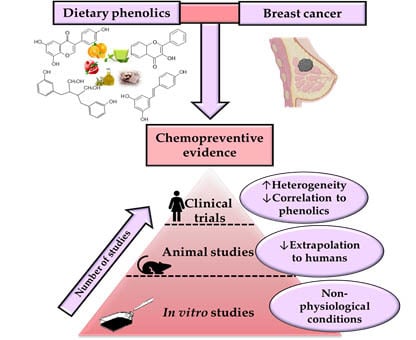
Dietary Phenolics against Breast Cancer. A Critical Evidence-Based Review and Future Perspectives
Abstract
:1. Breast Cancer: General Aspects
2. Epidemiological Evidence for Dietary (Poly)Phenols
3. Bioavailability of Dietary Phenolics and Their Occurrence in Human Breast Tissues
4. Evidence of the Chemopreventive Potential of Phenolic Compounds in Physiologically Relevant Preclinical Studies
5. Dietary (Poly)Phenols and Clinical Studies in Breast Cancer Patients
6. Identifying the Existing Gaps to Address Future Preclinical and Clinical Research
- (i)
- There is a need for multidisciplinary teams beyond researchers (oncologists, surgeons, pathologists, etc.) to conduct dietary interventions in BC patients. Indeed, this issue is inherent in every clinical trial dealing with cancer patients. In the case of ‘dietary interventions’, oncologists usually oppose the assay of high concentrations of dietary phenolics to patients undergoing chemotherapy due to the limited knowledge regarding the possible interactions between dietary compounds, including phenolics, and chemotherapy drugs. Overall, this challenging logistic yields a low number of recruited BC patients. Consequently, a restricted number of samples to explore or validate results, which is even more challenging when assaying molecules with relatively low anticancer activity compared to standard chemotherapy drugs.
- (ii)
- The high heterogeneity of the results obtained. Inter-individual variability is perhaps one of the most critical aspects to establish definite conclusions in clinical studies. The low number of volunteers, as well as the specific selection criteria of the volunteers selected in the different studies, are key factors to understand the high variability reported. Genetic polymorphisms or differences in the composition or functionality of the gut microbiota will also contribute to the different response of individuals to interventions with polyphenol-rich foods [48,144,145,146]. In this regard, a highly variable metabolism between individuals has been described for some phenolic compounds. This allows for grouping the population according to the excretion of the metabolized compound into “high or low producers”, as it occurs in the metabolism of the phenolic precursor-derived metabolite pairs hesperidin-hesperetin [147], lignans-enterolignans [148], ellagic acid-urolithins [149] and procyanidins-valerolactones [150,151]. Besides, specific metabotypes, attributed to the different composition and functionality of the gut microbiota, have been described in the case of urolithins [146,152] or isoflavones [153], allowing the stratification of individuals based on the production of specific metabolites associated with a particular gut microbiota ecology. Thus, the inter-individual variability related to bioavailability and metabolism of dietary phenolics is essential to comprehend the results in clinical studies and to identify whether the possible beneficial effects can be extrapolated to the whole population or only to certain specific individuals [146]. Future research should be conducted to evaluate associations between specific phenolic-related gut microbiota metabotypes and protective effects against BC.
- (iii)
- The difficulty of attributing chemopreventive effects to phenolics. Most human studies have been conducted with phenolic-containing products or extracts, which makes it challenging to identify whether single phenolics are (or not) actually responsible for the possible anti-tumour effect. Along this line, it is important to consider the differences in phenolic composition between plant foods, the difficulty in the estimation of the dietary intake of phenolics modulated by several factors such as food matrix, food processing-related factors, as well as the interaction between phenolics and the gut microbiota, and also other food components, such as proteins and carbohydrates that will undoubtedly influence the bioavailability and subsequent potential chemopreventive effects [45,154].
- (iv)
- There is not enough evidence regarding the possible interaction between dietary phenolics and conventional chemotherapy drugs. Despite the preclinical evidence that supports the potential benefits of dietary phenolics as possible adjuvants in BC management [155,156], to date, only three clinical studies have been conducted using a combination of phenolics and chemotherapeutic drugs [126,137,139]. Therefore, there is a need to evaluate the potential interactions between phenolics and chemotherapeutic drugs to support phenolics’ usefulness as adjuvants in BC treatment and further follow-up of patients to prevent relapses.
- (i)
- The difficulty of extrapolating the results obtained from animal research to humans. As in the case of human studies, several problems can be found in the BC animal models to evaluate the chemopreventive effect of phenolics: the methodology issues (low number of animals, and lack of controls, etc.), the heterogeneity of data, the difficulty of attributing chemopreventive effects to phenolics (use of whole-foods or extracts instead of single phenolics, determination of phenolics in breast tissues, etc.).
- (ii)
- In vitro studies must have physiological relevance to establishing potential effects and conclusions. Different aspects of in vitro research should be avoided: the use of both unrealistic concentration and metabolic forms of phenolics; the unsuitability of BC cell models using a single cell line instead of considering the heterogeneity of cell lines using multiple cell lines with different mutations and other genetic characteristics (ER/PR positive or negative, etc.), or even more realistic advanced models such as primary cell cultures, organoids, etc.; and testing single phenolic or derived metabolite without considering the real mixture of compounds present in vivo, as well as the food matrix effect, avoiding the possible synergistic, antagonistic, or additive effects among them. Therefore, following these principles, transferring the information on the pharmacokinetic properties and bioavailability of phytochemicals in breast tissues plays one of the critical roles for a better evaluation of their mechanism of action involved in their BC chemoprevention.
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| EGCG | Epigallocatechin Gallate |
| ER | Oestrogen Receptor |
| HER2 | Human Epidermal growth factor Receptor 2 |
| MTD | Maximum Tolerability Dose |
| PR | Progesterone Receptor |
| RSV | Resveratrol |
| TNBC | Triple Negative Breast Cancer |
References
- WHO Media Centre (Cancer—Fact Sheets). Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 3 May 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Breast Cancer Statistics. Available online: https://www.breastcancer.org/symptoms/understand_bc/statistics (accessed on 13 February 2020).
- Campeau, P.M.; Foulkes, W.D.; Tischkowitz, M.D. Hereditary breast cancer: New genetic developments, new therapeutic avenues. Hum. Genet. 2008, 124, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary natural products for prevention and treatment of breast cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [Green Version]
- Tamimi, R.M.; Spiegelman, D.; Smith-Warner, S.A.; Wang, M.; Pazaris, M.; Willett, W.C.; Eliassen, A.H.; Hunter, D.J. Population Attributable Risk of Modifiable and Nonmodifiable Breast Cancer Risk Factors in Postmenopausal Breast Cancer. Am. J. Epidemiol. 2016, 184, 884–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodai, B.I.; Tuso, P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm. J. 2015, 19, 48–79. [Google Scholar] [CrossRef] [Green Version]
- Timms, K.M.; Abkevich, V.; Hughes, E.; Neff, C.; Reid, J.; Morris, B.; Kalva, S.; Potter, J.; Tran, T.V.; Chenet, J.; et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014, 16, 475. [Google Scholar] [CrossRef] [Green Version]
- Rivenbark, A.G.; O’Connor, S.M.; Coleman, W.B. Molecular and cellular heterogeneity in breast cancer: Challenges for personalized medicine. Am. J. Pathol. 2013, 183, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Weigelt, B.; Geyer, F.C.; Reis-Filho, J.S. Histological types of breast cancer: How special are they? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z.; Shi, A.; Lu, C.; Song, T.; Zhang, Z.; Zhao, J. Breast Cancer: Epidemiology and Etiology. Cell Biochem. Biophys. 2015, 72, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, M.; Shien, T.; Iwata, H. TNM classification of malignant tumors (Breast Cancer Study Group). Jpn. J. Clin. Oncol. 2019, 49, 228–231. [Google Scholar] [CrossRef]
