Endocannabinoid-Epigenetic Cross-Talk: A Bridge toward Stress Coping
Abstract
:1. Introduction
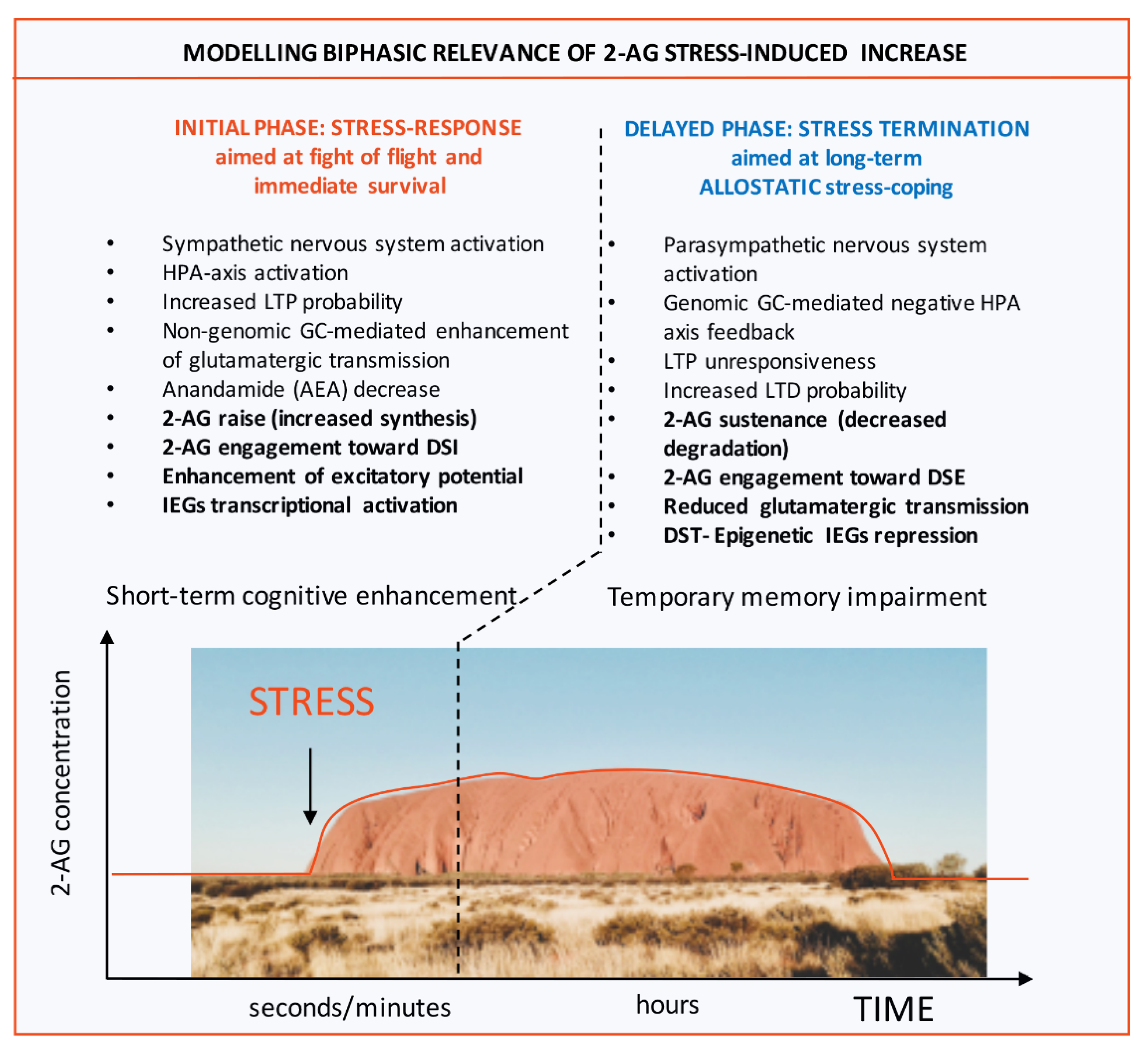
2. 2-AG Modulation of Stress Adaptation in the Hippocampus Is Mainly Devoted to Excitatory Control
3. Epigenetic Homeostatic System Protects against Excessive Excitatory Effects of Stress
4. ECS and Epigenetic Homeostatic System Cross-Regulation
5. Concerted Implications of ECS and LSD1 in Stress Vulnerability and Resiliency
6. Stress and tetrahydrocannabinol (THC) as Cannabinoid Signaling Desensitizers: Shared Epigenetic Language and Pathophysiological Implications
7. Concluding Remarks
Funding
Conflicts of Interest
References
- Bremner, J.D.; Randall, P.; Scott, T.M.; Bronen, R.A.; Seibyl, J.P.; Southwick, S.M.; Delaney, R.C.; McCarthy, G.; Charney, D.S.; Innis, R.B. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatry 1995, 152, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.L.; García-Bueno, B.; Caso, J.R.; Pérez-Nievas, B.G.; Leza, J.C. Stress-induced oxidative changes in brain. CNS Neurol. Disord. Drug Targets 2006, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, S.; Pérez Nievas, B.G.; Madrigal, J.L.; Manzanares, J.; Leza, J.C.; García-Bueno, B. Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology 2011, 36, 805–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Guggenhuber, S.; Romo-Parra, H.; Bindila, L.; Leschik, J.; Lomazzo, E.; Remmers, F.; Zimmermann, T.; Lerner, R.; Klugmann, M.; Pape, H.C.; et al. Impaired 2-AG Signaling in Hippocampal Glutamatergic Neurons: Aggravation of Anxiety-Like Behavior and Unaltered Seizure Susceptibility. Int. J. Neuropsychopharmacol. 2015, 19. [Google Scholar] [CrossRef] [Green Version]
- Bluett, R.J.; Báldi, R.; Haymer, A.; Gaulden, A.D.; Hartley, N.D.; Parrish, W.P.; Baechle, J.; Marcus, D.J.; Mardam-Bey, R.; Shonesy, B.C.; et al. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat. Commun. 2017, 8, 14782. [Google Scholar] [CrossRef] [Green Version]
- Rusconi, F.; Battaglioli, E. Acute Stress-Induced Epigenetic Modulations and Their Potential Protective Role Toward Depression. Front. Mol. Neurosci. 2018, 11, 184. [Google Scholar] [CrossRef]
- Borrelli, E.; Nestler, E.J.; Allis, C.D.; Sassone-Corsi, P. Decoding the epigenetic language of neuronal plasticity. Neuron 2008, 60, 961–974. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Eiland, L.; Hunter, R.G.; Miller, M.M. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012, 62, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Bagot, R.C.; Labonté, B.; Peña, C.J.; Nestler, E.J. Epigenetic signaling in psychiatric disorders: Stress and depression. Dialogues Clin. Neurosci. 2014, 16, 281–295. [Google Scholar]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 2016, 41, 80–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedse, G.; Hill, M.N.; Patel, S. 2-Arachidonoylglycerol Modulation of Anxiety and Stress Adaptation: From Grass Roots to Novel Therapeutics. Biol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Alger, B.E.; Tang, A.H. Do cannabinoids reduce brain power? Nat. Neurosci. 2012, 15, 499–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alger, B.E. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): What we still do not know. J. Physiol. 2012, 590, 2203–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Vasilyev, D.V.; Goncalves, M.B.; Howell, F.V.; Hobbs, C.; Reisenberg, M.; Shen, R.; Zhang, M.Y.; Strassle, B.W.; Lu, P.; et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 2010, 30, 2017–2024. [Google Scholar] [CrossRef]
