Induced Periosteum-Mimicking Membrane with Cell Barrier and Multipotential Stromal Cell (MSC) Homing Functionalities
Abstract
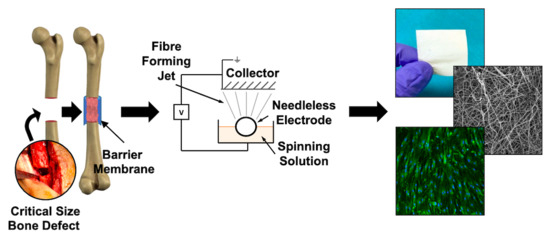
:1. Introduction
2. Results
2.1. Architecture of Human Periosteum and Induced Membrane
2.2. Free Surface Electrospun Membrane Characterisation
2.3. PL Absorption and Release Profile of PCL3%-E
2.4. Cellular Interactions with PCL3%-E
3. Discussion
4. Materials and Methods
4.1. Human Tissue Collection and Ethics
4.2. Histology Analysis of Periosteum and Induced Membrane
4.3. Free Surface Electrospun Membrane Manufacture and Processing
4.4. Physical Characterisation of the FSE Membranes
4.5. PCL3%-E Membrane Absorbance and Release of Platelet Lysate
4.6. In Vitro MSC Attachment onto PCL3%-E
4.7. Modified Transwell Barrier Function Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMA | Bone marrow aspirate |
| BV/mm2 | Blood vessels per mm2 |
| CSBD | Critical size bone defects |
| FSE | Free surface electrospinning |
| FWHM | Full width at half maximum |
| IM | Induced membrane |
| MSC | Multipotential stromal cells |
| P3 | Passage 3 |
| PL | Platelet lysate |
| PRP | Platelet rich plasma |
| PSR | Picro Sirius Red |
| PCL | poly(ε-caprolactone) |
| PMMA | Polymethyl methacrylate |
| SEM | Scanning electron microscopy |
| SBF | Simulated body fluid |
| UTS | Ultimate tensile strength |
| WCA | Water contact angle |
References
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, A.; Pape, H.C. Bone defects caused by high-energy injuries, bone loss, infected nonunions, and nonunions. Orthop. Clin. N. Am. 2010, 41, 1–4. [Google Scholar] [CrossRef]
- Calori, G.M.; Albisetti, W.; Agus, A.; Iori, S.; Tagliabue, L. Risk factors contributing to fracture non-unions. Injury 2007, 38, S11–S18. [Google Scholar] [CrossRef]
- Mills, L.A.; Simpson, A.H.R.W. The relative incidence of fracture non-union in the Scottish population (5.17 million): A 5-year epidemiological study. BMJ Open 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzioupis, C.; Giannoudis, P.V. Prevalence of long-bone non-unions. Injury 2007, 38, S3–S9. [Google Scholar] [CrossRef]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The risk of non-union per fracture: Current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Bigham, A.S.; Shadkhast, M.; Sadegh, A.B.; Shafiei, Z.; Lakzian, A.; Khalegi, M.R. Evaluation of osteoinduction properties of the demineralized bovine foetal growth plate powder as a new xenogenic biomaterial in rat. Res. Vet. Sci. 2011, 91, 306–310. [Google Scholar] [CrossRef]
- Altaie, A.; Owston, H.; Jones, E. Use of platelet lysate for bone regeneration-are we ready for clinical translation? World J. Stem Cells 2016, 8, 47. [Google Scholar] [CrossRef]
- Douras, P.; Tosounidis, T.; Giannoudis, P.V. Application of the ‘diamond concept’ with fast bone marrow aspirate concentration for the treatment of medial malleolus non-union. Injury 2018, 49, 2326–2330. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Harwood, P.J.; Tosounidis, T.; Kanakaris, N.K. Restoration of long bone defects treated with the induced membrane technique: Protocol and outcomes. Injury 2016, 47, S53–S61. [Google Scholar] [CrossRef]
- Morelli, I.; Drago, L.; George, D.A.; Gallazzi, E.; Scarponi, S.; Romanò, C.L. Masquelet technique: Myth or reality? A systematic review and meta-analysis. Injury 2016, 47, S68–S76. [Google Scholar] [CrossRef]
