Farnesoid X Receptor Activation Stimulates Organic Cations Transport in Human Renal Proximal Tubular Cells
Abstract
:1. Introduction
2. Results
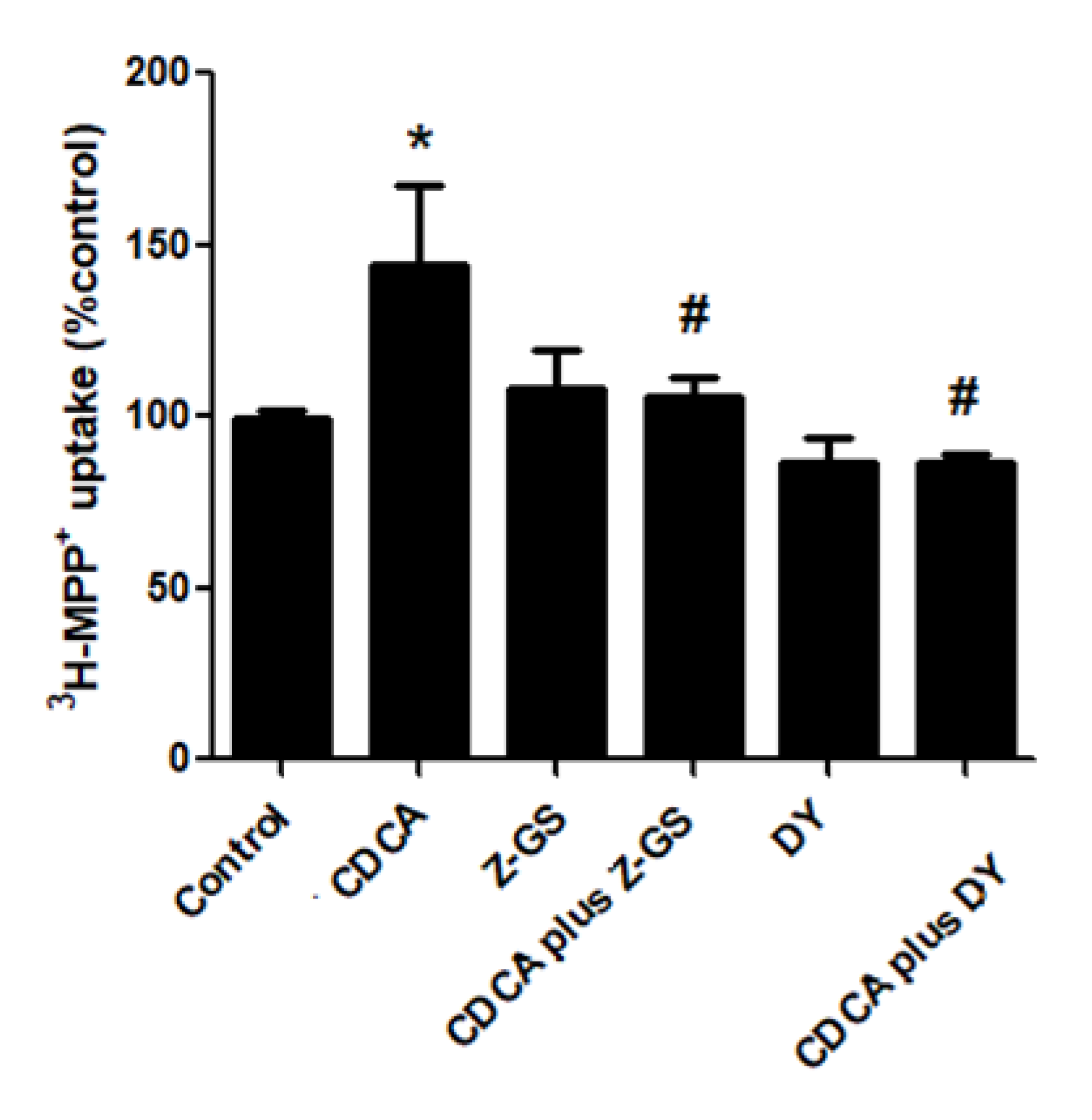
2.1. FXR Agonists Stimulate OCT2-Mediated 3H-MPP+ Uptake
2.2. Stimulatory Effects of FXR Agonists Require FXR Activation
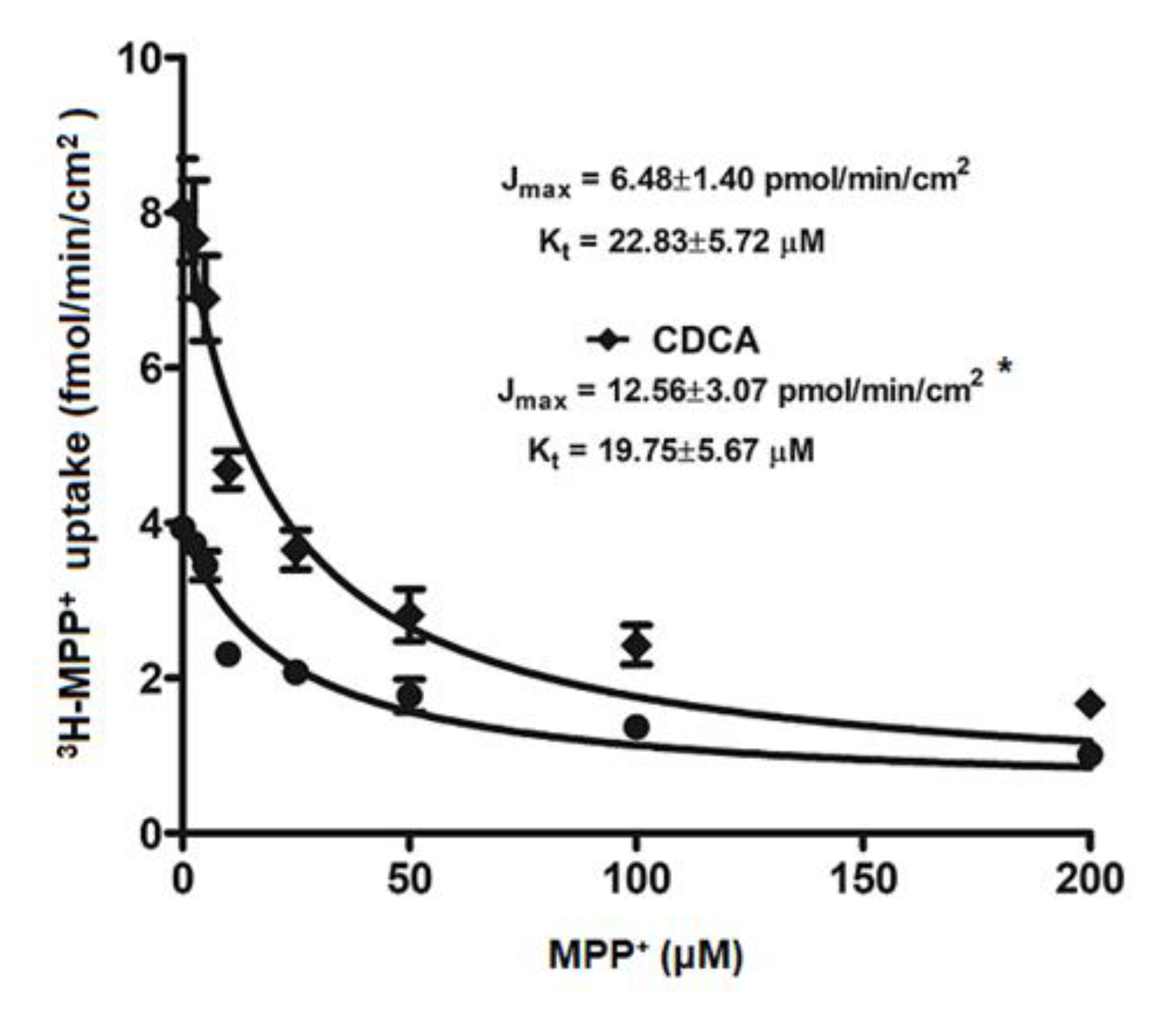
2.3. Kinetic Study on FXR Activation on OCT2-mediated 3H-MPP+ Uptake
2.4. FXR Activation Increases mRNA and Protein Expression of OCT2
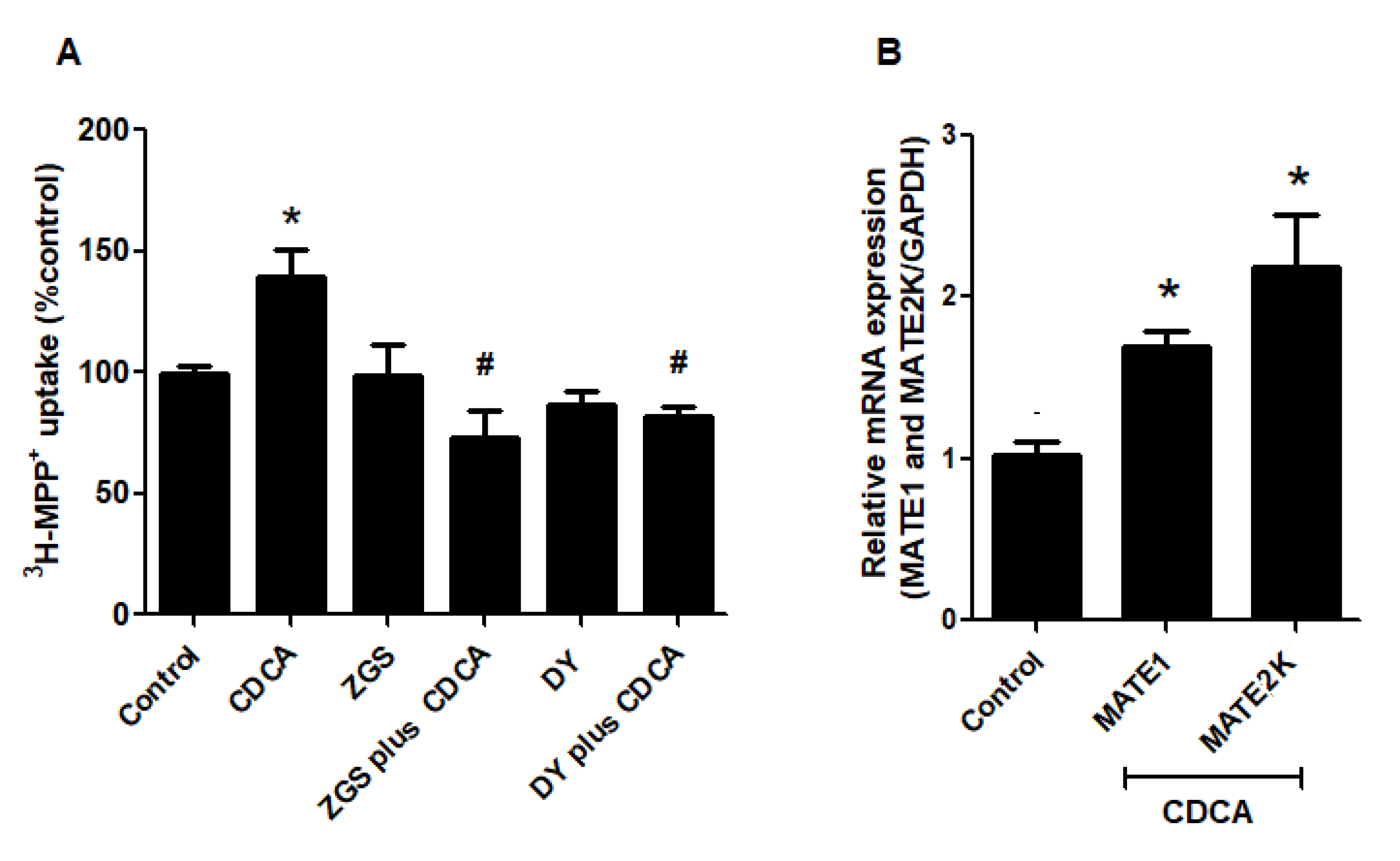
2.5. FXR Activation Increases Function and Expression of MATEs
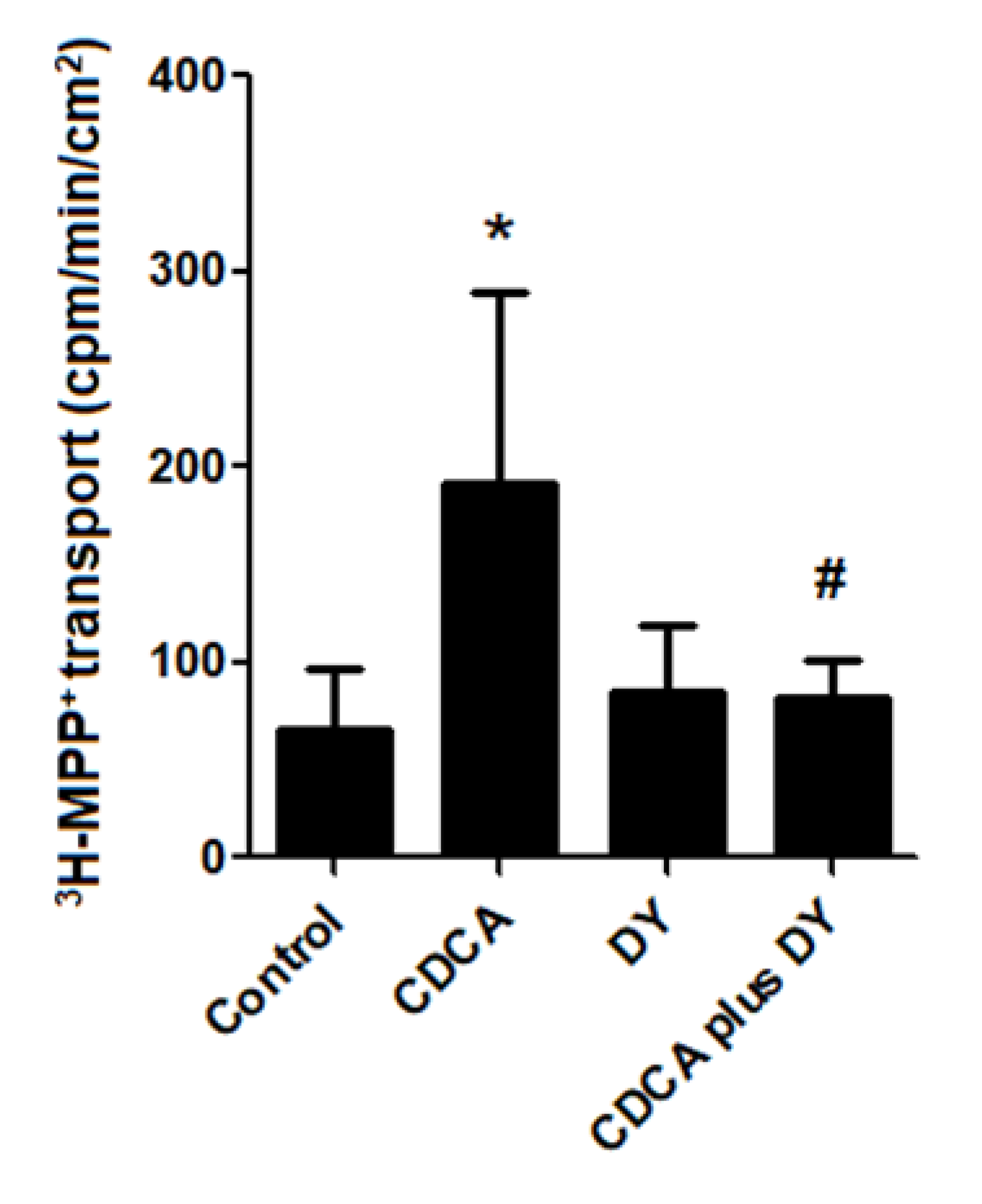
2.6. FXR Activation Stimulates Transepithelial Transport of 3H-MPP+
2.7. Pathological Concentration of Bile Acid Stimulates Renal OCT2 and MATEs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Cultures
4.3. Measurement of OCT2 Transport Function
4.4. Measurement of MATEs Transport Function
4.5. Basolateral to Apical Transport of 3H-MPP+
4.6. Kinetic Analysis of OCT2-mediated 3H-MPP+ Uptake
4.7. Real-Time PCR
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wright, S.H. Role of organic cation transporters in the renal handling of therapeutic agents and xenobiotics. Toxicol. Appl. Pharmacol. 2005, 204, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hosoyamada, M.; Sekine, T.; Kanai, Y.; Endou, H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am. J. Physiol. Content 1999, 276, F122–F128. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.W.; Schinkel, A.H. Pharmacological and physiological functions of the polyspecific organic cation transporters: Oct1, 2, and 3 (SLC22A1-3). J. Pharmacol. Exp. Ther. 2003, 308, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, S.H.; Dantzler, W.H. Molecular and cellular physiology of renal organic cation and anion transport. Physiol. Rev. 2004, 84, 987–1049. [Google Scholar] [CrossRef]
