Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications
Abstract
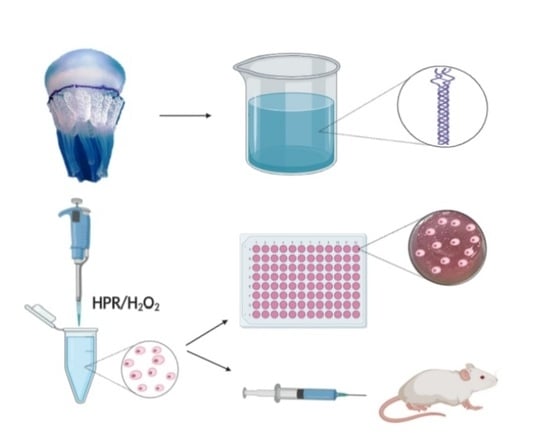
:1. Introduction
2. Results and Discussion
2.1. Purification and Characterization of R. pulmo Native Collagen
2.2. Functionalization Strategy for a Stable and Injectable Marine Hydrogel Formulation
2.3. Comparing Rat Tail Collagen Hydrogel (RTCh) and MCh for the Maintenance of Differentiated Chondrocytes
2.4. Cytoskeleton Organization and Gene Expression Analyses
3. Materials and Methods
3.1. R. pulmo Collagen Extraction
3.2. Characterization of R. pulmo Extracted Proteins
3.3. Natural Cross-Linking Degree Determination
3.4. Synthesis of Gelatin (Gtn)-HPA Conjugate
3.5. Enzyme-Mediated Injectable Hydrogels.
3.6. Marine Collagen Hydrogel (MCh) Preparation and Qualitative Evaluation of Injectability
3.7. Isolation of Nasal Septal Chondrocytes from Bovine Tissue
3.8. Immunofluorescence Assay
3.9. Cell Viability and Proliferation Assays
3.10. RTCh Preparation
3.11. Gel Contraction Assay
3.12. Glycosaminoglycans Identification of Electrophoretic Gel
3.13. Statistical Analysis
3.14. RNA Isolation and cDNA Synthesis
3.15. RT-qPCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hunziker, E.B. Articular cartilage repair: Are the intrinsic biological constraints undermining this process insuperable? Osteoarthr. Cartil. 1999, 7, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M.; et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: An observational first-in-human trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Vedicherla, S.; Buckley, C.T. Rapid chondrocyte isolation for tissue engineering applications: The effect of enzyme concentration and temporal exposure on the matrix forming capacity of nasal derived chondrocytes. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Pelttari, K.; Pippenger, B.; Mumme, M.; Feliciano, S.; Scotti, C.; Mainil-Varlet, P.; Procino, A.; Von Rechenberg, B.; Schwamborn, T.; Jakob, M.; et al. Adult human neural crest-derived cells for articular cartilage repair. Sci. Transl. Med. 2014, 6, 251ra119. [Google Scholar] [CrossRef]
- Pelttari, K.; Mumme, M.; Barbero, A.; Martin, I. Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine. Curr. Opin. Biotechnol. 2017, 47, 1–6. [Google Scholar] [CrossRef]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Carfì Pavia, F.; Ciappa, M.; Lepedda, A.; Fiorentino, S.; Rigogliuso, S.; Brucato, V.; Formato, M.; Ghersi, G.; La Carrubba, V. A poly-L-lactic acid/collagen/glycosaminoglycan matrix for tissue engineering applications. J. Cell. Plast. 2017, 53, 537–549. [Google Scholar] [CrossRef]
- Scaffaro, R.; Re, G.L.; Rigogliuso, S.; Ghersi, G. 3D Polylactide-Based Scaffolds for Studying Human Hepatocarcinoma Processes in vitro. Sci. Technol. Adv. Mater. 2012, 13, 045003. [Google Scholar] [CrossRef]
- Carfì Pavia, F.; La Carrubba, V.; Ghersi, G.; Brucato, V. Poly-left-lactic acid tubular scaffolds via diffusion induced phase separation: Control of morphology. Polym. Eng. Sci. 2013, 53, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Rigogliuso, S.; Carfi Pavia, F.; Brucato, V.; La Carrubba, V.; Favia, P.; Intranuovo, F.; Gristina, R.; Ghersi, G. Use of modified 3D scaffolds to improve cell adhesion and drive desired cell responses. Chem. Eng. Trans. 2012, 27, 415–420. [Google Scholar]
- Rigogliuso, S.; Carfi’ Pavia, F.; La Carrubba, V.; Brucato, V.; Ghersi, G. PLLA/Fibrin tubular scaffold: A new way for reliableendothelial cell seeding. In Proceedings of the LIAC Meeting on Vascular Research 2013, Lisbon, Portugal, 9–12 September 2014. [Google Scholar]
- Miao, Z.; Lu, Z.; Wu, H.; Liu, H.; Li, M.; Lei, D.; Zheng, L.; Zhao, J. Collagen, agarose, alginate, and Matrigel hydrogels as cell substrates for culture of chondrocytes in vitro: A comparative study. J. Cell. Biochem. 2018, 119, 7924–7933. [Google Scholar] [CrossRef]
- Ghersi, G. Roles of molecules involved in epithelial/mesenchymal transition during angiogenesis. Front. Biosci. 2008, 13, 2335–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [Green Version]