- Ley, P.; Gosselin, R.A.; Villar, R. The Masquelet induced-membrane technique: An option for a tertiary-referral conflict setting. J. Surg. Case Rep. 2019, 2019, rjz149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Ogiso, B.; Hughes, F.; Melcher, A.; McCulloch, C. Fibroblasts Inhibit Mineralised Bone Nodule Formation by Rat Bone Marrow Stromal Cells In Vitro. J. Cell. Physiol. 1991, 146, 442–450. [Google Scholar] [CrossRef]
- Kozlovsky, A.; Aboodi, G.; Moses, O.; Tal, H.; Artzi, Z.; Weinreb, M.; Nemcovsky, C.E. Bio-degradation of a resorbable collagen membrane (Bio-Gide ®) applied in a double-layer technique in rats. Clin. Oral Implants Res. 2009, 20, 1116–1123. [Google Scholar] [CrossRef]
- Ramalingam, S.; Basudan, A.; Babay, N.; Al-Rasheed, A.; Nooh, N.; Naghshbandi, J.; Aldahmash, A.; Atteya, M.; Al-Hezaimi, K. Efficacy of Mucograft vs Conventional Resorbable Collagen Membranes in Guided Bone Regeneration Around Standardized Calvarial Defects in Rats: A Histologic and Biomechanical Assessment. Int. J. Periodontics Restor. Dent. 2016, 36, s99–s107. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Cohen, A.; Hinsche, A.; Stratford, T.; Matthews, S.J.; Smith, R.M. Simultaneous bilateral femoral fractures: Systemic complications in 14 cases. Int. Orthop. 2000, 24, 264–267. [Google Scholar] [CrossRef] [Green Version]
- Shan, X.; Hu, D. Bone engineering by cell sheet technology to repair mandibular defects. Exp. Ther. Med. 2017, 14, 5007–5011. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.F.; Parent, J.B.; Lasko, C.E.; Zhen, X.; Knothe, U.R.; Lemaire, T.; Tate, M.L.K. Periosteum, bone’s ‘smart’ bounding membrane, exhibits direction-dependent permeability. J. Bone Miner. Res. 2013, 28, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef]
- Mercurio, A.D.; Motta, T.; Green, E.; Noble, G.; Hart, R.T.; Allen, M.J. Effects of extensive circumferential periosteal stripping on the microstructure and mechanical properties of the murine femoral cortex. J. Orthop. Res. 2012, 30, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, R.; Churchman, S.M.; Tan, H.B.; McGonagle, D.; Jones, E.; Giannoudis, P.V. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone 2013, 57, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers (Basel) 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer: Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Goh, B.T.; Teh, L.Y.; Tan, D.B.P.; Zhang, Z.; Teoh, S.H. Novel 3D polycaprolactone scaffold for ridge preservation-a pilot randomised controlled clinical trial. Clin. Oral Implants Res. 2015, 26, 271–277. [Google Scholar] [CrossRef]
- Chainani, A.; Hippensteel, K.J.; Kishan, A.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng. Part A 2013, 19, 2594–2604. [Google Scholar] [CrossRef] [Green Version]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Bazbouz, M.B.; Liang, H.; Tronci, G. A UV-cured nanofibrous membrane of vinylbenzylated gelatin-poly(ɛ-caprolactone) dimethacrylate co-network by scalable free surface electrospinning. Mater. Sci. Eng. C 2018, 91, 541–555. [Google Scholar] [CrossRef]
- Schenk, R.K.; Buser, D.; Hardwick, W.R.; Dahlin, C. Healing pattern of bone regeneration in membrane-protected defects: A histologic study in the canine mandible. Int. J. Oral Maxillofac. Implant. 1997, 9, 13–29. [Google Scholar]
- Froum, S.; Cho, S.-C.; Pariente, L. A Surgical Protocol for Guided Bone Regeneration Procedures: Using Absorbable Membranes to Minimize and Treat Complications. Dent. Learn. 2012, 6, 3–13. [Google Scholar]
