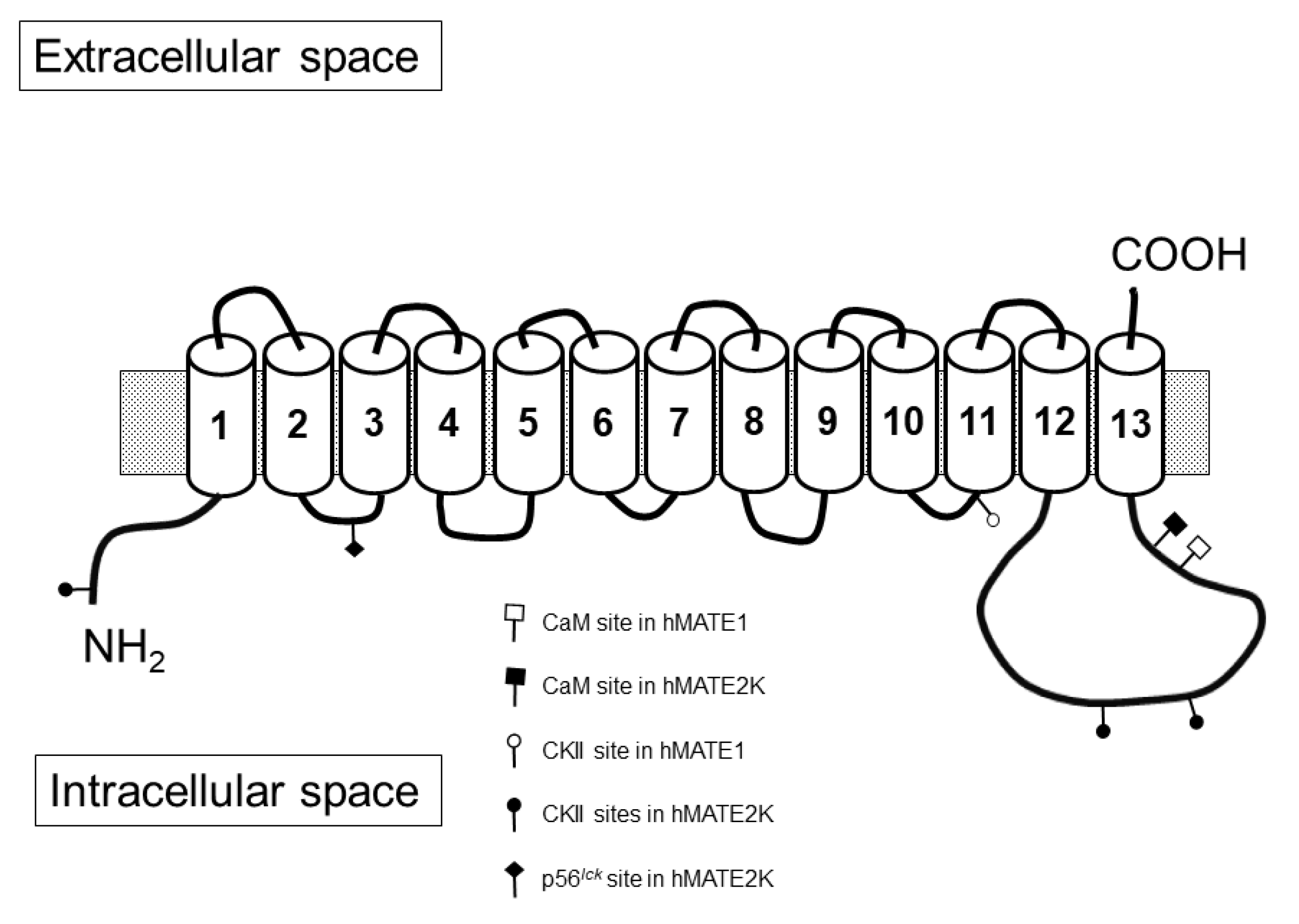
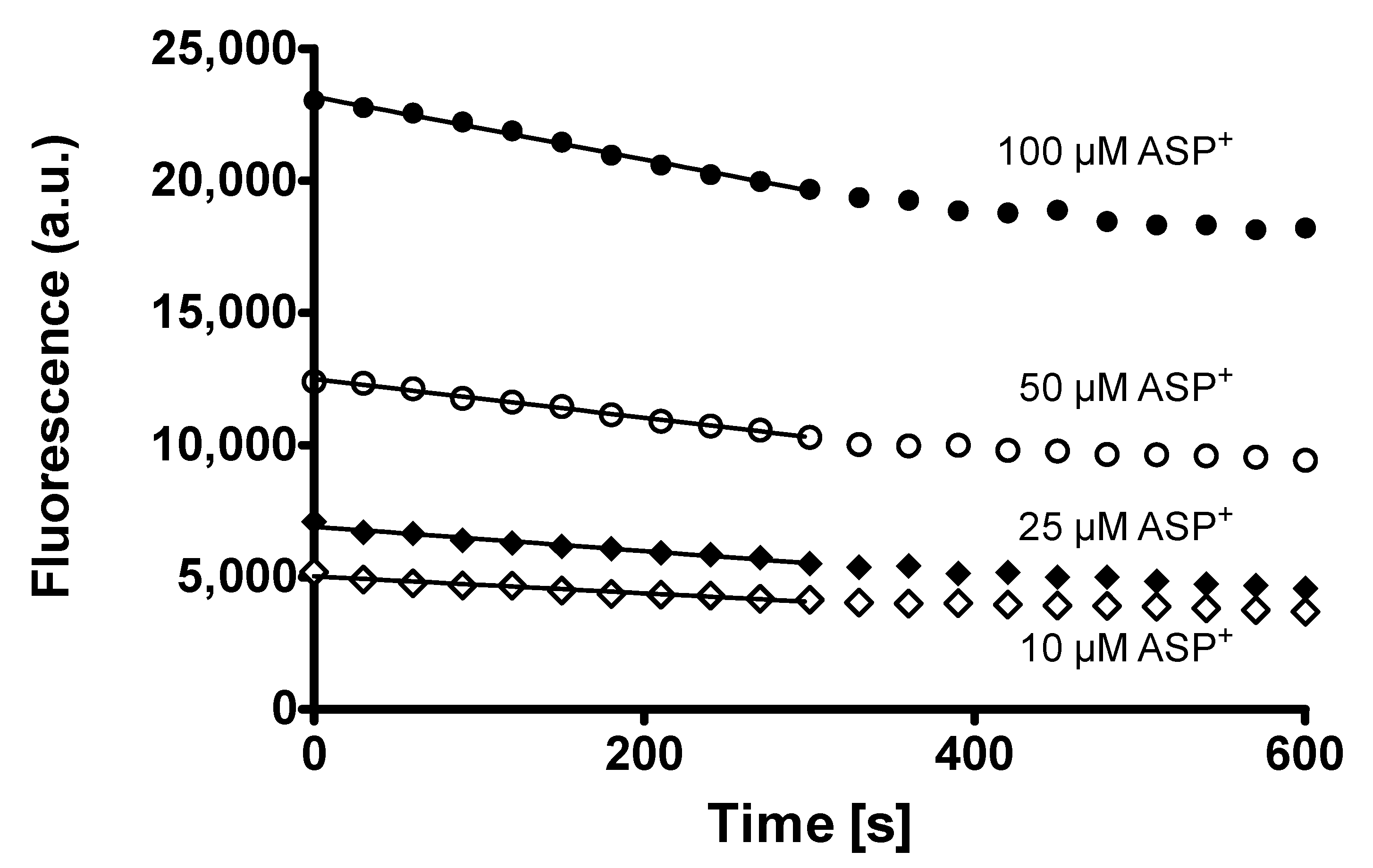
| Transport Direction | Transporter Affinity (Km) | |
|---|---|---|
| hMATE1 | hMATE2K | |
| Uptake | 14.2 ± 5.0 µM | 6.6 ± 1.0 µM |
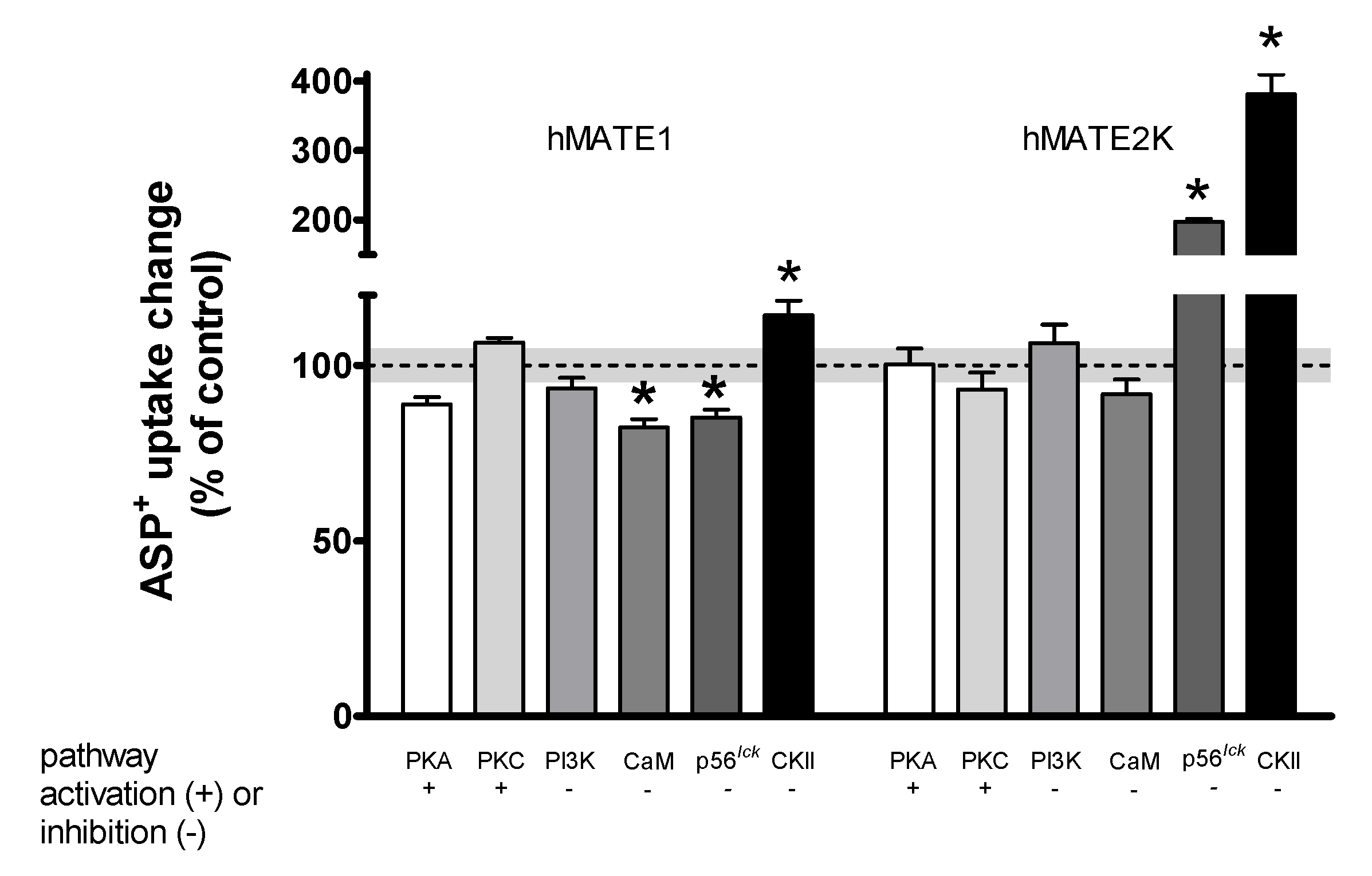
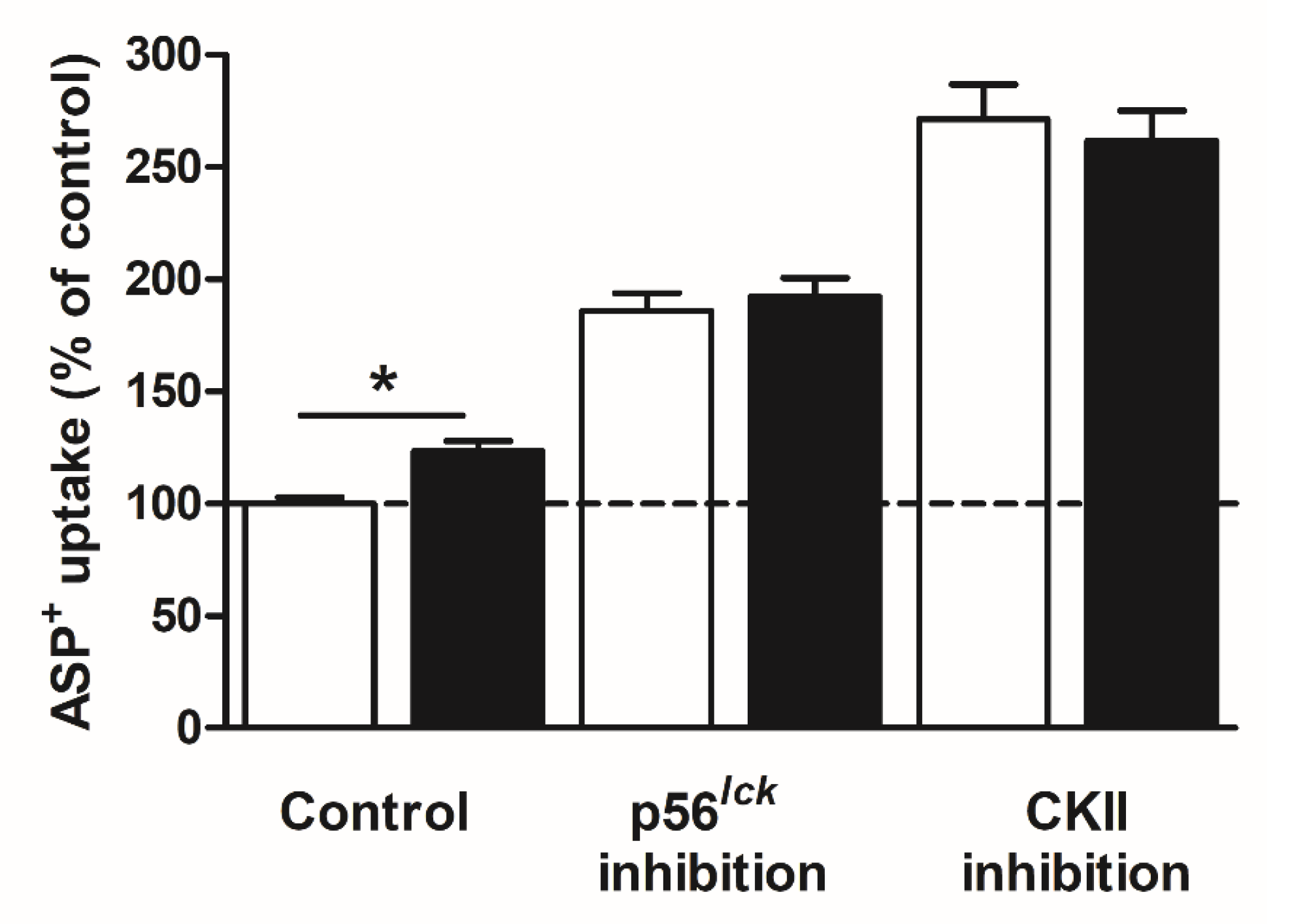
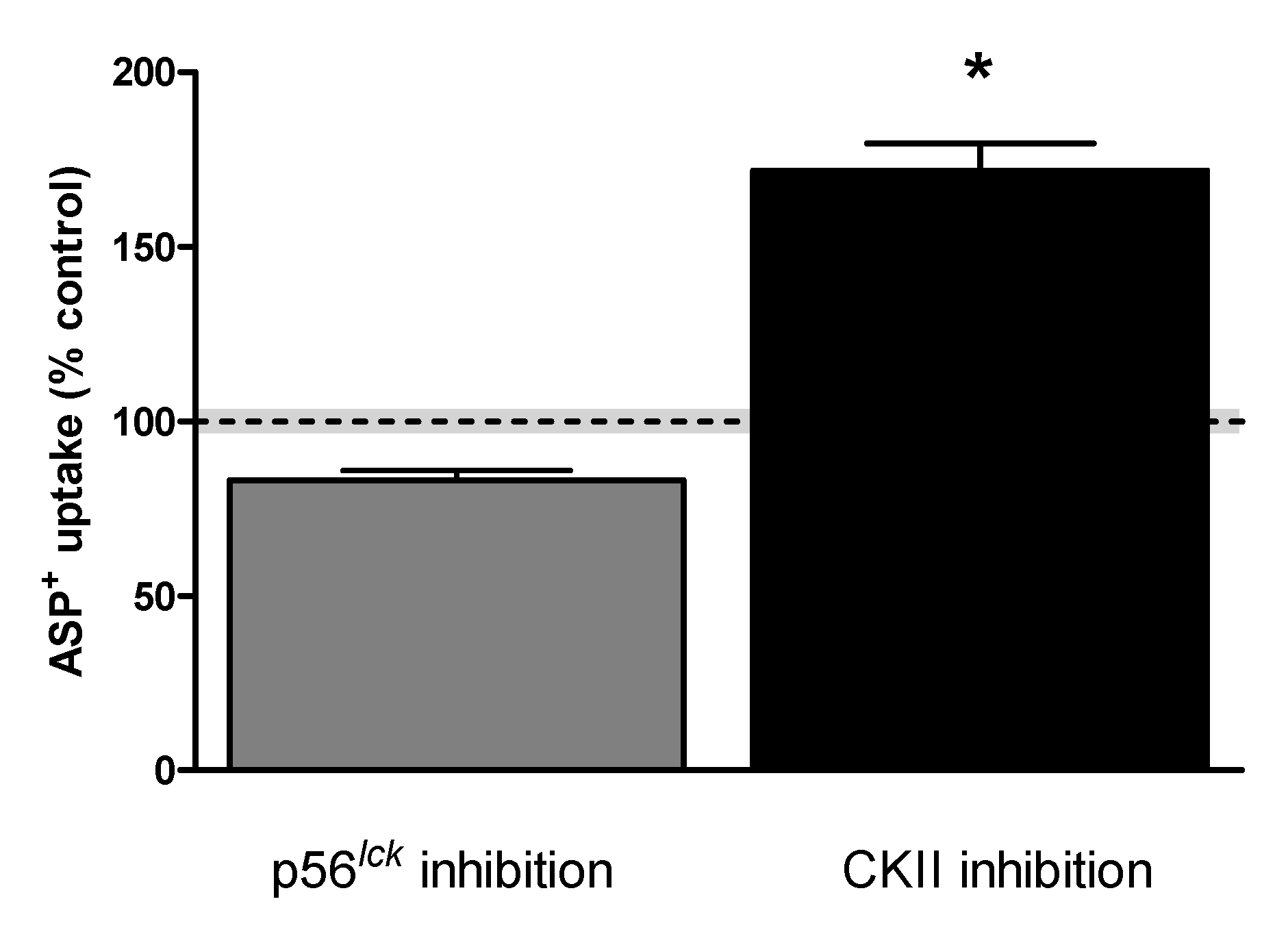