- Tanimura, A.; Yamazaki, M.; Hashimotodani, Y.; Uchigashima, M.; Kawata, S.; Abe, M.; Kita, Y.; Hashimoto, K.; Shimizu, T.; Watanabe, M.; et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 2010, 65, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Wang, W.; Zhong, P.; Blankman, J.L.; Cravatt, B.F.; Liu, Q.S. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J. Neurosci. 2011, 31, 13420–13430. [Google Scholar] [CrossRef] [Green Version]
- Zhong, P.; Pan, B.; Gao, X.P.; Blankman, J.L.; Cravatt, B.F.; Liu, Q.S. Genetic deletion of monoacylglycerol lipase alters endocannabinoid-mediated retrograde synaptic depression in the cerebellum. J. Physiol. 2011, 589, 4847–4855. [Google Scholar] [CrossRef]
- Bagley, J.; Moghaddam, B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: Effects of pretreatment with saline or diazepam. Neuroscience 1997, 77, 65–73. [Google Scholar] [CrossRef]
- Wang, M.; Hill, M.N.; Zhang, L.; Gorzalka, B.B.; Hillard, C.J.; Alger, B.E. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J. Psychopharmacol. 2012, 26, 56–70. [Google Scholar] [CrossRef]
- Dubreucq, S.; Matias, I.; Cardinal, P.; Häring, M.; Lutz, B.; Marsicano, G.; Chaouloff, F. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology 2012, 37, 1885–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruehle, S.; Remmers, F.; Romo-Parra, H.; Massa, F.; Wickert, M.; Wörtge, S.; Häring, M.; Kaiser, N.; Marsicano, G.; Pape, H.C.; et al. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: Distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J. Neurosci. 2013, 33, 10264–10277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamprath, K.; Plendl, W.; Marsicano, G.; Deussing, J.M.; Wurst, W.; Lutz, B.; Wotjak, C.T. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav. 2009, 8, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kamprath, K.; Marsicano, G.; Tang, J.; Monory, K.; Bisogno, T.; Di Marzo, V.; Lutz, B.; Wotjak, C.T. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J. Neurosci. 2006, 26, 6677–6686. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgänsberger, W.; et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.A.; Purrio, M.; Viveros, M.P.; Lutz, B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 2012, 37, 2624–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, D.M.; Campbell, A.M.; Park, C.R.; Halonen, J.; Zoladz, P.R. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007, 2007, 60803. [Google Scholar] [CrossRef] [Green Version]
- Saunderson, E.A.; Spiers, H.; Mifsud, K.R.; Gutierrez-Mecinas, M.; Trollope, A.F.; Shaikh, A.; Mill, J.; Reul, J.M. Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proc. Natl. Acad. Sci. USA 2016, 113, 4830–4835. [Google Scholar] [CrossRef] [Green Version]