- Engstrøm, M.J.; Opdahl, S.; Hagen, A.I.; Romundstad, P.R.; Akslen, L.A.; Haugen, O.A.; Vatten, L.J.; Bofin, A.M. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res. Treat. 2013, 140, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Cadoo, K.A.; Fornier, M.N.; Morris, P.G. Biological subtypes of breast cancer: Current concepts and implications for recurrence patterns. Q J. Nucl. Med. Mol. Imaging 2013, 57, 312–321. [Google Scholar]
- Marotti, J.D.; de Abreu, F.B.; Wells, W.A.; Tsongalis, G.J. Triple-negative breast cancer. Am. J. Pathol. 2017, 187, 2133–2138. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [Green Version]
- Di Cosimo, S.; Baselga, J. Management of breast cancer with targeted agents: Importance of heterogeneity. Nat. Rev. Clin. Oncol. 2010, 7, 139–147. [Google Scholar] [CrossRef]
- Balduzzi, S.; Mantarro, S.; Guarneri, V.; Tagliabue, L.; Pistotti, V.; Moja, L.; D’Amico, R. Trastuzumab containing regimens for metastatic breast cancer. Cochrane Database Syst. Rev. 2014, 12, CD006242. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Wang, Y.; Kiani, M.F.; Wang, B. Classification, treatment strategy, and associated drug resistance in breast cancer. Clin. Breast Cancer 2016, 16, 335–343. [Google Scholar] [CrossRef]
- Guarneri, V.; Conte, P. Metastatic breast cancer: Therapeutic options according to molecular subtypes and prior adjuvant therapy. Oncologist 2009, 14, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Irigaray, P.; Newby, J.A.; Clapp, R.; Hardell, L.; Howard, V.; Montagnier, L.; Epstein, S.; Belpomme, D. Lifestyle-related factors and environmental agents causing cancer: An overview. Biomed. Pharmacother. 2007, 61, 640–658. [Google Scholar] [CrossRef] [PubMed]
- Catsburg, C.; Kim, R.S.; Kirsh, V.A.; Soskolne, C.L.; Kreiger, N.; Rohan, T.E. Dietary patterns and breast cancer risk: A study in 2 cohorts. Am. J. Clin. Nutr. 2015, 101, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Norat, T. Fruits, vegetables and breast cancer risk: A systematic review and meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012, 134, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farvid, M.S.; Chen, W.Y.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int. J. Cancer 2019, 144, 1496–1510. [Google Scholar] [CrossRef]
- Emaus, M.J.; Peeter, P.H.; Bakker, M.F.; Overvad, K.; Tjonneland, A.; Olsen, A.; Romieu, I.; Ferrari, P.; Dossus, L.; Boutron-Ruault, M.C.; et al. Vegetable and fruit consumption and the risk of hormone receptor-defined breast cancer in the EPIC cohort. Am. J. Clin. Nutr. 2016, 103, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.; Spiegelman, D.; Baglietto, L.; Bernstein, L.; Boggs, D.A.; van den Brandt, P.A.; Buring, J.E.; Cerhan, J.R.; Gaudet, M.M.; Giles, G.G.; et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J. Natl. Cancer Inst. 2013, 105, 219–236. [Google Scholar] [CrossRef]
- Heath, A.K.; Muller, D.C.; van den Brandt, P.A.; Papadimitriou, N.; Critselis, E.; Gunter, M.; Vineis, P.; Weiderpass, E.; Fagherazzi, G.; Boeing, H.; et al. Nutrient-wide association study of 92 foods and nutrients and breast cancer risk. Breast Cancer Res. 2020, 22, 5. [Google Scholar] [CrossRef]
- Karavasiloglou, N.; Hüsing, A.; Masala, G.; van Gils, C.H.; Turzanski Fortner, R.; Chang-Claude, J.; Huybrechts, I.; Weiderpass, E.; Gunter, M.; Arveux, P.; et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and risk of in situ breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. BMC Med. 2019, 17, 221. [Google Scholar] [CrossRef]
- Farvid, M.S.; Chen, W.Y.; Michels, K.B.; Cho, E.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: Population based cohort study. BMJ 2016, 353, i2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothwell, J.A.; Knaze, V.; Zamora-Ros, R. Polyphenols: Dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Res. 2017, 75, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Qin, L.Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence. A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2011, 125, 315–323. [Google Scholar] [CrossRef]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micek, A.; Godos, J.; Brzostek, T.; Gniadek, A.; Favari, C.; Mena, P.; Libra, M.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary phytoestrogens and biomarkers of their intake in relation to cancer survival and recurrence: A comprehensive systematic review with meta-analysis. Nutr. Rev. 2020, nuaa043, (published online ahead of print, 6 July 2020). [Google Scholar] [CrossRef]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Castelló, A.; Boldo, E.; Pérez-Gómez, B.; Lope, V.; Altzibar, J.M.; Martín, V.; Castaño-Vinyals, G.; Guevara, M.; Dierssen-Sotos, T.; Tardón, A.; et al. Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 2017, 103, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.H.; Chiou, Y.S.; Chen, L.H.; Ho, C.T. Breast cancer chemoprevention by dietary natural phenolic compounds: Specific epigenetic related molecular targets. Mol. Nutr. Food Res. 2015, 59, 21–35. [Google Scholar] [CrossRef]
- Losada-Echeberría, M.; Herranz-López, M.; Micol, V.; Barrajón-Catalán, E. Polyphenols as Promising Drugs Against Main Breast Cancer Signatures. Antioxidants 2017, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás-Barberán, F.A.; Espín, J.C. Effect of Food Structure and Processing on (Poly)phenol-Gut Microbiota Interactions and the Effects on Human Health. Annu. Rev. Food Sci. Technol. 2019, 10, 221–238. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; Núñez-Sánchez, M.Á.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur. J. Nutr. 2014, 53, 853–864. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Physiological Relevance of the Antiproliferative and Estrogenic Effects of Dietary Polyphenol Aglycones versus Their Phase-II Metabolites on Breast Cancer Cells: A Call of Caution. J. Agric. Food Chem. 2018, 66, 8547–8555. [Google Scholar] [CrossRef]
- Maubach, J.; Bracke, M.E.; Heyerick, A.; Depypere, H.T.; Serreyn, R.F.; Mareel, M.M.; De Keukeleire, D. Quantitation of soy-derived phytoestrogens in human breast tissue and biological fluids by high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 784, 137–144. [Google Scholar] [CrossRef]
- Maubach, J.; Depypere, H.T.; Goeman, J.; Van Der Eycken, J.; Heyerick, A.; Bracke, M.E.; Blondeel, P.; De Keukeleire, D. Distribution of soy-derived phytoestrogens in human breast tissue and biological fluids. Obstet. Gynecol. 2004, 103, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Pumford, S.; Morton, M.; Turkes, A.; Griffiths, K. Determination of the isoflavonoids genistein and daidzein in biological samples by gas chromatography–mass spectrometry. Ann. Clin. Biochem. 2002, 39, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Urpi-Sarda, M.; Blondeel, P.; Roche, N.; Vanhaecke, L.; Possemiers, S.; Al-Maharik, N.; Botting, N.; De Keukeleire, D.; Bracke, M.; et al. Disposition of soy isoflavones in normal human breast tissue. Am. J. Clin. Nutr. 2010, 91, 976–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.C.; Churchwell, M.I.; Delclos, K.B.; Newbold, R.R.; Doerge, D.R. Mass spectrometric determination of genistein tissue distribution in diet-exposed Sprague-Dawley rats. J. Nutr. 2000, 130, 1963–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamartiniere, C.A.; Wang, J.; Smith-Johnson, M.; Eltoum, I.E. Daidzein: Bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol. Sci. 2002, 65, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Urpi-Sarda, M.; Morand, C.; Besson, C.; Kraft, G.; Viala, D.; Scalbert, A.; Besle, J.M.; Manach, C. Tissue distribution of isoflavones in ewes after consumption of red clover silage. Arch. Biochem. Biophys. 2008, 476, 205–210. [Google Scholar] [CrossRef]
- Yuan, L.; Wagatsuma, C.; Yoshida, M.; Miura, T.; Mukoda, T.; Fujii, H.; Sun, B.; Kim, J.H.; Surh, Y.J. Inhibition of human breast cancer growth by GCP (genistein combined polysaccharide) in xenogeneic athymic mice: Involvement of genistein biotransformation by beta-glucuronidase from tumor tissues. Mutat. Res. 2003, 523, 55–62. [Google Scholar] [CrossRef]