| Signaling Pathway | hOCT2 Uptake | hMATE1 Uptake | hMATE1 Efflux | hMATE2K Uptake | hMATE2K Efflux |
|---|---|---|---|---|---|
| PKA | ↓[24] | 0 | 0 | 0 | ↓ |
| PKC | ↓[24] | 0 | 0 | 0 | ↓ |
| CKII | ↑ | ↓ | ↑ | ↓ | 0 |
| CaM | ↑[24] | ↑ | 0 | 0 | 0 |
| p56lck | ↑[44] | ↑ | 0 | ↓ | 0 |
| PI3K | ↓[24] | 0 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantauskaitė, M.; Hucke, A.; Reike, M.; Ahmed Eltayeb, S.; Xiao, C.; Barz, V.; Ciarimboli, G. Rapid Regulation of Human Multidrug and Extrusion Transporters hMATE1 and hMATE2K. Int. J. Mol. Sci. 2020, 21, 5157. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21145157
Kantauskaitė M, Hucke A, Reike M, Ahmed Eltayeb S, Xiao C, Barz V, Ciarimboli G. Rapid Regulation of Human Multidrug and Extrusion Transporters hMATE1 and hMATE2K. International Journal of Molecular Sciences. 2020; 21(14):5157. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21145157
Chicago/Turabian StyleKantauskaitė, Marta, Anna Hucke, Moritz Reike, Sara Ahmed Eltayeb, Chuyan Xiao, Vivien Barz, and Giuliano Ciarimboli. 2020. "Rapid Regulation of Human Multidrug and Extrusion Transporters hMATE1 and hMATE2K" International Journal of Molecular Sciences 21, no. 14: 5157. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21145157