- Trollope, A.F.; Gutièrrez-Mecinas, M.; Mifsud, K.R.; Collins, A.; Saunderson, E.A.; Reul, J.M. Stress, epigenetic control of gene expression and memory formation. Exp. Neurol. 2012, 233, 3–11. [Google Scholar] [CrossRef]
- Wondolowski, J.; Dickman, D. Emerging links between homeostatic synaptic plasticity and neurological disease. Front. Cell Neurosci. 2013, 7, 223. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.G.; Murakami, G.; Dewell, S.; Seligsohn, M.; Baker, M.E.; Datson, N.A.; McEwen, B.S.; Pfaff, D.W. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc. Natl. Acad. Sci. USA 2012, 109, 17657–17662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusconi, F.; Grillo, B.; Ponzoni, L.; Bassani, S.; Toffolo, E.; Paganini, L.; Mallei, A.; Braida, D.; Passafaro, M.; Popoli, M.; et al. LSD1 modulates stress-evoked transcription of immediate early genes and emotional behavior. Proc. Natl. Acad. Sci. USA 2016, 113, 3651–3656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Kim, S.Y.; Artis, S.; Molfese, D.L.; Schumacher, A.; Sweatt, J.D.; Paylor, R.E.; Lubin, F.D. Histone methylation regulates memory formation. J. Neurosci. 2010, 30, 3589–3599. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forneris, F.; Binda, C.; Vanoni, M.A.; Mattevi, A.; Battaglioli, E. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005, 579, 2203–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zibetti, C.; Adamo, A.; Binda, C.; Forneris, F.; Toffolo, E.; Verpelli, C.; Ginelli, E.; Mattevi, A.; Sala, C.; Battaglioli, E. Alternative splicing of the histone demethylase LSD1/KDM1 contributes to the modulation of neurite morphogenesis in the mammalian nervous system. J. Neurosci. 2010, 30, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, L.L. Microexons go big. Cell 2014, 159, 1488–1489. [Google Scholar] [CrossRef] [Green Version]
- Irimia, M.; Blencowe, B.J. Alternative splicing: Decoding an expansive regulatory layer. Curr. Opin. Cell Biol. 2012, 24, 323–332. [Google Scholar] [CrossRef]
- Irimia, M.; Weatheritt, R.J.; Ellis, J.D.; Parikshak, N.N.; Gonatopoulos-Pournatzis, T.; Babor, M.; Quesnel-Vallières, M.; Tapial, J.; Raj, B.; O’Hanlon, D.; et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell 2014, 159, 1511–1523. [Google Scholar] [CrossRef] [Green Version]
- Gonatopoulos-Pournatzis, T.; Blencowe, B.J. Microexons: At the nexus of nervous system development, behaviour and autism spectrum disorder. Curr. Opin. Genet. Dev. 2020, 65, 22–33. [Google Scholar] [CrossRef]
- Toffolo, E.; Rusconi, F.; Paganini, L.; Tortorici, M.; Pilotto, S.; Heise, C.; Verpelli, C.; Tedeschi, G.; Maffioli, E.; Sala, C.; et al. Phosphorylation of neuronal Lysine-Specific Demethylase 1LSD1/KDM1A impairs transcriptional repression by regulating interaction with CoREST and histone deacetylases HDAC1/2. J. Neurochem. 2014, 128, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Telese, F.; Tan, Y.; Li, W.; Jin, C.; He, X.; Basnet, H.; Ma, Q.; Merkurjev, D.; Zhu, X.; et al. LSD1n is an H4K20 demethylase regulating memory formation via transcriptional elongation control. Nat. Neurosci. 2015, 18, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, F.; Grillo, B.; Toffolo, E.; Mattevi, A.; Battaglioli, E. NeuroLSD1: Splicing-Generated Epigenetic Enhancer of Neuroplasticity. Trends Neurosci. 2017, 40, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kano, M. Control of synaptic function by endocannabinoid-mediated retrograde signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 235–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longaretti, A.; Forastieri, C.; Gabaglio, M.; Rubino, T.; Battaglioli, E.; Rusconi, F. Termination of acute stress response by the endocannabinoid system is regulated through LSD1-mediated transcriptional repression of 2-AG hydrolases ABHD6 and MAGL. J. Neurochem. 2020, 35, e15000. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; McLaughlin, R.J.; Pan, B.; Fitzgerald, M.L.; Roberts, C.J.; Lee, T.T.; Karatsoreos, I.N.; Mackie, K.; Viau, V.; Pickel, V.M.; et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011, 31, 10506–10515. [Google Scholar] [CrossRef]
- Evanson, N.K.; Tasker, J.G.; Hill, M.N.; Hillard, C.J.; Herman, J.P. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 2010, 151, 4811–4819. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.V.; Abraham, W.C.; Maroun, M.; Stork, O.; Richter-Levin, G. Stress-induced metaplasticity: From synapses to behavior. Neuroscience 2013, 250, 112–120. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Campbell, A.M.; Park, C.R.; Schaefer, D.; Danysz, W.; Diamond, D.M. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol. Biochem. Behav. 2006, 85, 298–306. [Google Scholar] [CrossRef]
- Cadle, C.E.; Zoladz, P.R. Stress time-dependently influences the acquisition and retrieval of unrelated information by producing a memory of its own. Front. Psychol. 2015, 6, 910. [Google Scholar] [CrossRef] [Green Version]
- Morena, M.; De Castro, V.; Gray, J.M.; Palmery, M.; Trezza, V.; Roozendaal, B.; Hill, M.N.; Campolongo, P. Training-Associated Emotional Arousal Shapes Endocannabinoid Modulation of Spatial Memory Retrieval in Rats. J. Neurosci. 2015, 35, 13962–13974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratano, P.; Petrella, C.; Forti, F.; Passeri, P.P.; Morena, M.; Palmery, M.; Trezza, V.; Severini, C.; Campolongo, P. Pharmacological inhibition of 2-arachidonoilglycerol hydrolysis enhances memory consolidation in rats through CB2 receptor activation and mTOR signaling modulation. Neuropharmacology 2018, 138, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Péterfi, Z.; Urbán, G.M.; Papp, O.I.; Németh, B.; Monyer, H.; Szabó, G.; Erdélyi, F.; Mackie, K.; Freund, T.F.; Hájos, N.; et al. Endocannabinoid-mediated long-term depression of afferent excitatory synapses in hippocampal pyramidal cells and GABAergic interneurons. J. Neurosci. 2012, 32, 14448–14463. [Google Scholar] [CrossRef] [PubMed]
- Bedse, G.; Bluett, R.J.; Patrick, T.A.; Romness, N.K.; Gaulden, A.D.; Kingsley, P.J.; Plath, N.; Marnett, L.J.; Patel, S. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: Comparative profiling of FAAH, MAGL and dual inhibitors. Transl. Psychiatry 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Shonesy, B.C.; Bluett, R.J.; Winder, D.G.; Colbran, R.J. The Anxiolytic Actions of 2-Arachidonoylglycerol: Converging Evidence From Two Recent Genetic Endocannabinoid Deficiency Models. Biol. Psychiatry 2016, 79, e78–e79. [Google Scholar] [CrossRef]
- Shonesy, B.C.; Bluett, R.J.; Ramikie, T.S.; Báldi, R.; Hermanson, D.J.; Kingsley, P.J.; Marnett, L.J.; Winder, D.G.; Colbran, R.J.; Patel, S. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 2014, 9, 1644–1653. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Bierer, L.M.; Makotkine, I.; Golier, J.A.; Galea, S.; McEwen, B.S.; Hillard, C.J.; Yehuda, R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 2013, 38, 2952–2961. [Google Scholar] [CrossRef] [Green Version]
- Bagot, R.C.; Parise, E.M.; Peña, C.J.; Zhang, H.X.; Maze, I.; Chaudhury, D.; Persaud, B.; Cachope, R.; Bolaños-Guzmán, C.A.; Cheer, J.F.; et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 2015, 6, 7062. [Google Scholar] [CrossRef]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 2018, 559, 98–102. [Google Scholar] [CrossRef]
- Rubino, T.; Viganò, D.; Massi, P.; Parolaro, D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55,940. J. Neurochem. 2000, 75, 2080–2086. [Google Scholar] [CrossRef]
- Burston, J.J.; Wiley, J.L.; Craig, A.A.; Selley, D.E.; Sim-Selley, L.J. Regional enhancement of cannabinoid CB₁ receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br. J. Pharmacol. 2010, 161, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubino, T.; Viganò, D.; Realini, N.; Guidali, C.; Braida, D.; Capurro, V.; Castiglioni, C.; Cherubino, F.; Romualdi, P.; Candeletti, S.; et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: Behavioral and biochemical correlates. Neuropsychopharmacology 2008, 33, 2760–2771. [Google Scholar] [CrossRef]
- Bambico, F.R.; Nguyen, N.T.; Katz, N.; Gobbi, G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol. Dis. 2010, 37, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Rushlow, W.J.; Laviolette, S.R. What Can Rats Tell Us about Adolescent Cannabis Exposure? Insights from Preclinical Research. Can. J. Psychiatry 2016, 61, 328–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prini, P.; Rusconi, F.; Zamberletti, E.; Gabaglio, M.; Penna, F.; Fasano, M.; Battaglioli, E.; Parolaro, D.; Rubino, T. Adolescent THC exposure in female rats leads to cognitive deficits through a mechanism involving chromatin modifications in the prefrontal cortex. J. Psychiatry Neurosci. 2018, 43, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Rubino, T.; Prini, P.; Piscitelli, F.; Zamberletti, E.; Trusel, M.; Melis, M.; Sagheddu, C.; Ligresti, A.; Tonini, R.; Di Marzo, V.; et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol. Dis. 2015, 73, 60–69. [Google Scholar] [CrossRef]
- Miller, M.L.; Chadwick, B.; Dickstein, D.L.; Purushothaman, I.; Egervari, G.; Rahman, T.; Tessereau, C.; Hof, P.R.; Roussos, P.; Shen, L.; et al. Adolescent exposure to Δ9-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol. Psychiatry 2019, 24, 588–600. [Google Scholar] [CrossRef] [Green Version]
- Prini, P.; Penna, F.; Sciuccati, E.; Alberio, T.; Rubino, T. Chronic Δ⁸-THC Exposure Differently Affects Histone Modifications in the Adolescent and Adult Rat Brain. Int. J. Mol. Sci. 2017, 18, 2094. [Google Scholar] [CrossRef]
- Hunter, R.G.; McCarthy, K.J.; Milne, T.A.; