Precocious Downregulation of Krüppel-Homolog 1 in the Migratory Locust, Locusta migratoria, Gives Rise to An Adultoid Phenotype with Accelerated Ovarian Development but Disturbed Mating and Oviposition
Abstract
:1. Introduction
2. Results
2.1. Temporal Transcript Profiles of MEKRE93 and JH Biosynthesis Pathway Components
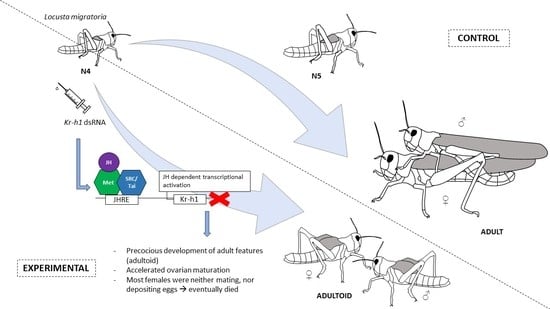
2.2. Knockdown of LmKr-h1 Results in Precocious Development of External Adult Features
2.3. Transcript Levels of MEKRE93 and JH Biosynthesis Pathway Components in dsRNA-Injected Fourth Nymphal and Freshly Molted Fifth Nymphal, Adultoid and Adult Female Locusts
2.4. The Adultoid Phenotype Shows an Accelerated Ovarian Maturation
2.5. Transcript Levels of MEKRE93 and JH Biosynthesis Pathway Components in Adultoid Females
2.6. Transcript Levels of Halloween Genes and Ecdysone Receptor Complex in Adultoids And Adults
2.7. Adultoid Females Have Severe Defects in Mating and Egg Laying
3. Discussion
3.1. DsLmKr-h1 Injections in Fourth Nymphal Stage Induced the Developmental Transition to Adultoids
3.2. Comparison Between Adultoid and Adult Female Locusts during Their First Gonadotrophic Cycle
3.2.1. An Accelerated Ovarian Maturation
3.2.2. Associated Changes in Gene Expression Profiles in Fat Body and CA
3.2.3. Accelerated Kr-h1 and Halloween Gene Expression in the Ovary
3.2.4. Severe Defects in Mating and Egg Laying
4. Materials and Methods
4.1. Rearing of Animals
4.2. Tissue Collection
4.3. RNA Extraction and cDNA Synthesis
4.4. Quantitative Real-Time PCR
4.5. RNA Interference Experiments
4.6. Observing Ecdysis
4.7. Measurement of Oocyte Length
4.8. Microscopy and Histological Analysis
4.9. Observation of Mating, Egg Deposition and Hatching
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 20E | 20-hydroxyecdysone |
| Ad | Adult |
| Ao | Adultoid |
| bHLH | Basic helix–loop–helix |
| CA | Corpora allata |
| cDNA | Complementary DNA |
| CYP15A1 | Methyl farnesoate epoxidase |
| D | Day |
| Dib | Disembodied |
| DNA | Deoxyribonucleic acid |
| ds | Double-stranded |
| dsRNA | Double-stranded RNA |
| E93 | Ecdysone-induced protein 93 |
| EcR | Ecdysone receptor |
| GFP | Green fluorescent protein |
| JH | Juvenile hormone |
| JHAMT | Juvenile hormone acid O-methyltransferase |
| Kr-h1 | Krüppel-homolog 1 |
| MEKRE93 | Methoprene-tolerant-Krüppel homolog 1-E93 |
| Met | Methoprene-tolerant |
| mRNA | Messenger RNA |
| N | Nymph |
| PAS | Per-Arnt-Sim |
| PCR | Polymerase chain reaction |
| Phm | Phantom |
| qRT-PCR | Quantitative reverse transcription (real-time) PCR |
| RNA | Ribonucleic acid |
| RNAi | RNA interference |
| RP49 | Ribosomal protein 49 |
| RXR | Retinoid-X-receptor |
| Sad | Shadow |
| Shd | Shade |
| Spo | Spook |
| Tai | Taiman |
| TubA1 | Tubulin A1 |
| USP | Ultraspiracle |
| Vg | Vitellogenin |
References
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012, 179, 477–484. [Google Scholar] [CrossRef]
- Hill, R.J.; Billas, I.M.L.; Bonneton, F.; Graham, L.D.; Lawrence, M.C. Ecdysone Receptors: From the Ashburner Model to Structural Biology. Annu. Rev. Entomol. 2013, 58, 251–271. [Google Scholar] [CrossRef]
- Wilson, T.G.; Fabian, J. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev. Biol. 1986, 118, 190–201. [Google Scholar] [CrossRef]
- Charles, J.-P.; Iwema, T.; Epa, V.C.; Takaki, K.; Rynes, J.; Jindra, M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. USA 2011, 108, 21128–21133. [Google Scholar] [CrossRef] [Green Version]