- Di Prima, G.; Saladino, S.; Bongiovì, F.; Adamo, G.; Ghersi, G.; Pitarresi, G.; Giammona, G. Novel inulin-based mucoadhesive micelles loaded with corticosteroids as potential transcorneal permeation enhancers. Eur. J. Pharm. Biopharm. 2017, 117, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X. Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Biotechnol. Bioeng. 2015, 112, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, L.S.; Järvinen, E.; Muhonen, V.; Collin, E.C.; Pandit, A.S.; Kiviranta, I.; Yliperttula, M.; Urtti, A. An injectable, in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Deliv. Transl. Res. 2014, 4, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Dispenza, C.; Rigogliuso, S.; Grimaldi, N.; Sabatino, M.A.; Bulone, D.; Bondì, M.L.; Ghersi, G. Structure and biological evaluation of amino-functionalized PVP nanogels for fast cellular internalization. React. Funct. Polym. 2013, 73, 1103–1113. [Google Scholar] [CrossRef]
- Adamo, G.; Grimaldi, N.; Campora, S.; Bulone, D.; Bondì, M.L.; Al-Sheikhly, M.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Multi-functional nanogels for tumor targeting and redox-sensitive drug and siRNA delivery. Molecules 2016, 21, 1594. [Google Scholar] [CrossRef]
- Campora, S.; Mauro, N.; Griffiths, P.; Giammona, G.; Ghersi, G. Graphene nanosystems as supports in siRNA Delivery. Chem. Eng. Trans. 2018, 64, 415–420. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Nicosia, A.; Maggio, T.; Costa, S.; Salamone, M.; Tagliavia, M.; Mazzola, S.; Gianguzza, F.; Cuttitta, A. Maintenance of a protein structure in the dynamic evolution of TIMPs over 600 million years. Genome Biol. Evol. 2016, 8, 1056–1071. [Google Scholar] [CrossRef] [Green Version]
- Sarras, M.P.; Deutzmann, R. Hydra and Niccolo Paganini (1782–1840)—Two peas in a pod? The molecular basis of extracellular matrix structure in the invertebrate, hydra. BioEssays 2001, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Silvipriya, K.S.; Kumar, K.K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; James, S. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Easterbrook, C.; Maddern, G. Porcine and bovine surgical products: Jewish, muslim, and hindu perspectives. Arch. Surg. 2008, 143, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [Green Version]
- Pugliano, M. Combined Jellyfish Collagen Type II, Human Stem Cells and Tgf-Β3 as a Therapeutic Implant for Cartilage Repair. J. Stem Cell Res. Ther. 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices. J. Tissue Eng. Regen. Med. 2017, 11, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Calejo, M.T.; Almeida, A.J.; Fernandes, A.I. Exploring a new jellyfish collagen in the production of microparticles for protein delivery. J. Microencapsul. 2012, 29, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Paradiso, F.; Fitzgerald, J.; Yao, S.; Barry, F.; Taraballi, F.; Gonzalez, D.; Conlan, R.S.; Francis, L. Marine collagen substrates for 2D and 3D ovarian cancer cell systems. Front. Bioeng. Biotechnol. 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaithilingam, J.; Sanjuan-Alberte, P.; Campora, S.; Rance, G.A.; Jiang, L.; Thorpe, J.; Burroughs, L.; Tuck, C.J.; Denning, C.; Wildman, R.D.; et al. Multifunctional bioinstructive 3D architectures to modulate cellular behavior. Adv. Funct. Mater. 2019, 29, 1902016. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum kishinouye for use in hemostatic applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [Green Version]
- Salamone, M.; Seidita, G.; Cuttitta, A.; Rigogliuso, S.; Mazzola, S.; Bertuzzi, F.; Ghersi, G. A new method to value efficiency of enzyme blends for pancreatic tissue digestion. Transplant. Proc. 2010, 42, 2043–2048. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.S.; Chung, J.E.; Pui-Yik Chan, P.; Kurisawa, M. Injectable biodegradable hydrogels with tunable mechanical properties for the stimulation of neurogenesic differentiation of human mesenchymal stem cells in 3D culture. Biomaterials 2010, 31, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Shibata, Y.; Pugdee, K.; Abiko, Y.; Ogata, Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 2007, 59, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.C.; Arnhold, S. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histochem. Cell Biol. 2010, 134, 545–554. [Google Scholar] [CrossRef]
- Jin, G.Z.; Kim, H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Duan, L.; Zhu, W.; Xiong, J.; Wang, D. Extracellular matrix production in vitro in cartilage tissue engineering. J. Transl. Med. 2014, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Schuh, E.; Kramer, J.; Rohwedel, J.; Notbohm, H.; Müller, R.; Gutsmann, T.; Rotter, N. Effect of matrix elasticity on the maintenance of the chondrogenic phenotype. Tissue Eng. Part A 2010, 16, 1281–1290. [Google Scholar] [CrossRef]