- Yokoo, S.; Yonezawa, A.; Masuda, S.; Fukatsu, A.; Katsura, T.; Inui, K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem. Pharmacol. 2007, 74, 477–487. [Google Scholar] [CrossRef]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef]
- Terada, T.; Inui, K. Physiological and pharmacokinetic roles of H+/organic cation antiporters (MATE/SLC47A). Biochem. Pharmacol. 2008, 75, 1689–1696. [Google Scholar] [CrossRef]
- Yonezawa, A.; Inui, K.-I. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br. J. Pharmacol. 2011, 164, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Ciarimboli, G.; Deuster, D.; Knief, A.; Sperling, M.; Holtkamp, M.; Edemir, B.; Pavenstädt, H.; Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen, A.A.; Schinkel, A.H.; et al. Organic cation transporter 2 mediates cisplatin-induced oto—and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010, 176, 1169–1180. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Lancaster, C.S.; Schlatter, E.; Franke, R.M.; Sprowl, J.A.; Pavenstädt, H.; Massmann, V.; Guckel, D.; Mathijssen, R.H.J.; Yang, W.; et al. Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin. Cancer Res. 2012, 18, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Misaka, S.; Knop, J.; Singer, K.; Hoier, E.; Keiser, M.; Muller, F.; Glaeser, H.; Konig, J.; Fromm, M.F. The nonmetabolized beta-blocker nadolol is a substrate of Oct1, Oct2, Mate1, Mate2-k, and P-glycoprotein, but not of OATP1B1 and OATP1B3. Mol. Pharm. 2016, 13, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Yoda, N.; Morokado, F.; Komori, H.; Nakanishi, T.; Tamai, I. Changes of drug pharmacokinetics mediated by downregulation of kidney organic cation transporters Mate1 and Oct2 in a rat model of hyperuricemia. PLoS ONE 2019, 14, e0214862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonezawa, A.; Masuda, S.; Yokoo, S.; Katsura, T.; Inui, K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1–3 and multidrug and toxin extrusion family). J. Pharmacol. Exp. Ther. 2006, 319, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanyuk, A.; Livio, F.; Biollaz, J.; Buclin, T. Renal drug transporters and drug interactions. Clin. Pharmacokinet. 2017, 56, 825–892. [Google Scholar] [CrossRef]
- Jonker, J.W.; Wagenaar, E.; van Eijl, S.; Schinkel, A.H. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol. Cell. Biol. 2003, 23, 7902–7908. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.; Bello, C.L.; Mangravite, L.M.; Feng, B.; Giacomini, K.M. Functional characteristics and steroid hormone-mediated regulation of an organic cation transporter in Madin-Darby canine kidney cells. J. Pharmacol. Exp. Ther. 2001, 299, 392–398. [Google Scholar]
- Asaka, J.-I.; Terada, T.; Okuda, M.; Katsura, T.; Inui, K. Androgen receptor is responsible for rat organic cation transporter 2 gene regulation but not for rOCT1 and rOCT3. Pharm. Res. 2006, 23, 697–704. [Google Scholar] [CrossRef]
- Wongwan, T.; Kittayaruksakul, S.; Asavapanumas, N.; Chatsudthipong, V.; Soodvilai, S. Activation of liver X receptor inhibits Oct2-mediated organic cation transport in renal proximal tubular cells. Pflügers Archiv. Eur. J. Physiol. 2017, 469, 1471–1481. [Google Scholar] [CrossRef]
- Fukuda, Y.; Kaishima, M.; Ohnishi, T.; Tohyama, K.; Chisaki, I.; Nakayama, Y.; Ogasawara-Shimizu, M.; Kawamata, Y. Fluid shear stress stimulates MATE2-K expression via Nrf2 pathway activation. Biochem. Biophys. Res. Commun. 2017, 484, 358–364. [Google Scholar] [CrossRef]
- Mencarelli, A.; Fiorucci, S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J. Cell. Mol. Med. 2009, 14, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Ananthanarayanan, M.; Balasubramanian, N.; Makishima, M.; Mangelsdorf, D.J.; Suchy, F.J. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 2001, 276, 28857–28865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kast, H.R.; Goodwin, B.; Tarr, P.T.; Jones, S.A.; Anisfeld, A.M.; Stoltz, C.M.; Tontonoz, P.; Kliewer, S.; Willson, T.M.; Edwards, P.A. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 2001, 277, 2908–2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, J.L.; Trauner, M.; Mennone, A.; Soroka, C.J.; Cai, S.-Y.; Tarek, M.; Zollner, G.; Lee, J.Y.; Ballatori, N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1124–G1130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zhang, Y.; Nelson, S.F.; Gonzales, F.J.; Edwards, P.A. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J. Lipid Res. 2006, 47, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Y.; Huang, S.; Gao, M.; Liu, J.; Jia, X.; Han, Q.; Zheng, S.; Miao, Y.; Li, S.; Weng, H.; et al. Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 2277–2282. [Google Scholar] [CrossRef] [Green Version]
- Ferrigno, A.; Di Pasqua, L.G.; Berardo, C.; Siciliano, V.; Rizzo, V.; Adorini, L.; Richelmi, P.; Vairetti, M.P. The farnesoid X receptor agonist obeticholic acid upregulates biliary excretion of asymmetric dimethylarginine via MATE-1 during hepatic ischemia/reperfusion injury. PLoS ONE 2018, 13, e0191430. [Google Scholar] [CrossRef]
- Denk, G.U.; Soroka, C.J.; Mennone, A.; Koepsell, H.; Beuers, U.; Boyer, J.L. Down-regulation of the organic cation transporter 1 of rat liver in obstructive cholestasis. Hepatology 2004, 39, 1382–1389. [Google Scholar] [CrossRef]
- Nies, A.T.; Koepsell, H.; Winter, S.; Burk, O.; Klein, K.; Kerb, R.; Zanger, U.M.; Keppler, D.; Schwab, M.; Schaeffeler, E. Expression of organic cation transporters Oct1 (SLC22A1) and Oct3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 2009, 50, 1227–1240. [Google Scholar] [CrossRef]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile acids activated receptors regulate innate immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef] [Green Version]
- Makino, I.; Nakagawa, S.; Mashimo, K. Conjugated and unconjugated serum bile acid levels n patients with hepatobiliary diseases. Gastroenterology 1969, 56, 1033–1039. [Google Scholar] [CrossRef]
- Rizzo, G.; Renga, B.; Mencarelli, A.; Pellicciari, R.; Fiorucci, S. Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr. Drug Targets Immune Endocr. Metab. Disord. 2005, 5, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.H.; Choi, H.S.; Joo, S.Y.; Kim, I.J.; Kim, C.S.; Choi, J.S.; Ma, S.K.; Lee, J.; Kim, S.W. Farnesoid x receptor ligand prevents cisplatin-induced kidney injury by enhancing small heterodimer partner. PLoS ONE 2014, 9, e86553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1 and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Nies, A.T.; Koepsell, H.; Damme, K.; Schwab, M. Organic cation transporters (Octs, Mates), in vitro and in vivo evidence for the importance in drug therapy. Arrestins Pharmacol. Ther. Potential 2010, 201, 105–167. [Google Scholar] [CrossRef]
- Aschauer, L.; Carta, G.; Vogelsang, N.; Schlatter, E.; Jennings, P. Expression of xenobiotic transporters in the human renal proximal tubule cell line RPTEC/TERT1. Toxicol. Vitr. 2015, 30, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Huang, L.; Zhao, A.; Lew, J.-L.; Yu, J.; Sahoo, S.; Meinke, P.T.; Royo, I.; Peláez, F.; Wright, S.D. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 2003, 278, 10214–10220. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.D.; Lin, W.; Forman, B.M.; Chen, T. Identification of trisubstituted-pyrazol carboxamide analogs as novel and potent antagonists of farnesoid x receptor. Bioorganic Med. Chem. 2014, 22, 2919–2938. [Google Scholar] [CrossRef] [Green Version]
- Claudel, T.; Staels, B.; Kuipers, F. The farnesoid x receptor. Arter. Thromb. Vasc. Biol. 2005, 25, 2020–2030. [Google Scholar] [CrossRef]
- Kurata, T.; Muraki, Y.; Mizutani, H.; Iwamoto, T.; Okuda, M. Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: The role of renal organic cation transporter Oct2. Drug Metab. Pharmacokinet. 2010, 25, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Wieser, M.; Stadler, G.; Jennings, P.; Streubel, B.; Pfaller, W.; Ambros, P.F.; Riedl, C.; Katinger, H.; Grillari, J.; Grillari-Voglauer, R.; et al. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am. J. Physiol. 2008, 295, F1365–F1375. [Google Scholar] [CrossRef] [Green Version]
- Yasujima, T.; Ohta, K.-Y.; Inoue, K.; Ishimaru, M.; Yuasa, H. Evaluation of 4′,6-diamidino-2-phenylindole as a fluorescent probe substrate for rapid assays of the functionality of human multidrug and toxin extrusion proteins. Drug Metab. Dispos. 2010, 38, 715–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, K.; Wagner, C.; Haddad, G.; Burnekova, O.; Geibel, J. Intracellular pH activates 690 membrane-bound Na (+)/H (+) exchanger and vacuolar H (+)-ATPase in human embryonic kidney (HEK) 691 cells. Cell. Physiol. Biochem. 2003, 13, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Terada, T.; Yonezawa, A.; Tanihara, Y.; Kishimoto, K.; Katsura, T.; Ogawa, O.; Inui, K.-I. Identification and functional characterization of a new human kidney–specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol. 2006, 17, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- Malo, C.; Berteloot, A. Analysis of kinetic data in transport studies: New insights from kinetic studies of Na+-d-glucose cotransport in human intestinal brush-border membrane vesicles using a fast sampling, rapid filtration apparatus. J. Membr. Biol. 1991, 122, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Target | Forward Primer (5′-3′) | Reverse Primer (3′-5′) |
|---|---|---|
| hOCT2 | 5-AGTCTGCCTGGTCAATGCT-3 | 5-AGGAATGGCGTGATGATGC-3 |
| hMATE1 | 5-TGCTCCTGGGGGTCTTCTTA-3 | 5-GTGGGCCTGTGAATTGTGTG-3 |
| hMATE2-K | 5-TTGCACAGACCGTCTTCCTC-3 | 5-TGAGGAAGCTCCCGATCTCA-3 |
| hSHP | 5-GGCTTCAATGCTGTCTGGAGT-3 | 5-CTGGCACATCGGGGTTGAAGA-3 |
| hGAPDH | 5-CAAGCTCATTTCCTGGTATGAC-3 | 5-GTGTGGTGGGGGACTGAGTGTGG-3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongwan, T.; Chatsudthipong, V.; Soodvilai, S. Farnesoid X Receptor Activation Stimulates Organic Cations Transport in Human Renal Proximal Tubular Cells. Int. J. Mol. Sci. 2020, 21, 6078. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176078
Wongwan T, Chatsudthipong V, Soodvilai S. Farnesoid X Receptor Activation Stimulates Organic Cations Transport in Human Renal Proximal Tubular Cells. International Journal of Molecular Sciences. 2020; 21(17):6078. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176078
Chicago/Turabian StyleWongwan, Teerasak, Varanuj Chatsudthipong, and Sunhapas Soodvilai. 2020. "Farnesoid X Receptor Activation Stimulates Organic Cations Transport in Human Renal Proximal Tubular Cells" International Journal of Molecular Sciences 21, no. 17: 6078. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176078