- Godlewski, J.; Wang, S.; Wilson, T.G. Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem. Biophys. Res. Commun. 2006, 342, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Z.; Wang, Z.; Deng, S.; Zhou, S. Krüppel-homolog 1 mediates juvenile hormone action to promote vitellogenesis and oocyte maturation in the migratory locust. Insect Biochem. Mol. Biol. 2014, 52, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Konopova, B.; Jindra, M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2007, 104, 10488–10493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minakuchi, C.; Namiki, T.; Shinoda, T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 2009, 325, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Chafino, S.; Ureña, E.; Casanova, J.; Casacuberta, D.M.; Franch-Marro, X.; Martín, D. Upregulation of E93 Gene Expression Acts as the Trigger for Metamorphosis Independently of the Threshold Size in the Beetle Tribolium castaneum. Cell Rep. 2019, 27, 1039–1049.e2. [Google Scholar] [CrossRef]
- Konopova, B.; Smykal, V.; Jindra, M. Common and Distinct Roles of Juvenile Hormone Signaling Genes in Metamorphosis of Holometabolous and Hemimetabolous Insects. PLoS ONE 2011, 6, e28728. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Fernandez, J.; Bellés, X. Role of Methoprene-Tolerant (Met) in Adult Morphogenesis and in Adult Ecdysis of Blattella germanica. PLoS ONE 2014, 9, e103614. [Google Scholar] [CrossRef] [Green Version]
- Smykal, V.; Daimon, T.; Kayukawa, T.; Takaki, K.; Shinoda, T.; Jindra, M. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev. Biol. 2014, 390, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villalobos-Sambucaro, M.J.; Riccillo, F.L.; Calderón-Fernández, G.M.; Sterkel, M.; Diambra, L.; Ronderos, J.R. Genomic and functional characterization of a methoprene-tolerant gene in the kissing-bug Rhodnius prolixus. Gen. Comp. Endocrinol. 2015, 216, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Fernandez, J.; Bellés, X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 2011, 1, 163. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, Y.; Tomonari, S.; Watanabe, T.; Noji, S.; Mito, T. Regulatory mechanisms underlying the specification of the pupal-homologous stage in a hemimetabolous insect. Philos. Trans. R. Soc. B: Biol. Sci. 2019, 374, 20190225. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Yuan, S.Y.; Nanda, S.; Wang, W.X.; Lai, F.X.; Fu, Q.; Wan, P.J. The Roles of E93 and Kr-h1 in Metamorphosis of Nilaparvata lugens. Front. Physiol. 2018, 9, 1677. [Google Scholar] [CrossRef] [Green Version]
- Gujar, H.; Palli, S.R. Krüppel homolog 1 and E93 mediate Juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci. Rep. 2016, 6, 26092. [Google Scholar] [CrossRef]
- Jin, M.-N.; Xue, J.; Yao, Y.; Lin, X.-D. Molecular Characterization and Functional Analysis of Krüppel-homolog 1 (Kr-h1) in the Brown Planthopper, Nilaparvata lugens (Stål). J. Integr. Agric. 2014, 13, 1972–1981. [Google Scholar] [CrossRef]
- Ureña, E.; Manjón, C.; Franch-Marro, X.; Martín, D. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc. Natl. Acad. Sci. USA 2014, 111, 7024–7029. [Google Scholar] [CrossRef] [Green Version]
- Ureña, E.; Chafino, S.; Manjón, C.; Franch-Marro, X.; Martín, D. The Occurrence of the Holometabolous Pupal Stage Requires the Interaction between E93, Krüppel-Homolog 1 and Broad-Complex. PLoS Genet. 2016, 12, e1006020. [Google Scholar] [CrossRef] [Green Version]
- Bellés, X. Krüppel homolog 1 and E93: The doorkeeper and the key to insect metamorphosis. Arch. Insect Biochem. Physiol. 2019, 103, e21609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Yao, Y.; Wang, B. Methoprene-tolerant (Met) and Krüpple-homologue 1 (Kr-h1) are required for ovariole development and egg maturation in the brown plant hopper. Sci. Rep. 2015, 5, 18064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gujar, H.; Palli, S.R. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci. Rep. 2016, 6, 35546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Li, Y.; Zhu, L.; Zhu, F.; Lei, C.; Wang, X.-P. Juvenile hormone facilitates the antagonism between adult reproduction and diapause through the methoprene-tolerant gene in the female Colaphellus bowringi. Insect Biochem. Mol. Biol. 2016, 74, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Marchal, E.; Hult, E.F.; Huang, J.; Pang, Z.; Stay, B.; Tobe, S.S. Methoprene-Tolerant (Met) Knockdown in the Adult Female Cockroach, Diploptera punctata Completely Inhibits Ovarian Development. PLoS ONE 2014, 9, e106737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Z.; Saha, T.T.; Roy, S.; Shin, S.W.; Backman, T.W.H.; Girke, T.; White, K.P.; Raikhel, A.S. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc. Natl. Acad. Sci. USA 2013, 110, E2173–E2181. [Google Scholar] [CrossRef] [Green Version]
- Smykal, V.; Bajgar, A.; Provazník, J.; Fexova, S.; Buricova, M.; Takaki, K.; Hodkova, M.; Jindra, M.; Dolezel, D. Juvenile hormone signaling during reproduction and development of the linden bug, Pyrrhocoris apterus. Insect Biochem. Mol. Biol. 2014, 45, 69–76. [Google Scholar] [CrossRef]
- Bilen, J.; Atallah, J.; Azanchi, R.; Levine, J.D.; Riddiford, L.M. Regulation of onset of female mating and sex pheromone production by juvenile hormone in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2013, 110, 18321–18326. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, R.; Sun, Z.; Bai, H.; Palli, S.R. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.; Xu, J.; Bai, H.; Zhu, F.; Palli, S.R. Juvenile Hormone Regulates Vitellogenin Gene Expression through Insulin-like Peptide Signaling Pathway in the Red Flour Beetle, Tribolium castaneum. J. Biol. Chem. 2011, 286, 41924–41936. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zhang, W.-N.; Liu, C.; Chen, L.; Xu, Y.; Xiao, H.; Liang, G. Methoprene-Tolerant (Met) Is Indispensable for Larval Metamorphosis and Female Reproduction in the Cotton Bollworm Helicoverpa armigera. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naghdi, M.; Maestro, J.-L.; Belles, X.; Bandani, A. Transduction of the vitellogenic signal of juvenile hormone by Methoprene-tolerant in the cockroach Blattella germanica (L.) (Dictyoptera, Blattellidae). Arthropods 2016, 5, 130–136. [Google Scholar]
- Saiki, R.; Gotoh, H.; Toga, K.; Miura, T.; Maekawa, K. High juvenile hormone titre and abdominal activation of JH signalling may induce reproduction of termite neotenics. Insect Mol. Biol. 2015, 24, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Saha, T.T.; Zhang, Y.; Zhang, C.; Raikhel, A.S. Juvenile hormone and its receptor methoprene-tolerant promote ribosomal biogenesis and vitellogenesis in the Aedes aegypti mosquito. J. Biol. Chem. 2017, 292, 10306–10315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Hou, Y.; Saha, T.T.; Pei, G.; Raikhel, A.S.; Zou, Z. Hormone and receptor interplay in the regulation of mosquito lipid metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, E2709–E2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; He, H.; Qu, X.; Cai, Y.; Ding, W.; Qiu, L.; Li, Y. RNA interference-mediated knockdown of the transcription factor Krüppel homologue 1 suppresses vitellogenesis in Chilo suppressalis. Insect Mol. Biol. 2019, 29, 183–192. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Song, J.; Kang, L.; Zhou, S. An isoform of Taiman that contains a PRD-repeat motif is indispensable for transducing the vitellogenic juvenile hormone signal in Locusta migratoria. Insect Biochem. Mol. Biol. 2017, 82, 31–40. [Google Scholar] [CrossRef]
- Gijbels, M.; Lenaerts, C.; Vanden Broeck, J.; Marchal, E. Juvenile Hormone receptor Met is essential for ovarian maturation in the Desert Locust, Schistocerca gregaria. Sci. Rep. 2019, 9, 10797. [Google Scholar] [CrossRef]
- Wyatt, G.R.; Braun, R.P.; Zhang, J. Priming effect in gene activation by juvenile hormone in locust fat body. Arch. Insect Biochem. Physiol. 1996, 32, 633–640. [Google Scholar] [CrossRef]
- Davey, K.G.; Sevala, V.L.; Gordon, D.R. The action of juvenile hormone and antigonadotropin on the follicle cells of Locusta migratoria. Invertebr. Reprod. Dev. 1993, 24, 39–45. [Google Scholar] [CrossRef]
- Jing, Y.-P.; An, H.; Zhang, S.; Ningbo, W.; Shutang, Z. Protein kinase C mediates juvenile hormone-dependent phosphorylation of Na+/K+-ATPase to induce ovarian follicular patency for yolk protein uptake. J. Biol. Chem. 2018, 293, 20112–20122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevala, V.L.; Davey, K.G. Action of juvenile hormone on the follicle cells ofRhodnius prolixus: Evidence for a novel regulatory mechanism involving protein kinase C. Cell. Mol. Life Sci. 1989, 45, 355–356. [Google Scholar] [CrossRef]
- Sevala, V.L.; Davey, K.; Prestwich, G.D. Photoaffinity labeling and characterization of a juvenile hormone binding protein in the membranes of follicle cells of Locusta migratoria. Insect Biochem. Mol. Biol. 1995, 25, 267–273. [Google Scholar] [CrossRef]
- Seidelmann, K.; Helbing, C.; Göbeler, N.; Weinert, H. Sequential oogenesis is controlled by an oviduct factor in the locusts Locusta migratoria and Schistocerca gregaria: Overcoming the doctrine that patency in follicle cells is induced by juvenile hormone. J. Insect Physiol. 2016, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lanot, R.; Thiebold, J.; Lagueux, M.; Goltzené, F.; Hoffmann, J.A. Involvement of ecdysone in the control of meiotic reinitiation in oocytes of Locusta migratoria (Insecta, orthoptera). Dev. Biol. 1987, 121, 174–181. [Google Scholar] [CrossRef]
- Isaac, R.E.; Rees, H.H. Isolation and identification of ecdysteroid phosphates and acetylecdysteroid phosphates from developing eggs of the locust, Schistocerca gregaria. Biochem. J. 1984, 221, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, A.I.; Vedrová, A.; Sehnal, F. Ecdysteroids during ovarian development and embryogenesis in solitary and gregarious Schistocerca gregaria. Arch. Insect Biochem. Physiol. 1999, 41, 134–143. [Google Scholar] [CrossRef]
- Lagueux, M.; Harry, P.; Hoffmann, J.A. Ecdysteroids are bound to vitellin in newly laid eggs of locusta. Mol. Cell. Endocrinol. 1981, 24, 325–338. [Google Scholar] [CrossRef]
- Lenaerts, C.; Marchal, E.; Peeters, P.; Vanden Broeck, J. The ecdysone receptor complex is essential for the reproductive success in the female desert locust, Schistocerca gregaria. Sci. Rep. 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Chapuis, M.-P.; Loiseau, A.; Michalakis, Y.; Lecoq, M.; Franc, A.; Estoup, A. Outbreaks, gene flow and effective population size in the migratory locust, Locusta migratoria: A regional-scale comparative survey. Mol. Ecol. 2009, 18, 792–800. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.A.; Cease, A.J.; Latchininsky, A.V.; Ayali, A.; Berry, K.; Buhl, J.; De Keyser, R.; Foquet, B.; Hadrich, J.C.; Matheson, T.; et al. From Molecules to Management: Mechanisms and Consequences of Locust Phase Polyphenism. Adv. Insect Physiol. 2017, 53, 167–285. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Madagascar Locust Crisis. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/emergencies/crisis/madagascar-locust (accessed on 24 July 2020).
- Roussi, A. The Battle to contain gigantic locust swarms. Nature 2020, 576, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephen, S. ‘Biblical’ swarm of locusts plague Italian farmlands in worst infestation since WWII. Fox News. Available online: https://www.foxnews.com/world/biblical-locusts-italy-sardinia-farmers (accessed on 24 July 2020).
- Carlone, M.; Sestito, D. ‘There’s nothing left’ – Sardinian farmland stripped by locust swarms. CGTN. Available online: https://newseu.cgtn.com/news/2020-06-25/-There-s-nothing-left-Sardinian-farmland-stripped-by-locust-swarms-RzaYxkBVuw/index.html (accessed on 24 July 2020).
- Ren, D.; Cai, Z.; Song, J.; Wu, Z.; Zhou, S. dsRNA uptake and persistence account for tissue-dependent susceptibility to RNA interference in the migratory locust, Locusta migratoria. Insect Mol. Biol. 2013, 23, 175–184. [Google Scholar] [CrossRef]
- Wynant, N.; Verlinden, H.; Breugelmans, B.; Simonet, G.; Vanden Broeck, J. Tissue-dependence and sensitivity of the systemic RNA interference response in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2012, 42, 911–917. [Google Scholar] [CrossRef]
- Minakuchi, C.; Zhou, X.; Riddiford, L.M. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 2008, 125, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Peel, A.D.; Akam, M. The dynamics of yolk deposition in the desert locust Schistocerca gregaria. J. Insect Physiol. 2007, 53, 436–443. [Google Scholar] [CrossRef]
- Tobe, S.S.; Pratt, G.E. Corpus allatum activity in vitro during ovarian maturation in the desert locust, Schistocerca gregaria. J. Exp. Biol. 1975, 62, 611–627. [Google Scholar]
- Guo, W.; Wu, Z.; Yang, L.; Cai, Z.; Zhao, L.; Zhou, S. Juvenile hormone–dependent Kazal-type serine protease inhibitor Greglin safeguards insect vitellogenesis and egg production. FASEB J. 2018, 33, 917–927. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Z.; Song, J.; Jiang, F.; Wang, Z.; Deng, S.; Walker, V.K.; Zhou, S. Juvenile Hormone-Receptor Complex Acts on Mcm4 and Mcm7 to Promote Polyploidy and Vitellogenesis in the Migratory Locust. PLoS Genet. 2014, 10, e1004702. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Guo, W.; Yang, L.; He, Q.; Zhou, S. Juvenile hormone promotes locust fat body cell polyploidization and vitellogenesis by activating the transcription of Cdk6 and E2f1. Insect Biochem. Mol. Biol. 2018, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Li, Y.; Gao, H.; Lin, X. The Direct Interaction between E93 and Kr-h1 Mediated Their Antagonistic Effect on Ovary Development of the Brown Planthopper. Int. J. Mol. Sci. 2019, 20, 2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinjoh, T.; Kaneko, Y.; Itoyama, K.; Mita, K.; Hiruma, K.; Shinoda, T. Control of juvenile hormone biosynthesis in Bombyx mori: Cloning of the enzymes in the mevalonate pathway and assessment of their developmental expression in the corpora allata. Insect Biochem. Mol. Biol. 2007, 37, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, J.G.; Nouzova, M.; Yoshiyama, M.; Shinoda, T.; Hernandez-Martinez, S.; Dolghih, E.; Turjanski, A.G.; Roitberg, A.E.; Priestap, H.; Pérez, M.; et al. Molecular and functional characterization of a juvenile hormone acid methyltransferase expressed in the corpora allata of mosquitoes. Insect Biochem. Mol. Biol. 2009, 39, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Minakuchi, C.; Namiki, T.; Yoshiyama, M.; Shinoda, T. RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J. 2008, 275, 2919–2931. [Google Scholar] [CrossRef]
- Niwa, R.; Niimi, T.; Honda, N.; Yoshiyama, M.; Itoyama, K.; Kataoka, H.; Shinoda, T. Juvenile hormone acid O-methyltransferase in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2008, 38, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.; Ma, L.; Li, S.; Cao, M.-X.; Jiang, R.-J. Juvenile hormone acid methyl transferase is a key regulatory enzyme for juvenile hormone synthesis in the Eri silkworm, Samia cynthica ricini. Arch. Insect Biochem. Physiol. 2008, 69, 143–154. [Google Scholar] [CrossRef]
- Marchal, E.; Zhang, J.; Badisco, L.; Verlinden, H.; Hult, E.F.; Van Wielendaele, P.; Yagi, K.J.; Tobe, S.S.; Vanden Broeck, J. Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2011, 41, 219–227. [Google Scholar] [CrossRef]
- Kappler, C.; Goltzené, F.; Lagueux, M.; Hetru, C.; Hoffmann, J.A. Role of the follicle cells and the oocytes in ecdysone biosynthesis and esterification in vitellogenic females of Locusta migratoria. Int. J. Invertebr. Reprod. Dev. 1986, 9, 17–34. [Google Scholar] [CrossRef]