- Henrich, D.; Seebach, C.; Nau, C.; Basan, S.; Relja, B.; Wilhelm, K.; Schaible, A.; Frank, J.; Barker, J.; Marzi, I. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J. Tissue Eng. Regen. Med. 2016, 10, E382–E396. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, G.; Shang, P.; Shen, Y.; Nie, P.; Peng, L.; Xu, H. Histological characteristics of induced membranes in subcutaneous, intramuscular sites and bone defect. Orthop. Traumatol. Surg. Res. 2013, 99, 959–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.A.; Kinsey, S.E.; English, A.; Jones, R.A.; Straszynski, L.; Meredith, D.M.; Markham, A.F.; Jack, A.; Emery, P.; Mcgonagle, D. Isolation and Characterization of Bone Marrow Multipotential Mesenchymal Progenitor Cells. Arthritis Rheum. 2002, 46, 3349–3360. [Google Scholar] [CrossRef]
- Walker, P.A.; Jimenez, F.; Gerber, M.H.; Gill, B.S.; Savitz, S.I.; Cox, C.S.; Al, W.E.T. Effect of Needle Diameter and Flow Rate on Rat and Human Mesenchymal Stromal Cell Characterization and Viability. Tissue Eng. Part C 2010, 16, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Siegel, G.; Kluba, T.; Hermanutz-klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.L.; Jeon, H.; Wang, A.; Yan, Z.; Yu, J.; Grigoropoulous, C.; Li, S. Femtosecond Laser Ablation Enhances Cell Infiltration into Three-Dimensional Electrospun Scaffolds. Acta Biomater. 2012, 8, 2648–2658. [Google Scholar] [CrossRef] [Green Version]
- Kong, B.; Sun, W.; Chen, G.; Tang, S.; Li, M.; Shao, Z.; Mi, S. Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, I.A.; Kalaf, E.A.G.; Bowlin, G.L.; Sell, S.A. Platelet-rich plasma in bone regeneration: Engineering the delivery for improved clinical efficacy. Biomed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed]
- El-Jawhari, J.J.; Cuthbert, R.; McGonagle, D.; Jones, E.; Giannoudis, P.V. The CD45lowCD271high Cell Prevalence in Bone Marrow Samples May Provide a Useful Measurement of the Bone Marrow Quality for Cartilage and Bone Regenerative Therapy. J. Bone Jt. Surg. Am. Vol. 2017, 99, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.R.; Madhusoothanan, M.; Sekaran, G. Electrospinning of Type I Collagen and PCL Nanofibers Using Acetic Acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Nica, C.; Lin, Z.; Sculean, A.; Asparuhova, M.B. Adsorption and release of growth factors from four different porcine-derived collagen matrices. Materials (Basel) 2020, 13, 2635. [Google Scholar] [CrossRef] [PubMed]
- Moisley, K.M.; El-Jawhari, J.J.; Owston, H.; Tronci, G.; Russell, S.J.; Jones, E.A.; Giannoudis, P.V. Optimising proliferation and migration of mesenchymal stem cells using platelet products: A rational approach to bone regeneration. J. Orthop. Res. 2019, 37, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Zhang, W.; Yang, T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol. Res. 2015, 48, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Owston, H.E.; Ganguly, P.; Tronci, G.; Russell, S.J.; Giannoudis, P.V.; Jones, E.A. Colony Formation, Migratory, and Differentiation Characteristics of Multipotential Stromal Cells (MSCs) from ‘Clinically Accessible’ Human Periosteum Compared to Donor-Matched Bone Marrow MSCs. Stem Cell Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Tate, M.K. Concise Review: The Periosteum: Tapping into a Reservoir of Clinically Useful Progenitor Cells. Stem Cells Transl. Med. 2012, 1, 480–491. [Google Scholar] [CrossRef]
- Colnot, C.; Zhang, X.; Tate, M.L.K. Current insights on the regenerative potential of the periosteum: Molecular, cellular, and endogenous engineering approaches. J. Orthop. Res. 2012, 30, 1869–1878. [Google Scholar] [CrossRef] [Green Version]
- Filion, T.M.; Song, J. A Sulfated Nanofibrous Mesh Supporting the Osteogenic Differentiation of Periosteum-Derived Cells. J. Biomater. Tissue Eng. 2013, 3, 486–493. [Google Scholar] [CrossRef]
- Stachewicz, U.; Qiao, T.; Rawlinson, S.C.F.; Viga, F.; Almeida, D.M. 3D imaging of cell interactions with electrospun PLGA nanofiber membranes for bone regeneration. Acta Biomater. 2015, 27, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Fujie, T.; Saito, A.; Takeoka, S.; Hou, Y.; Shu, Y.; Chen, M.; Wu, H.; Khademhosseini, A. Periosteum-Mimetic Structures Made from Freestanding Microgrooved Nanosheets. Adv. Mater. 2014, 26, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Foolen, J.; van Donkelaar, C.C.; Nowlan, N.; Murphy, P.; Huiskes, R.; Ito, K. Collagen orientation in periosteum and perichondrium is aligned with preferential directions of tissue growth. J. Orthop. Res. 2008, 26, 1263–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilas, D.C.; Churchman, S.M.; Baboolal, T.; Giannoudis, P.V.; Aderinto, J.; McGonagle, D.; Jones, E. The simultaneous analysis of mesenchymal stem cells and early osteocytes accumulation in osteoarthritic femoral head sclerotic bone. Rheumatology 2019. [Google Scholar] [CrossRef] [Green Version]
- Walser, J.; Ferguson, S.J. Oriented nanofibrous membranes for tissue engineering applications: Electrospinning with secondary field control. J. Mech. Behav. Biomed. Mater. 2016, 58, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Gong, P.; Lin, Y.; Qu, Y.; Li, J.; Kong, X.; Chen, Z.; Man, Y. Nanofibrous electrospun barrier membrane promotes osteogenic differentiation of human mesenchymal stem cells. J. Bioact. Compat. Polym. 2011, 26, 607–618. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Catledge, S.A.; Xu, Y.; Hennessy, K.M.; Thomas, V.; Jablonsky, M.J.; Chowdhury, S.; Stanishevsky, A.V.; Vohra, Y.K.; et al. Mesenchymal stem cell responses to bone-mimetic electrospun matrices composed of polycaprolactone, collagen I and nanoparticulate hydroxyapatite. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Caballero, M.; Pappa, A.; Roden, K.; Krochmal, D.J.; van Aalst, J.A. Osteoinduction of Umbilical Cord and Palate Periosteum- Derived Mesenchymal Stem Cells on Poly-Co-Glycolytic Acid Nano-Microfibers. Ann. Plast. Surg. 2014, 72, S176–S183. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Huang, C.; Huang, Y.; Li, G.; Chi, C.; Ye, J.; Xie, W.; Shi, R.; Zhang, L. Core-sheath micro/nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 124–136. [Google Scholar] [CrossRef]
- Sun, F.; Chen, J.; Jin, S.; Wang, J.; Man, Y.; Li, J.; Zou, Q.; Li, Y.; Zuo, Y. Development of biomimetic trilayer fibrous membranes for guided bone regeneration. J. Mater. Chem. B 2019, 7, 665–675. [Google Scholar] [CrossRef]
- Ma, S.; Chen, Z.; Qiao, F.; Sun, Y.; Yang, X.; Deng, X.; Cen, L.; Cai, Q.; Wu, M.; Zhang, X.; et al. Guided bone regeneration with tripolyphosphate cross-linked asymmetric chitosan membrane. J. Dent. 2014, 42, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (e-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials 2011, 32, 8108–8117. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Cooper-White, J.J. Increasing electrospun scaffold pore size with tailored collectors for improved cell penetration. Acta Biomater. 2011, 7, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, T.; He, N.; Wang, J.; Chen, W.; He, L.; Huang, C.; I-Hamshary, H.A.E.; Al-Deyab, S.S.; Ke, Q.; et al. Three-dimensional polycaprolactone scaffold via needleless electrospinning promotes cell proliferation and infiltration. Colloids Surf. B Biointerfaces 2014, 121, 432–443. [Google Scholar] [CrossRef]
- Ameer, J.M.; Anil, K.P.R.; Kasoju, N. Strategies to tune electrospun scaffold porosity for effective cell response in tissue engineering. J. Funct. Biomater. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonvallet, P.P.; Schultz, M.J.; Mitchell, E.H.; Bain, J.L.; Culpepper, B.K.; Thomas, S.J.; Bellis, S.L. Microporous dermal-mimetic electrospun scaffolds pre-seeded with fibroblasts promote tissue regeneration in full-thickness skin wounds. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Gee, A. Regulation of Regenerative Medicine Products. Adv. Exp. Med. Biol. 2018, 1098, 189–198. [Google Scholar]
- Graziano, A.; Carinci, F.; Scolaro, S.; D’Aquino, R. Periodontal tissue generation using autologous dental ligament micro-grafts: Case report with 6 months follow-up. Ann. Oral. Maxillofac. Surg. 2013, 1, 20–25. [Google Scholar] [CrossRef]
- Barbier, L.; Ramos, E.; Mendiola, J.; Rodriguez, O.; Santamaria, G.; Santamaria, J.; Arteagoitia, I. Autologous dental pulp mesenchymal stem cells for inferior third molar post-extraction socket healing: A split-mouth randomised clinical trial. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e469–e477. [Google Scholar] [CrossRef]
- Lammens, J.; Maréchal, M.; Delport, H.; Geris, L.; Oppermann, H.; Vukicevic, S.; Luyten, F.P. A cell-based combination product for the repair of large bone defects. Bone 2020, 138, 115511. [Google Scholar] [CrossRef]
- Cuthbert, R.; Boxall, S.; Tan, H.B.; Giannoudis, P.V.; McGonagle, D.; Jones, E. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy 2012, 14, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minuth, W.W.; Denk, L. Supportive development of functional tissues for biomedical research using the MINUSHEET® perfusion system. Clin. Transl. Med. 2012, 1, 22–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owston, H.E.; Moisley, K.M.; Tronci, G.; Russell, S.J.; Giannoudis, P.V.; Jones, E. Induced Periosteum-Mimicking Membrane with Cell Barrier and Multipotential Stromal Cell (MSC) Homing Functionalities. Int. J. Mol. Sci. 2020, 21, 5233. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155233
Owston HE, Moisley KM, Tronci G, Russell SJ, Giannoudis PV, Jones E. Induced Periosteum-Mimicking Membrane with Cell Barrier and Multipotential Stromal Cell (MSC) Homing Functionalities. International Journal of Molecular Sciences. 2020; 21(15):5233. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155233
Chicago/Turabian StyleOwston, Heather E., Katrina M. Moisley, Giuseppe Tronci, Stephen J. Russell, Peter V. Giannoudis, and Elena Jones. 2020. "Induced Periosteum-Mimicking Membrane with Cell Barrier and Multipotential Stromal Cell (MSC) Homing Functionalities" International Journal of Molecular Sciences 21, no. 15: 5233. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155233