- Freyria, A.M.; Ronzière, M.C.; Cortial, D.; Galois, L.; Hartmann, D.; Herbage, D.; Mallein-Gerin, F. Comparative phenotypic analysis of articular chondrocytes cultured within type i or type II collagen scaffolds. Tissue Eng. Part A 2009, 15, 1233–1245. [Google Scholar] [CrossRef]
- Costa, E.; González-García, C.; Gómez Ribelles, J.L.; Salmerón-Sánchez, M. Maintenance of chondrocyte phenotype during expansion on PLLA microtopographies. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Salamone, M.; Rigogliuso, S.; Nicosia, A.; Tagliavia, M.; Campora, S.; Cinà, P.; Bruno, C.; Ghersi, G. Neural crest-derived chondrocytes isolation for tissue engineering in regenerative medicine. Cells 2020, 9, 962. [Google Scholar] [CrossRef]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; De Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, L.J.; Wheatley, S.; Muscat, G.E.O.; Conway-Campbell, J.; Bowles, J.; Wright, E.; Bell, D.M.; Tam, P.P.L.; Cheah, K.S.E.; Koopman, P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997, 183, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Mukaida, T.; Urabe, K.; Naruse, K.; Aikawa, J.; Katano, M.; Hyon, S.H.; Itoman, M. Influence of three-dimensional culture in a type II collagen sponge on primary cultured and dedifferentiated chondrocytes. J. Orthop. Sci. 2005, 10, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Pieper, J.S.; Van Der Kraan, P.M.; Hafmans, T.; Kamp, J.; Buma, P.; Van Susante, J.L.C.; Van Den Berg, W.B.; Veerkamp, J.H.; Van Kuppevelt, T.H. Crosslinked type II collagen matrices: Preparation, characterization, and potential for cartilage engineering. Biomaterials 2002, 23, 3183–3192. [Google Scholar] [CrossRef]
- Kanazawa, T.; Furumatsu, T.; Hachioji, M.; Oohashi, T.; Ninomiya, Y.; Ozaki, T. Mechanical stretch enhances COL2A1 expression on chromatin by inducing SOX9 nuclear translocalization in inner meniscus cells. J. Orthop. Res. 2012, 30, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Wang, C.; Acton, A.J.; Eckert, G.J.; Trippel, S.B. Role of Sox9 in growth factor regulation of articular chondrocytes. J. Cell. Biochem. 2015, 116, 1391–1400. [Google Scholar] [CrossRef] [Green Version]
- Salamone, M.; Saladino, S.; Pampalone, M.; Campora, S.; Ghersi, G. Tissue dissociation and primary cells isolation using recombinant collagenases class I and II. Chem. Eng. Trans. 2014, 38, 247–252. [Google Scholar] [CrossRef]
- Volpe, L.; Salamone, M.; Giardina, A.; Ghersi, G. Optimization of a biotechnological process for production and purification of two recombinant proteins: Col G and Col H. Chem. Eng. Trans. 2016, 49, 61–66. [Google Scholar] [CrossRef]
- Andrade, J.P.S.; Oliveira, C.P.; Tovar, A.M.F.; de Mourão, P.A.S.; Vilanova, E. A color-code for glycosaminoglycans identification by means of polyacrylamide gel electrophoresis stained with the cationic carbocyanine dye Stains-all. Electrophoresis 2018, 39, 666–669. [Google Scholar] [CrossRef]







| Primers | Sequences (5′–3′) | Accession Number |
|---|---|---|
| GAPDH | ATCTCGCTCCTGGAAGATG a TCGGAGTGAACGGATTCG b | NM_001034034 |
| 18S | TTCGATGGTAGTCGCTGTGC a TTGGATGTGGTAGCCGTTTC b | NR 036642 |
| Acan | CATCCCCTGCTACTTCATCG a CCTTCTCCTTGGAAATGCG b | NM_173981 |
| Col1A2 | GGATGGTCACCCTGGAAAAC a CCCCTAATGCCCTTGAAGC b | NM_174520 |
| Col2A1 | TGATCGTGGTGACAAAGGTG a ATCTGGGCAGCAAAGTTTCC b | NM_001001135 |
| Sox9 | ACGCAGATTCCCAAGACAC a GGTTTCCAGTCCAGTTTCG b | XM_014478986 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigogliuso, S.; Salamone, M.; Barbarino, E.; Barbarino, M.; Nicosia, A.; Ghersi, G. Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 5798. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165798
Rigogliuso S, Salamone M, Barbarino E, Barbarino M, Nicosia A, Ghersi G. Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications. International Journal of Molecular Sciences. 2020; 21(16):5798. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165798
Chicago/Turabian StyleRigogliuso, Salvatrice, Monica Salamone, Enza Barbarino, Maria Barbarino, Aldo Nicosia, and Giulio Ghersi. 2020. "Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications" International Journal of Molecular Sciences 21, no. 16: 5798. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165798