- Lagueux, M.; Hirn, M.; Hoffmann, J.A. Ecdysone during ovarian development in Locusta migratoria. J. Insect Physiol. 1977, 23, 109–119. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Warren, J.T. A Molecular Genetic Approach to the Biosynthesis of the Insect Steroid Molting Hormone. Vitam. Horm. 2005, 73, 31–57. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Matsuda, T.; Yoshiyama, T.; Namiki, T.; Mita, K.; Fujimoto, Y.; Kataoka, H. CYP306A1, a Cytochrome P450 Enzyme, Is Essential for Ecdysteroid Biosynthesis in the Prothoracic Glands ofBombyxandDrosophila. J. Biol. Chem. 2004, 279, 35942–35949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, J.T.; Petryk, A.; Marqués, G.; Parvy, J.-P.; Shinoda, T.; Itoyama, K.; Kobayashi, J.; Jarcho, M.; Li, Y.; O’Connor, M.B.; et al. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: A P450 enzyme critical in ecdysone biosynthesis. Insect Biochem. Mol. Biol. 2004, 34, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Rewitz, K.F.; Shinoda, T.; Itoyama, K.; Petryk, A.; Rybczynski, R.; Jarcho, M.; Warren, J.T.; Marqués, G.; Shimell, M.J.; et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 2006, 298, 555–570. [Google Scholar] [CrossRef] [Green Version]
- Hult, E.F.; Huang, J.; Marchal, E.; Lam, J.; Tobe, S.S. RXR/USP and EcR are critical for the regulation of reproduction and the control of JH biosynthesis in Diploptera punctata. J. Insect Physiol. 2015, 80, 48–60. [Google Scholar] [CrossRef]
- Bellés, X.; Cassier, P.; Cerda, X.; Pascual, N.; Andre, M.; Rósso, Y.; Piulachs, M. Induction of choriogenesis by 20-hydroxyecdysone in the german cockroach. Tissue Cell 1993, 25, 195–204. [Google Scholar] [CrossRef]
- Lange, A.B. Neural mechanisms coordinating the female reproductive system in the locust. Front Biosci 2009, 14, 4401–4415. [Google Scholar] [CrossRef] [Green Version]
- Davey, K.G. The interaction of feeding and mating in the hormonal control of egg production in Rhodnius prolixus. J. Insect Physiol. 2007, 53, 208–215. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Forknall, N.; Patrick, C.; Yang, T.; Parekh, R.; Bock, D.; Dickson, B. Neural circuitry linking mating and egg laying in Drosophila females. Nature 2020, 579, 101–105. [Google Scholar] [CrossRef]
- Bergerard, J.; Seugé, J. La parthénogenèse accidentelle chez Locusta migratoria L. Bull Biol Fr Belg 1959, 93, 16–37. [Google Scholar]
- Pardo, M.C.; López-León, M.D.; Cabrero, J.; Camacho, J.P. Cytological and developmental analysis of tychoparthenogenesis in Locusta migratoria. Hered. 1995, 75, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billen, J. Morphology and ultrastructure of the Dufour gland in workers of social wasps (Hymenoptera, Vespidae). Arthropod Struct. Dev. 2006, 35, 77–84. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gijbels, M.; Schellens, S.; Schellekens, T.; Bruyninckx, E.; Marchal, E.; Vanden Broeck, J. Precocious Downregulation of Krüppel-Homolog 1 in the Migratory Locust, Locusta migratoria, Gives Rise to An Adultoid Phenotype with Accelerated Ovarian Development but Disturbed Mating and Oviposition. Int. J. Mol. Sci. 2020, 21, 6058. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176058
Gijbels M, Schellens S, Schellekens T, Bruyninckx E, Marchal E, Vanden Broeck J. Precocious Downregulation of Krüppel-Homolog 1 in the Migratory Locust, Locusta migratoria, Gives Rise to An Adultoid Phenotype with Accelerated Ovarian Development but Disturbed Mating and Oviposition. International Journal of Molecular Sciences. 2020; 21(17):6058. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176058
Chicago/Turabian StyleGijbels, Marijke, Sam Schellens, Tine Schellekens, Evert Bruyninckx, Elisabeth Marchal, and Jozef Vanden Broeck. 2020. "Precocious Downregulation of Krüppel-Homolog 1 in the Migratory Locust, Locusta migratoria, Gives Rise to An Adultoid Phenotype with Accelerated Ovarian Development but Disturbed Mating and Oviposition" International Journal of Molecular Sciences 21, no. 17: 6058. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176058