- Bolca, S.; Li, J.; Nikolic, D.; Roche, N.; Blondeel, P.; Possemiers, S.; De Keukeleire, D.; Bracke, M.; Heyerick, A.; van Breemen, R.B.; et al. Disposition of hop prenylflavonoids in human breast tissue. Mol. Nutr. Food Res. 2010, 54, S284–S294. [Google Scholar] [CrossRef] [Green Version]
- Overk, C.R.; Guo, J.; Chadwick, L.R.; Lantvit, D.D.; Minassi, A.; Appendino, G.; Chen, S.N.; Lankin, D.C.; Farnsworth, N.R.; Pauli, G.F.; et al. In vivo estrogenic comparisons of Trifolium pratense (red clover), Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin. Chem. Biol. Interact. 2008, 176, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Dietz, B.M.; Hagos, G.K.; Eskra, J.N.; Wijewickrama, G.T.; Anderson, J.R.; Nikolic, D.; Wright, B.; Chen, S.-N.; Pauli, G.F.; van Breemen, R.B.; et al. Differential regulation of detoxification enzymes in hepatic and mammary tissue by hops (Humulus lupulus) in vitro and in vivo. Mol. Nutr. Food Res. 2013, 57, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Albin, N.; Massaad, L.; Toussaint, C.; Mathieu, M.C.; Morizet, J.; Parise, O.; Gouyette, A.; Chabot, G.G. Main Drug-Metabolizing Enzyme Systems in Human Breast Tumors and Peritumoral Tissues. Cancer Res. 1993, 53, 3541. [Google Scholar] [PubMed]
- Shimoi, K.; Nakayama, T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005, 400, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; Guerrieri-Gonzaga, A.; Gandini, S.; Johansson, H.; Serrano, D.; Cazzaniga, M.; Aristarco, V.; Puccio, A.; Mora, S.; Caldarella, P.; et al. A presurgical study of oral silybin-phosphatidylcholine in patients with early breast cancer. Cancer Prev. Res. 2016, 9, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzeroni, M.; Guerrieri-Gonzaga, A.; Gandini, S.; Johansson, H.; Serrano, D.; Cazzaniga, M.; Aristarco, V.; Macis, D.; Mora, S.; Caldarella, P.; et al. A Presurgical Study of Lecithin Formulation of Green Tea Extract in Women with Early Breast Cancer. Cancer Prev. Res. 2017, 10, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Sartippour, M.R.; Pietras, R.; Marquez-Garban, D.C.; Chen, H.-W.; Heber, D.; Henning, S.M.; Sartippour, G.; Zhang, L.; Lu, M.; Weinberg, O.; et al. The combination of green tea and tamoxifen is effective against breast cancer. Carcinogenesis 2006, 27, 2424–2433. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Greaves, P.; Cooke, D.N.; Edwards, R.; Steward, W.P.; Gescher, A.J.; Marczylo, T.H. Breast Cancer Prevention by Green Tea Catechins and Black Tea Theaflavins in the C3(1) SV40 T,t Antigen Transgenic Mouse Model Is Accompanied by Increased Apoptosis and a Decrease in Oxidative DNA Adducts. J. Agric. Food Chem. 2007, 55, 3378–3385. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, e1801239. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Romo-Vaquero, M.; González-Sarrías, A.; Espín, J.C. Kinetic disposition of dietary polyphenols and methylxanthines in the rat mammary tissue. J. Funct. Foods 2019, 61, 103516. [Google Scholar] [CrossRef]
- Lærke, H.N.; Mortensen, M.A.; Hedemann, M.S.; Bach-Knudsen, K.E.; Penalvo, J.L.; Adlercreutz, H. Quantitative aspects of the metabolism of lignans in pigs fed fibre-enriched rye and wheat bread. Br. J. Nutr. 2009, 102, 985–994. [Google Scholar] [CrossRef]
- García-Mateos, D.; García-Villalba, R.; Marañón, J.A.; Espín, J.C.; Merino, G.; Álvarez, A.I. The Breast Cancer Resistance Protein (BCRP/ABCG2) influences the levels of enterolignans and their metabolites in plasma, milk and mammary gland. J. Funct. Foods 2017, 35, 648–654. [Google Scholar] [CrossRef]
- Liu, G.; Khanna, V.; Kirtane, A.; Grill, A.; Panyam, J. Chemopreventive efficacy of oral curcumin: A prodrug hypothesis. FASEB J. 2019, 33, 9453–9465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunihiro, A.G.; Brickey, J.A.; Frye, J.B.; Luis, P.B.; Schneider, C.; Funk, J.L. Curcumin, but not curcumin-glucuronide, inhibits Smad signaling in TGFβ-dependent bone metastatic breast cancer cells and is enriched in bone compared to other tissues. J. Nutr. Biochem. 2019, 63, 150–156. [Google Scholar] [CrossRef] [PubMed]
- De Pace, R.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S. Anticancer activities of (-)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.; Walters, J.; Brownlow, B.; Elbayoumi, T. Enhanced cytotoxicity of optimized liposomal genistein via specific induction of apoptosis in breast, ovarian and prostate carcinomas. J. Drug Target 2013, 21, 1001–1011. [Google Scholar] [CrossRef]
- Khan, M.N.; Haggag, Y.A.; Lane, M.E.; McCarron, P.A.; Tambuwala, M.M. Polymeric Nano-Encapsulation of Curcumin Enhances its Anti-Cancer Activity in Breast (MDA-MB231) and Lung (A549) Cancer Cells Through Reduction in Expression of HIF-1α and Nuclear p65 (Rel A). Curr. Drug Deliv. 2018, 15, 286–295. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Espín, J.C. In Vitro Research on Dietary Polyphenols and Health: A Call of Caution and a Guide on How To Proceed. J. Agric. Food Chem. 2018, 66, 7857–7858. [Google Scholar] [CrossRef]
- Power, K.A.; Saarinen, N.M.; Chen, J.M.; Thompson, L.U. Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. Int. J. Cancer 2018, 118, 1316–1320. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Power, K.; Chen, J.; Thompson, L.U. Flaxseed attenuates the tumor growth stimulating effect of soy protein in ovariectomized athymic mice with MCF-7 human breast cancer xenografts. Int. J. Cancer 2006, 119, 925–931. [Google Scholar] [CrossRef]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; Le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The combination of epigallocatechin gallate and curcumin suppresses ERα-breast cancer cell growth in vitro and in vivo. Int. J. Cancer 2007, 122, 1966–1971. [Google Scholar] [CrossRef]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Cubano, L.A.; Dharmawardhane, S.F. Combined Resveratrol, Quercetin, and Catechin Treatment Reduces Breast Tumor Growth in a Nude Mouse Model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, P.; Satapathy, S.R.; Siddharth, S.; Das, D.; Nayak, A.; Kundu, C.N. Resveratrol and curcumin synergistically induces apoptosis in cigarette smoke condensate transformed breast epithelial cells through a p21Waf1/Cip1 mediated inhibition of Hh-Gli signaling. Int. J. Biochem. Cell Biol. 2015, 66, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Kushwah, V.; Thanki, K.; Jain, S. Triple antioxidant SNEDDS formulation with enhanced oral bioavailability: Implication of chemoprevention of breast cancer. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Fritz, W. Dietary genistein: Perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis 1998, 19, 2151–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowlands, J.C.; He, L.; Hakkak, R.; Ronis, M.J.J.; Badger, T.M. Soy and Whey Proteins Downregulate DMBA-Induced Liver and Mammary Gland CYP1 Expression in Female Rats. J. Nutr. 2001, 131, 3281–3287. [Google Scholar] [CrossRef] [Green Version]
- Papoutsis, A.J.; Selmin, O.I.; Borg, J.L.; Romagnolo, D.F. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: Preventive effects of resveratrol: GESTATIONAL EXPOSURE TO THE AhR AGONIST 2,3,7,8-TETRACHLORODIBENZO-p-DIOXIN. Mol. Carcinog. 2015, 54, 261–269. [Google Scholar] [CrossRef]
- Jung, K.-J.; Wallig, M.A.; Singletary, K.W. Purple grape juice inhibits 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 2006, 233, 279–288. [Google Scholar] [CrossRef]
- Chatterjee, M.; Das, S.; Janarthan, M.; Ramachandran, H.K.; Chatterjee, M. Role of 5-lipoxygenase in resveratrol mediated suppression of 7,12-dimethylbenz(α)anthracene-induced mammary carcinogenesis in rats. Eur. J. Pharmacol. 2011, 668, 99–106. [Google Scholar] [CrossRef]
- Rahal, O.M.; Machado, H.L.; Montales, M.T.E.; Pabona, J.M.P.; Heard, M.E.; Nagarajan, S.; Simmen, R.C.M. Dietary suppression of the mammary CD29hiCD24+ epithelial subpopulation and its cytokine/chemokine transcriptional signatures modifies mammary tumor risk in MMTV-Wnt1 transgenic mice. Stem Cell Res. 2013, 11, 1149–1162. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Chan, F.L.; Chen, S.; Leung, L.K. The citrus flavonone hesperetin inhibits growth of aromatase-expressing MCF-7 tumor in ovariectomized athymic mice. J. Nutr. Biochem. 2012, 23, 1230–1237. [Google Scholar] [CrossRef]
- Li, F.; Wong, T.Y.; Lin, S.; Chow, S.; Cheung, W.; Chan, F.L.; Chen, S.; Leung, L.K. Coadministrating Luteolin Minimizes the Side Effects of the Aromatase Inhibitor Letrozole. J. Pharmacol. Exp. Ther. 2014, 351, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Mai, Z.; Blackburn, G.L.; Zhou, J.-R. Soy phytochemicals synergistically enhance the preventive effect of tamoxifen on the growth of estrogen-dependent human breast carcinoma in mice. Carcinogenesis 2007, 28, 1217–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, A.; Bishayee, A. Mechanism of Breast Cancer Preventive Action of Pomegranate: Disruption of Estrogen Receptor and Wnt/β-Catenin Signaling Pathways. Molecules 2015, 20, 22315–22328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzat, S.M.; Shouman, S.A.; Elkhoely, A.; Attia, Y.M.; Elsesy, M.S.; El Senousy, A.S.; Choucry, M.A.; El Gayed, S.H.; El Sayed, A.A.; Sattar, E.A.; et al. Anticancer potentiality of lignan rich fraction of six Flaxseed cultivars. Sci. Rep. 2018, 8, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Saggar, J.K.; Corey, P.; Thompson, L.U. Flaxseed and Pure Secoisolariciresinol Diglucoside, but Not Flaxseed Hull, Reduce Human Breast Tumor Growth (MCF-7) in Athymic Mice. J. Nutr. 2009, 139, 2061–2066. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.; Beebe, J.; Schwartzbaum, J. Chemoprevention of breast cancer by cyclooxygenase and lipoxygenase inhibitors. World Acad. Sci. J. 2020, 2, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The Role of Cytokines in Breast Cancer Development and Progression. J. Interferon Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002, 62, 4945–4954. [Google Scholar]
- Ke, J.-Y.; Banh, T.; Hsiao, Y.-H.; Cole, R.M.; Straka, S.R.; Yee, L.D.; Belury, M.A. Citrus flavonoid naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol. Nutr. Food Res. 2017, 61, 1600934. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, K.; Yu, Z.; Ren, H.; Zhao, L.; Li, Z.; Guo, Q.; Lu, N. Wogonoside inhibits invasion and migration through suppressing TRAF2/4 expression in breast cancer. J. Exp. Clin. Cancer Res. 2017, 36, 103. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Lin, H.; Yang, Y.; Zhang, S.; Wu, X.; Wang, M.; Ji, L.; Lu, L.; Yu, L.; Han, G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int. Immunopharmacol. 2010, 10, 819–826. [Google Scholar] [CrossRef]
- Forghani, P.; Khorramizadeh, M.R.; Waller, E.K. Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med. 2014, 3, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Jin, L.; Lu, L.; Lu, X.; Zhang, C.; Zhang, F.; Liang, W. Naringenin reduces lung metastasis in a breast cancer resection model. Protein Cell 2011, 2, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yan, F.; Zhao, Y.; Chen, X.; Sun, S.; Wang, Y.; Ying, L. Green Tea Polyphenol EGCG Attenuates MDSCs-mediated Immunosuppression through Canonical and Non-Canonical Pathways in a 4T1 Murine Breast Cancer Model. Nutrients 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, B.; Wang, L.; Jin, Y.; Zhen, H.; Xu, P.; Xu, Y.; Li, C.; Xu, H. Role of Metabolism in the Effects of Genistein and Its Phase II Conjugates on the Growth of Human Breast Cell Lines. AAPS J. 2012, 14, 329–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Kroon, P.A.; Shao, H.; Needs, P.W.; Yang, X. Differential Effects of Quercetin and Two of Its Derivatives, Isorhamnetin and Isorhamnetin-3-glucuronide, in Inhibiting the Proliferation of Human Breast-Cancer MCF-7 Cells. J. Agric. Food Chem. 2018, 66, 7181–7189. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Conjugated Physiological Resveratrol Metabolites Induce Senescence in Breast Cancer Cells: Role of p53/p21 and p16/Rb Pathways, and ABC Transporters. Mol. Nutr. Food Res. 2019, 63, 1900629. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. Tissue deconjugation of urolithin A glucuronide to free urolithin A in systemic inflammation. Food Funct. 2019, 10, 3135–3141. [Google Scholar] [CrossRef] [Green Version]
- Miksits, M.; Wlcek, K.; Svoboda, M.; Kunert, O.; Haslinger, E.; Thalhammer, T.; Szekeres, T.; Jäger, W. Antitumor Activity of Resveratrol and its Sulfated Metabolites against Human Breast Cancer Cells. Planta Med. 2009, 75, 1227–1230. [Google Scholar] [CrossRef]
- Hoshino, J.; Park, E.-J.; Kondratyuk, T.P.; Marler, L.; Pezzuto, J.M.; van Breemen, R.B.; Mo, S.; Li, Y.; Cushman, M. Selective Synthesis and Biological Evaluation of Sulfate-Conjugated Resveratrol Metabolites. J. Med. Chem. 2010, 53, 5033–5043. [Google Scholar] [CrossRef] [Green Version]
- Ruotolo, R.; Calani, L.; Fietta, E.; Brighenti, F.; Crozier, A.; Meda, C.; Maggi, A.; Ottonello, S.; Del Rio, D. Anti-estrogenic activity of a human resveratrol metabolite. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ren, D.; Deng, S.; Yang, X. Differential effects of baicalein and its sulfated derivatives in inhibiting proliferation of human breast cancer MCF-7 cells. Chem. Biol. Interact. 2014, 221, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.; Fernandes, I.; González-Manzano, S.; de Freitas, V.; Mateus, N.; Santos-Buelga, C. Anti-proliferative effects of quercetin and catechin metabolites. Food Funct. 2014, 5, 797. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Calani, L.; Brighenti, F.; Crozier, A.; Ottonello, S.; Del Rio, D. Glucuronidation does not suppress the estrogenic activity of quercetin in yeast and human breast cancer cell model systems. Arch. Biochem. Biophys. 2014, 559, 62–67. [Google Scholar] [CrossRef]
- Yamazaki, S.; Miyoshi, N.; Kawabata, K.; Yasuda, M.; Shimoi, K. Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking b2-adrenergic signaling. Arch. Biochem. Biophys. 2014, 557, 18–27. [Google Scholar] [CrossRef]
- Yamazaki, S.; Sakakibara, H.; Takemura, H.; Yasuda, M.; Shimoi, K. Quercetin-3-O-glucronide inhibits noradrenaline binding to α2-adrenergic receptor, thus suppressing DNA damage induced by treatment with 4-hydroxyestradiol and noradrenaline in MCF-10A cells. J. Steroid Biochem. Mol. Biol. 2014, 143, 122–129. [Google Scholar] [CrossRef]
- Kinjo, J.; Tsuchihashi, R.; Morito, K.; Hirose, T.; Aomori, T.; Nagao, T.; Okabe, H.; Nohara, T.; Masamune, Y. Interactions of phytoestrogens with estrogen receptors alpha and beta (III). Estrogenic activities of soy isoflavone aglycones and their metabolites isolated from human urine. Biol. Pharm. Bull. 2004, 27, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Pritchett, L.; Atherton, K.; Mutch, E.; Ford, D. Glucuronidation of the soyabean isoflavones genistein and daidzein by human liver is related to levels of UGT1A1 and UGT1A9 activity and alters isoflavone response in the MCF-7 human breast cancer cell line. J. Nutr. Biochem. 2008, 19, 739–745. [Google Scholar] [CrossRef]
- Pugazhendhi, D.; Watson, K.A.; Mills, S.; Botting, N.; Pope, G.S.; Darbre, P.D. Effect of sulphation on the oestrogen agonist activity of the phytoestrogens genistein and daidzein in MCF-7 human breast cancer cells. J. Endocrinol. 2008, 197, 503–515. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Hou, Y.C.; Lin, C.-H.; Hsu, Y.-A.; Sheu, J.J.C.; Lai, C.-H.; Chen, B.-H.; Lee Chao, P.-D.; Wan, L.; Tsai, F.-J. Puerariae radix isoflavones and their metabolites inhibit growth and induce apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2009, 378, 683–688. [Google Scholar] [CrossRef]
- Islam, M.A.; Bekele, R.; vanden Berg, J.H.J.; Kuswanti, Y.; Thapa, O.; Soltani, S.; van Leeuwen, F.X.R.; Rietjens, I.M.C.M.; Murk, A.J. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicol. In Vitro 2015, 29, 706–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalesi, E.; Cipolletti, M.; Cracco, P.; Fiocchetti, M.; Marino, M. Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers 2020, 12, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, L.U.; Chen, J.M.; Li, T.; Strasser-Weippl, K.; Goss, P.E. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin. Cancer Res. 2005, 11, 3828–3835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCann, S.E.; Edge, S.B.; Hicks, D.G.; Thompson, L.U.; Morrison, C.D.; Fetterly, G.; Andrews, C.; Clark, K.; Wilton, J.; Kulkarni, S. A pilot study comparing the effect of flaxseed, aromatase inhibitor, and the combination on breast tumor biomarkers. Nutr. Cancer. 2014, 66, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Ferreira Almeida, C.; Oliveira, A.; João Ramos, M.; Fernandes, P.A.; Teixeira, N.; Amaral, C. Estrogen receptor-positive (ER+) breast cancer treatment: Are multi-target compounds the next promising approach? Biochem. Pharmacol. 2020, 177, 113989. [Google Scholar] [CrossRef]
- Yu, S.S.; Spicer, D.V.; Hawes, D.; Tseng, C.C.; Yang, C.S.; Pike, M.C.; Wu, A.H. Biological effects of green tea capsule supplementation in pre-surgery postmenopausal breast cancer patients. Front. Oncol. 2013, 3, 298. [Google Scholar] [CrossRef] [Green Version]
- Crew, K.D.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Hudis, C.; McArthur, H.L.; Chang, J.; Rimawi, M.; Vornik, L.; et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of Ppolyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev. Res. (Phila) 2012, 5, 1144–1154. [Google Scholar] [CrossRef] [Green Version]
- Crew, K.D.; Ho, K.A.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Sneige, N.; Hudis, C.; McArthur, H.L.; Chang, J.; et al. Effects of a green tea extract, Polyphenon E, on systemic biomarkers of growth factor signalling in women with hormone receptor-negative breast cancer. J. Hum. Nutr. Diet 2015, 28, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.R.; Wahlin-Jacobsen, S. Testosterone in women--the clinical significance. Lancet Diabetes Endocrinol. 2015, 3, 980–992. [Google Scholar] [CrossRef]
- Glaser, R.; Dimitrakakis, C. Testosterone and breast cancer prevention. Maturitas 2015, 82, 291–295. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan Wolf, J.; Heckler, C.E.; Guido, J.J.; Peoples, A.R.; Gewandter, J.S.; Ling, M.; Vinciguerra, V.P.; Anderson, T.; Evans, L.; Wade, J.; et al. Oral curcumin for radiation dermatitis: A URCC NCORP study of 686 breast cancer patients. Support. Care Cancer 2018, 6, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Martin, S.; Pearson, A.; Bagchi, D.; Earl, J.; Gothard, L.; Hall, E.; Porter, L.; Yarnold, J. Double-blind, placebo-controlled, randomised phase II trial of IH636 grape seed proanthocyanidin extract (GSPE) in patients with radiation-induced breast induration. Radiother. Oncol. 2006, 79, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Ballestra, B.; Listorti, C.; Cappelletti, V.; Reduzzi, C.; Scaperrotta, G.P.; Pulice, I.; Ferrari, E.G.A.; Folli, S.; Mariani, L.; et al. Red clover and lifestyle changes to contrast menopausal symptoms in premenopausal patients with hormone-sensitive breast cancer receiving tamoxifen. Breast Cancer Res. Treat. 2020, 180, 157–165. [Google Scholar] [CrossRef]
- Hardman, W.E.; Primerano, D.A.; Legenza, M.T.; Morgan, J.; Fan, J.; Denvir, J. Dietary walnut altered gene expressions related to tumor growth, survival, and metastasis in breast cancer patients: A pilot clinical trial. Nutr. Res. 2019, 66, 82–94. [Google Scholar] [CrossRef]
- Martínez, N.; Herrera, M.; Frías, L.; Provencio, M.; Pérez-Carrión, R.; Díaz, V.; Morse, M.; Crespo, M.C. A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: Results of a pilot study. Clin. Transl. Oncol. 2019, 21, 489–498. [Google Scholar] [CrossRef]
- Núñez-Sánchez, M.A.; González-Sarrías, A.; Romo-Vaquero, M.; García-Villalba, R.; Selma, M.V.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Dietary phenolics against colorectal cancer—From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol. Nutr. Food Res. 2015, 59, 1274–1291. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; González-Sarrías, A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; et al. Gene expression changes in colon tissues from colorectal cancer patients following the intake of an ellagitannin-containing pomegranate extract: A randomized clinical trial. J. Nutr. Biochem. 2017, 42, 126–133. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A.; et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibney, E.R.; Milenkovic, D.; Combet, E.; Ruskovska, T.; Greyling, A.; González-Sarrías, A.; de Roos, B.; Tomás-Barberán, F.; Morand, C.; Rodriguez-Mateos, A. Factors influencing the cardiometabolic response to (poly)phenols and phytosterols: A review of the COST Action POSITIVe activities. Eur. J. Nutr. 2019, 58 (Suppl. 2), 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- Vallejo, F.; Larrosa, M.; Escudero, E.; Zafrilla, M.P.; Cerdá, B.; Boza, J.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans. J. Agric. Food Chem. 2010, 58, 6516–6524. [Google Scholar] [CrossRef]
- Quartieri, A.; García-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomàs-Barberàn, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Espín, J.C.; García-Villalba, R. The Human Metabolism of Nuts Proanthocyanidins does not Reveal Urinary Metabolites Consistent with Distinctive Gut Microbiota Metabotypes. Mol. Nutr. Food Res. 2019, 63, e1800819. [Google Scholar] [CrossRef]
- Mena, P.; Ludwig, I.A.; Tomatis, V.B.; Acharjee, A.; Calani, L.; Rosi, A.; Brighenti, F.; Ray, S.; Griffin, J.L.; Bluck, L.J.; et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes. Eur. J. Nutr. 2019, 58, 1529–1543. [Google Scholar] [CrossRef] [Green Version]
- Romo-Vaquero, M.; Cortés-Martín, A.; Loria-Kohen, V.; Ramírez-de-Molina, A.; García-Mantrana, I.; Collado, M.C.; Espín, J.C.; Selma, M.V. Deciphering the Human Gut Microbiome of Urolithin Metabotypes: Association with Enterotypes and Potential Cardiometabolic Health Implications. Mol. Nutr. Food Res. 2019, 63, e1800958. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann.-Ist. Super Sanita. 2007, 43, 348–361. [Google Scholar]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mena, P.; Del Rio, D. Gold Standards for Realistic (Poly) phenol Research. J. Agric. Food Chem. 2018, 66, 8221–8223. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Pratap, K.; Sinha, J.; Desiraju, K.; Bahal, D.; Kukreti, R. Critical evaluation of challenges and future use of animals in experimentation for biomedical research. Int. J. Immunopathol. Pharmacol. 2016, 29, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, A.; Da Costa, F. Molecular Approaches to Explore Natural and Food-Compound Modulators in Cancer Epigenetics and Metabolism. In Foodinformatics; Martinez-Mayorga, K., Medina-Franco, J., Eds.; Springer: Cham, Switzerland, 2014; pp. 131–149. [Google Scholar]
- Bayram, B.; González-Sarrías, A.; Istas, G.; Garcia-Aloy, M.; Morand, C.; Tuohy, K.; García-Villalba, R.; Mena, P. Breakthroughs in the Health Effects of Plant Food Bioactives: A Perspective on Microbiomics, Nutri(epi)genomics, and Metabolomics. J. Agric. Food Chem. 2018, 66, 10686–10692. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
| Group and Sample Size | Diet/Compound Administration | Extraction and Analytical Conditions | Identified and(or) Quantified Phenolic Metabolites | References |
|---|---|---|---|---|
| 8 women undergoing breast biopsy or cancer surgery | Intake of four soy-supplemented bread rolls per day, providing approximately 45 mg of isoflavonoids, for 14 days prior to surgery (n = 4) Placebo group (n = 4). | Breast cancer tissues were extracted and further hydrolyzed by enzymatic treatment 1. Quantitative analyses were performed by GC/MS using specific standards. | The mean daidzein concentration in non-soy-supplemented patients was 0.021 (range 0.017–0.028) nmol/g compared with 0.145 (range 0.083–0.218) nmol/g in soy-supplemented patients. | [54] |
| 3 healthy women undergoing breast reductions | Soy-based food supplement containing 100 mg of genistein/genistin, 37 mg of daidzein/daidzin, and 15 mg of glycitein/glycitin (more than 90% as glycosides (single and triple dose, n = 1 each) or a placebo tablet (n = 1) for 5 days before aesthetic breast surgery. | Breast tissues were extracted and further hydrolyzed by enzymatic treatment 1. Analyses were performed by HPLC-DAD and the compounds were identified by comparison of the retention time with the respective standards (UV spectra). | Genistein and equol concentrations were 4.16 µg/g and 52.98 µg/g, respectively, after soy-based food supplement (single dose), whereas daidzein was below the limit of detection. Genistein, equol and daidzein concentrations were 35.1 µg/g, 681.7 µg/g and 16.0 µg/g, respectively, after soy-based food supplement (triple dose). | [52] |
| 28 volunteers before aesthetic breast surgery | Soy-based food supplement containing 100 mg of genistein/genistin, 37 mg of daidzein/daidzin, and 15 mg of glycitein/glycitin (more than 90% as glycosides, for 5 evenings before aesthetic breast surgery (n = 9). Placebo group (n = 19). | Breast tissues were extracted and further hydrolyzed by enzymatic treatment 1. Analyses were performed by HPLC-MS and the compounds were quantified using specific standards. | The median daidzein and equol concentrations were 7.03 nmol/L and 2.44 nmol/L, respectively, in soy-supplemented subjects. Genistein was not detectable. The median daidzein concentration was 5.44 nmol/L in the placebo group. Equol and genistein were only detectable in some subjects of the placebo group, with equol ten-fold values higher than genistein. No significant differences were found between the 2 groups. | [53] |
| 31 healthy women undergoing an aesthetic breast reduction | Soy milk (n = 11; 250 mL containing 16.98 mg genistein and 5.40 mg daidzein (per dose)), soy supplement (n = 10; 5.27 mg genistein and 17.56 mg daidzein aglycone (per dose), or control (n = 10, no soy product). 3 doses of soy milk or soy supplements were taken daily for 5 days before an aesthetic breast reduction. | Breast tissues were dissected into fractions (adipose and glandular tissue) and were both non-hydrolyzed and hydrolyzed by enzymatic treatment 1. Quantitative analyses were performed by HPLC-MS/MS and the compounds were quantified using specific standards of aglycones, but not conjugated forms. | Total isoflavones showed a breast adipose/glandular tissue distribution of 40:60. In hydrolyzed breast adipose and glandular tissues, total genistein and daidzein concentrations ranged between 92.33–493.8 pmol/g and 22.15–770.8 pmol/g. Total equol and dihydrodaidzein were only detected in few subjects (up to 559.4 pmol/g and up to 368.8 ± 171.1 pmol/g. In non-hydrolyzed breast adipose and glandular tissues only traces of genistein and daidzein aglycones were observed (20–25 pmol/g total isoflavone aglycones), whereas an overall total glucuronidation of 98% was estimated (900–1150 pmol/g total isoflavone glucuronides), mainly genistein-7-O-glucuronide and daidzein-7-O-glucuronide. Neither glucuronides nor aglycones of microbial daidzein metabolites (equol and O-DMA) were detected after isoflavone supplementation. | [55] |
| 21 healthy women undergoing an aesthetic breast reduction | Hop-supplemented group (containing 2.04 mg xanthohumol (XN), 1.20 mg isoxanthohumol (IX), and 0.1 mg 8-prenylnaringenin (8-PN) per supplement) (n = 11) or control group (n = 10). Three supplements were taken daily for 5 days before surgery. | Breast tissues were dissected into fractions (adipose and glandular tissue) and were both non-hydrolyzed and hydrolyzed by enzymatic treatment 1. Qualitative analyses were performed by HPLC-MS/MS and the compounds were quantified using specific standards for aglycones, but not conjugated forms. | Total prenylflavonoids showed a breast adipose/glandular tissue distribution of 38:62. Total XN and IX concentrations ranged between 0.26 and 5.14 pmol/g and 1.16 and 83.67 pmol/g in hydrolyzed breast tissue, respectively. 8-PN was only detected in samples of moderate and strong 8-PN producers (0.78–4.83 pmol/g). An extensive glucuronidation was observed (>90%). | [60] |
| 12 early breast cancer patients | Orally bioavailable complex of silybin–phosphatidylcholine (2.8 g/day) for 4 weeks prior to surgery. | Breast tissues (tumour and normal) were extracted and further hydrolyzed both with and without enzymatic treatment 1. Qualitative analyses were performed by HPLC-MS/MS and the free and total silybin were quantified using specific standard. | The median total and free silybin concentration in breast cancer tissues were 131 ng/mg (IQR, 35–869) and 33 ng/g (IQR, 4–158), respectively. Concentrations were higher in the tumour as compared with the adjacent normal tissue (total and free silybin concentration were 11 ng/mL (IQR, 0–34) and 0 ng/g (IQR, 0–19), respectively). | [65] |
| 12 early breast cancer patients | Patients received 300 mg of a caffeine-free green tea catechin extract, equivalent to 44.9 mg of epigallocatechin-3-O-gallate (EGCG) daily, for 4 weeks prior to surgery. | Breast tissues (tumour and normal) were extracted and further both non-hydrolyzed and hydrolyzed by enzymatic treatment 1. Quantitative analyses were performed by HPLC-MS/MS and the compounds were quantified as free (unconjugated) EGCG, thereafter through enzymatic hydrolysis as total EGCG (free and conjugated) using EGCG standard. | Total EGCG was detectable in all tumour tissue samples (median total EGCG 3.18 ng/g (IQR, 2.76–4.58)) and higher amount than in adjacent normal tissue (under the limit of detectability, minimum, 0 ng/g; maximum, 2.85 ng/g). Free EGCG concentrations were under the limit of detectability in tumour tissue but present in adjacent normal breast tissue (median = 1.07 ng/g; IQR, 0–1.25). | [66] |
| 27 breast cancer patients | Breast cancer patients consumed a cocktail of plant extracts (cocoa, pomegranate, lemon, orange, grapeseed, and olive) plus resveratrol, providing 37 different phenolics (473.7 mg), theobromine and caffeine (19.7 mg) (n = 19) from diagnosis to surgical resection (6 ± 2 days). Control group did not consume extracts (n = 8). | Normal and tumour glandular breast tissues were extracted and further both non-hydrolyzed and hydrolyzed by enzymatic treatment 1. Quantitative analyses were performed by UPLC-ESI-QTOF-MS and carried out by peak area integration of their EIC using calibration curves of specific (free and conjugated metabolites) standards. | A total of 39 and 33 metabolites were identified in normal and tumour tissues, respectively. Some representative metabolites detected in tumour tissues (median and range, pmol/g) were urolithin-A-3-O-glucuronide (26.2; 3.2–66.5), 2,5-dihydroxybenzoic acid (40.2; 27.7–52.2), RSV-3-O-sulphate (86.4; 7.8–224.4), dihydroRSV-3-O-glucuronide (109.9; 10.3–229.4), and HP 3′-O-glucuronide 12.9 (2.7–14.1). No significantly different conjugation profiling was found in tumour vs. normal tissues. Overall, all compounds were mostly glucuronidated and(or) sulphated in tumour and normal tissues, 85.5% and 86.6%, respectively. Among these conjugates, the percentage of sulphated was slightly higher in tumour (42%) than in normal tissues (31%). Quantitative analysis after hydrolysis was only possible for 2,5- and 2,6-dihydroxybenzoic acids, HP, urolithin A, isourolithin A, and urolithin B. | [69] |
| Breast Cellular Model | Compound Assayed | Dose/Duration | Main Outcomes | References |
|---|---|---|---|---|
| Resveratrol | ||||
| MCF-7, ZR-75-1, MDA-MB-231 (breast cancer cell lines) and MCF-10A (normal cell line) | RSV, RSV 3-sulph, RSV 4′-sulph, RSV 3,4′-disulph. | 1–350 µM; 48 h | RSV: IC50 = 67.6–82.2 µM against all breast cancer cells; IC50 = 20 µM against MCF-10A. RSV 3-sulph: IC50 = 189–258 µM against MCF-7 and MDA-MB-231; IC50 = 228.3 µM against MCF-10A. RSV 4′-sulph and RSV 3,4′-disulph: no cytotoxic effect against breast cancer cells; IC50 = 202.4–202.9 µM against MCF-10A. | [111] |
| MCF-7 | RSV, RSV 3-sulph, RSV 4′-sulph, RSV 3,5 disulph, RSV 3,4′-disulph, RSV 3,4′,5-trisulph. | 340 µM; 72 h | Cytotoxic effect (only RSV and RSV 3-sulph). | [112] |
| Saccharomyces cerevisiae strain Y190 co-transformed with ERα LBD and hTif2 coactivator and MCF-7 | RSV, RSV 3-gluc, RSV 3-glur, RSV 3-sulph, RSV 4′-sulph. | 0.05–100 µM; 18–24 h | RSV 3-sulph showed anti-oestrogenic activity at 10 and 50 µM (with a marked preference for ERα at 10–100 µM), and weak oestrogenic activity. RSV showed oestrogenic activity in MCF-7 (5–10 µM). | [113] |
| MCF-7 and MDA-MB-231 | RSV, RSV 3-glur, RSV 3-sulph, RSV 4′-sulph, DH-RSV, DH-RSV 3-glur. | 10 and 50 µM; 3 and 7 days | ↓Proliferation in MDA-MB-231 cells (only RSV and DH-RSV). Oestrogenic/anti-oestrogenic effect in MCF-7 (only RSV and DH-RSV). | [51] |
| MCF-7, MDA-MB-231 and MCF-10A (normal cell line) | RSV, RSV 3-glur, RSV 3-sulph, RSV 4′-sulph, DH-RSV, DH-RSV 3-glur. | 0.5, 1, and 10 µM; 1–14 days | The effects were only observed in MCF-7 cells for all compounds: ↓Clonogenicity; cell cycle arrest; senescence induction; modulation of p53/p21 and p16/Rb pathways. In MDA-MB-231 cells (only RSV at 10 µM): ↓clonogenicity. | [109] |
| Flavanones | ||||
| MCF-7 and normal mammary epithelial cells H184B5F5/M10 | Baicalein, baicalein 7-O-sulph, and baicalein-8-sodium sulphonate. | 50, 100, and 200 µM; 24 h | Effects in MCF-7 cells: ↓cell viability; ↑LDH release; induction of cell cycle arrest; induction of morphological changes; ↑apoptosis; ↑ROS; ↑caspase-3, -9 activity. Effects on H184B5F5/M10 cells: No cytotoxic. | [114] |
| MCF-7 and MDA-MB-231 | HP, HP 3′-glur, HP 7-glur, HP 3′-sulph, HP 7-sulph. | 10 and 50 µM; 3 and 7 days | ↓Proliferation in MDA-MB-231 cells (only HP at 50 µM). Oestrogenic/anti-oestrogenic effect in MCF-7 (only HP at 10 and 50 µM). | [51] |
| Quercetin | ||||
| MCF-7 | Quer, Quer-3-O-gluc, Quer 3-O-glur, Quer 4′-O-sulph, Tamarixetin, Isorhamnetin. | 50 µM; 48 h | ↓Cell proliferation (no effect of Quer-3-O-gluc and Quer 3-O-glur). | [115] |
| Saccharomyces cerevisiae expressing ERα-Tif2 or ERβ-Tif2 and MCF-7 | Quer, rutin, isoquercitrin, Quer 3-O-glur, Quer 3-O-sulph. | 0.1–100 µM; 18–24 h | Quer 3-O-glur (IC50 = 2.1 ± 0.38 µM) and Quer (IC50 = 2.4 ± 0.93 µM) showed oestrogenic activity in MCF-7 cells. Quer 3-O-glur showed ERα-Tif2 (IC50 = 103 ± 2.24 µM) and ERβ-Tif2 (IC50 = 96 ± 1.2 µM) agonistic activity. Isoquercitrin showed similar ERβ-Tif2 agonistic activity than Quer 3-O-glur. Quer showed weak ERβ-Tif2 (IC50 = 5.3 ± 0.9 µM) agonistic activity. | [116] |
| MDA-MB-231 | Quer 3-O-glur (alone or together with A and NA). | 10−10–10−4 M (binding assay) and 0.01–1 µM (cell assay); 1–24 h | Quer 3-O-glur showed competitive binding of [3H]-NA to β2-AR (10-4–102 µM). ↓ROS formation; ↓HMOX1, MMP-2, and MMP-9 gene expression; ↓intracellular cAMP level; ↓p-ERK 1/2 and p-P38; ↓RAS activation; ↓invasive capacity of MDA-MB-231; ↓MMP-9 activity. | [117] |
| MCF-10A (normal cell line) | Quer and Quer 3-O-glur (alone or together with 4-OHE2 and NA). | 10−10–10−4 M (binding assay) and 0.05–10 µM (cell assay); 2 h | Quer and Quer 3-O-glur showed competitive binding of [3H]-NA to α2-AR (10−4–102 µM). ↓γ-H2AX and AP sites activation. | [118] |
| MCF-7 and normal mammary epithelial cells H184B5F5/M10 | Quer, Isorhamnetin, and Isorhamnetin 3-O-glucuronide. | 25, 50, and 100 µM; 24–48 h | Effects in MCF-7 cells: ↓Cell viability; ↑LDH release: induction of cell cycle arrest; induction of morphological changes; ↑apoptosis; ↑ROS. Effects on H184B5F5/M10 cells: No cytotoxic effects. | [107] |
| MCF-7 and normal mammary epithelial cells H184B5F5/M10 | Quer, Quer 3-O-glur, and Quer 3-O-sulph. | 25, 50 and 100 µM; 24–48 h | Effects in MCF-7 cells: ↓cell growth; ↑LDH release; ↑ROS; ↑apoptosis; induction of cell cycle arrest; induction of morphological changes. Effects on H184B5F5/M10 cells: No cytotoxic effects. | [108] |
| Urolithins | ||||
| MCF-7 and MDA-MB-231 | Uro-A, Uro-A 3-glur, Uro-A 8-glur, Uro-A 3-sulph, IsoUro-A, IsoUro-A 3-glur, IsoUro-A 9-glur, Uro-B, Uro-B 3-glur, Uro-B 3-sulph. | 10 and 50 µM; 3 and 7 days | ↓Proliferation in MCF-7 (Uro-A and IsoUro-A) and MDA-MB-231 (free forms and conjugates at 50 µM). Oestrogenic activity in MCF-7 (only free forms at 10 and 50 µM). Anti-oestrogenic activity in MCF-7 (Uro-A and its conjugates, IsoUro-A and its conjugates, and Uro-B at 10 and 50 µM). | [51] |
| Catechin and epicatechin | ||||
| MCF-7 | Epi, Epi-3′-O-sulph, 3′-O-methyl-Epi, 4′-O-methyl-Epi, catechin, 3′-O-methyl-catechin. | 100 µM; 48 h | ↓Cell proliferation (only 4′-O-methyl-Epi). | [115] |
| Isoflavones | ||||
| MCF-7 | GEN, GEN 4′-O-glur, GEN 7-O-glur, GEN 4′-O-sulph-7-O-glur, GEN 4′-O-sulph, GEN 7-O-sulph, GEN 4′,7-di-O-sulph, DAZ, DAZ 4′-O-glur, DAZ 7-O-glur, DAZ 4′-O-sulph-7-O-glur, DAZ 4′-O-sulph, DAZ 7-O-sulph, DAZ 4′,7-di-O-sulph, Glycitein, Glycitein 7-O-glur, DH-DAZ, DH-DAZ 7-O-glur, O-DMA, O-DMA 7-O-glur. | 10–1000 µM; 24 h | Low stimulatory MCF-7 growth, β-gal induction, and binding to ERs: sulphates of GEN and glucuronides of glycitein and DH-DAZ. Moderate binding, but low (or lack) MCF-7 growth and β -gal induction: glucuronides of GEN, DAZ, and O-DMA. Moderate MCF-7 growth and β-gal induction, but low (or lack) binding: DAZ 4′-O-sulph-7-O-glur. O-DMA was the most active compound stimulating MCF-7 growth, binding to hERβ, and inducing β-gal. | [119] |
| MCF-7 | GEN, GEN 7-O-glur, DAZ, DAZ 7-O-glur. | 16 µM; 72 h | ↑Cytotoxicity (only DAZ). | [120] |
| Isoflavones | ||||
| MCF-7 | GEN, GEN 7-O-sulph, GEN 4′-O-sulph, DAZ, DAZ 7-O-sulph, DAZ 4′-O-sulph, equol, equol-7-sulph (in the presence of 4 × 10−10 M [2,4,6,7-3H] oestradiol). | 10−7–10−4 M; 18–24 h (binding and gene expression assay) and 7 or 14 days (proliferation assay) | The sulphation in position 7 reduced the oestrogen capacity of GEN and equol in all assays. GEN 4′-O-sulph showed lower proliferative activity (at 1 and 10 µM), and similar or even higher binding affinity to ER (compared to GEN). DAZ 4′-O-sulph showed lower binding affinity to ER and increased proliferative activity (at 10 µM). DAZ 7-O-sulph showed similar or even higher affinity to ER and similar proliferative activity (at 1 and 10 µM) (compared to DAZ). | [121] |
| HS578T, MDA-MB-231, and MCF-7 | Puerarin, GEN, DAZ, and mix of DAZ glucuronides/sulphates. | 12.5–100 µM (free compounds) and 2.35 µM the mix of DAZ conjugates; 24–72 h | Effect of only with free compounds: (IC50 = 29–71 µM) ↓cell viability; induction of cell cycle arrest; ↑apoptosis; ↑caspase-3 activity. Effect of mix of DAZ conjugates: (IC50 = 2.35 µM) ↓cell proliferation; induction of cell cycle arrest; ↑apoptosis; ↑caspase-9, p53, p21, and Bax. | [122] |
| MCF-7, T47D, and MCF-10A (normal cell line) | GEN, GEN 7-O-sulph, GEN 4′-O-sulph, GEN 7-O-glur. | 5.12 × 10−3–80 µM for GEN and 2.2–4.5 µM for conjugates; 3 days | Dissimilar effects of GEN cell proliferation: ↑ at low concentrations and ↓ at higher concentrations. GEN 7-O-glur stimulated cells growth. This effect was related to deconjugation. GEN 7-O-sulph, GEN 4′-O-sulph exerted no effects. | [106] |
| T47D and T47D-ERβ (tetracycline dependent ERβ expression) | GEN, GEN 7-O-glur, DAZ, DAZ 7-O-glur. | 10−5–1000 µM; 48 h | Dissimilar effects on proliferation: ↓ at low concentrations and ↑ at higher concentrations. Lower proliferative potency than 17β-oestradiol: PC50 in T47D-wt: 17β-oestradiol = 4.2×10−6 µM; GEN = 0.19 µM; GEN 7-O-glur = 21.4 µM; DAZ = 0.186 µM; DAZ 7-O-glur = 107 µM. PC50 in T47ERβ: 17β-oestradiol = 2.45×10−7 µM; GEN = 0.2 µM; GEN 7-O-glur = 7.8 µM; DAZ = 0.035 µM; DAZ 7-O-glur = >400 µM. | [123] |
| Isoflavones | ||||
| MCF-7 and T47D | DAZ, DAZ 4′-O-sulph, DAZ 7-O-sulph, DAZ 4′,7-di-O-sulph, equol, O-DMA. | 0.1, 1, and 10 µM; 1–24 h | Different effects on NGB: ↑equol, O-DMA, DAZ 7-O-sulph, DAZ 4′,7-di-O-sulph and ↓DAZ and DAZ 4′-O-sulph. Effects of DAZ, DAZ 4′-O-sulph and equol on protein phosphorylation: ↑p-ERα/ERα (all compounds); ↑p-Akt/Akt (only equol; 1 h treatment); ↑p-p38/p38 (all molecules; 1 and 24 h treatment). DAZ, DAZ 4′-O-sulph and equol preserve PAX activity in MCF-7 cells (↓NGB, ↑PARP-1 cleavage, and ↓cell number) in the presence of 17β-oestradiol. Effects of the mix of metabolites: 1 Mix of sulphates metabolites: ↓NGB by in the presence or absence of 1 µM DAZ; the mix of sulphates together with 1 µM DAZ preserve PAX effects (↑PARP-1 cleavage) in the presence of 17β-oestradiol. 2 Mix of gut metabolites: ↑NGB by equol + O-DMA (1 µM each) in the presence or absence of 1 µM DAZ; ↓PAX effects (↑PARP-1 cleavage) in the presence of 17β-oestradiol. | [124] |
| Cohort and Sample Size | Trial Design | Objective | Outcomes | References |
|---|---|---|---|---|
| Newly biopsy-diagnosed breast cancer patients (n = 32), with no hormone therapy | Design: Pre-surgery, randomized double-blind, placebo-controlled clinical trial. Product and dose: Patients (n = 19) consumed flaxseed (25 g/d) or placebo (n = 13). Follow-up: 37 and 39 days in the flaxseed and placebo groups, respectively. | Effect of flaxseed on tumour biomarkers. | Significant reductions in Ki-67 labelling index (34.2%) and in c-erbB2 expression, (71%), and an increase in apoptosis (30.7%) were observed in the flaxseed, but not in the placebo group. | [125] |
| Patients (n = 66) with tissue hardness due to radiotherapy for early breast cancer (at least 24 months prior to trial entry), with no active disease | Design: Phase II, placebo-controlled, randomized trial. Product and dose: Capsules containing a grape seed proanthocyanidin extract 100 mg three times a day orally, or placebo. Follow-up: 6 months. | Effect on the surface area of palpable breast induration after 12 months. Secondary endpoints: change in photographic breast appearance and patient self-assessment of breast hardness, pain and tenderness. | No significant difference between treatment and control groups in terms of external assessments of tissue hardness, breast appearance or patient self-assessments of breast hardness, pain or tenderness. | [136] |
| Patients (n = 14) with metastatic breast cancer | Design: Open label, phase I, non-controlled trial. Product and dose: Intravenous docetaxel plus oral curcumin (escalated dose until toxicity limit is reached). Follow-up: Docetaxel 100 mg/m2 was administered every 3 weeks (w) for six cycles. Curcumin was given orally for 7 consecutive days (d) (from d-4 to d+2) for six cycles (a total of 63 cycles). | Establishment of the maximal tolerated dose (MTD) of oral curcumin plus intravenous docetaxel. | MTD of curcumin was 8 g/day, in combination with docetaxel 100 mg/m2 administered every 3 w for six cycles. Recommended curcumin dose: 6 g/d for 7 consecutive d every 3 w in combination with a standard dose of docetaxel. Decrease of plasmatic CEA and VEGF. | [133] |
| Patients (n = 40) with resected stage I-III ER- and PR- breast cancer with no active disease | Design: Randomized, phase IB, double-blinded, placebo-controlled, and dose-escalation study. Product and dose: Capsules (green tea extract) containing EGCG (treated group, n = 30). Daily oral dose was 800 mg (n = 16), 1200 mg (n = 11), and 1600 mg (n = 3) EGCG. Control group (n = 10): placebo. Follow-up: 6 months. | Establishment of the MTD for EGCG. | MTD was 1200 mg/d EGCG for 6 months. No significant change in SHBG, IGF-1, IGFBP-3, ER, Ki-67 proliferation index or mammographic density | [129] |
| Patients (n = 28) with ductal carcinoma in situ or primary invasive stage I or II breast cancer | Design: Pre-surgery, controlled study. Product and dose: 3 capsules/d (green tea extract) containing EGCG (treated group, n = 13). Daily oral dose was 940 mg EGCG. Control group (n = 15): no capsules. Follow-up: Average duration of green tea intake was 35 days. | Short-term effects of green tea supplementation on cancer biomarkers. | Significant decrease of Ki-67 in the tea group, but only in normal tissue. No effects on apoptosis (caspase-3), and angiogenesis (CD34) markers in benign or malignant tissue. | [128] |
| Patients (n = 30) with non-inflammatory breast cancer or carcinoma in situ and prescribed RT without concurrent chemotherapy | Design: Randomized, double-blind, placebo-controlled clinical trial. Product and dose: Patients (n = 14) consumed 6 g/d curcumin extract (a daily dose of 4.7 g curcumin, 0.9 g demethoxycurcumin, and 0.15 g bisdemethoxycurcumin) or placebo (n = 16). Follow-up: 6 months (30 RT sessions) | Effect of curcumin to reduce RDS. | Significant reduction of RDS and moist desquamation at the end of treatment vs. placebo (mean RDS = 2.6 vs. 3.4; and 28.6% vs. 87.5%; respectively). | [134] |
| Postmenopausal women (n = 24) with newly-diagnosed, and resectable, ER+ breast cancer | Design: Pre-surgery, 2 × 2 factorial, randomized, placebo-controlled trial. Product and dose: 25 g/d ground flaxseed + 1/d placebo pill (n = 6); 1 mg/d anastrozole (aromatase inhibitor) (n = 7); 25 g/d ground flaxseed + 1 mg/d anastrozole (n = 6); or 1/d placebo pill control (n = 5). Follow-up: Mean of 18.8 days. | Effects of flaxseed and the aromatase inhibitor, anastrozole, on steroid hormones and tumour-related biomarkers. | Mean ERβ expression was approximately 40% lower from pre- to post-intervention in the flaxseed (FS) + anastrozole (AI) group only. Significant negative association for androstenedione in the FS + AI group vs. placebo, and for dehydroepiandrosterone with AI treatment. | [126] |
| Patients (n = 34) with resected stage I-III ER- and PR- breast cancer with no active disease. (Ancillary study to that of Crew et al. [129] | Design: Randomized, phase IB, double-blinded, placebo-controlled, and dose-escalation study. Product and dose: Capsules (green tea extract) containing EGCG (treated group, n = 26). Daily oral dose was 800 mg (n = 14), 1200 mg (n = 11), and 1600 mg (n = 1) EGCG. Control group (n = 8): placebo. Follow-up: 6 months | Effect of EGCG on cancer biomarkers risk. | Significant transient decrease of serum HGF (only after 2 months of treatment) in EGCG consumers. No significant effects on VEGF, serum cholesterol and triglycerides, oxidative damage, and inflammatory biomarkers. | [130] |
| Patients (n = 12) with newly diagnosed breast cancer, ER+, not eligible for neoadjuvant treatment | Design: Pre-surgery (non-controlled) dietary intervention. Product and dose: Silybin-phosphatidylcholine complex (2.8 g/d) given orally. Follow-up: 4 w before surgery. | Effects on NO, IGF-1 and Ki-67. | No effects on NO, IGF-1 and Ki-67 were observed. | [65] |
| Patients (n = 12) with newly diagnosed breast cancer, not eligible for neoadjuvant treatment. | Design: Pre-surgery (non-controlled) dietary intervention. Product and dose: Catechin (65.1 mg/d) and EGCG (44.9 mg/d) given orally (300 mg tea extract). Follow-up: 4 w before surgery. | Effect on cell proliferation, angiogenesis, oxidative stress, chronic inflammation, and adiposity-related endocrine mechanism. | Significant increase of testosterone. No effect in the rest of markers. | [66] |
| Patients (n = 578) with non-inflammatory breast cancer or carcinoma in situ, and prescribed fractionated radiation therapy (RT) without concurrent chemotherapy | Design: Phase II, randomized, double-blind, placebo-controlled clinical trial. Product and dose: Patients (n = 283) consumed either 6 g/d curcumin extract (a daily dose of 5.4 g curcumin, 0.48 g dimethoxy curcumin, and 0.12 g bisdemethoxy curcumin), or placebo (n = 295). Follow-up: 6 months (30 RT sessions). | Confirmatory study on the effect of curcumin to reduce radiation dermatitis severity. | Curcumin did not reduce radiation dermatitis severity at the end of RT compared to placebo. | [135] |
| ER+ and(or) PR+ postmenopausal women (n = 45) with resected breast cancer at early stage (with no active disease), and receiving adjuvant hormonal therapy | Design: Open-label, single-arm (no placebo-controlled). Product and dose: Three daily capsules, containing 460 mg of fish oil (EPA and DHA), 125 mg of olive extract (12.5 mg hydroxytyrosol), and 50 mg extract of curcumin (47.5 mg curcuminoids) Follow-up: 30 days. | Effect on inflammation and pain. | Significant decrease of plasma CRP (from 8.2 ± 6.4 mg/L at baseline to 5.3 ± 3.2 mg/L), and pain (21.5%) after 30 days. | [139] |
| Breast cancer patients (n = 10) | Design: Pre-surgery, two arms, controlled study. Product and dose: Patients (n = 5) consumed walnuts (around 60 g/d) or not (i.e., controls, n = 5). Follow-up: About 15 days. | Effect of walnut consumption on gene expression in breast cancer tissue. | Significant change of 456 genes in the tumour due to walnut consumption. Activation of pathways that promote apoptosis and cell adhesion, and inhibition of pathways that promote cell proliferation and migration. | [138] |
| Patients (n = 81) with histologically confirmed operable ER+ breast cancer, with no distant metastasis, and receiving tamoxifen. | Design: Randomized, double-blind, and placebo-controlled trial. Product and dose: Patients (n = 42) consumed a red clover extract (80 mg isoflavones/d) or placebo (n = 39). Follow-up: 2 years | Effect of isoflavones from red clover extract and lifestyle change to reduce side-effects of tamoxifen treatment. | The reductions in BMI and waist circumference were significantly greater in the treatment than placebo group. No differences between groups in the rest of determinations: MRS, HDLc, insulin, total cholesterol, LDLc, triglycerides, insulin resistance, sex hormone levels, endometrial thickness and breast density. | [137] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; Espín, J.C.; González-Sarrías, A. Dietary Phenolics against Breast Cancer. A Critical Evidence-Based Review and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 5718. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165718
Ávila-Gálvez MÁ, Giménez-Bastida JA, Espín JC, González-Sarrías A. Dietary Phenolics against Breast Cancer. A Critical Evidence-Based Review and Future Perspectives. International Journal of Molecular Sciences. 2020; 21(16):5718. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165718
Chicago/Turabian StyleÁvila-Gálvez, María Ángeles, Juan Antonio Giménez-Bastida, Juan Carlos Espín, and Antonio González-Sarrías. 2020. "Dietary Phenolics against Breast Cancer. A Critical Evidence-Based Review and Future Perspectives" International Journal of Molecular Sciences 21, no. 16: 5718. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165718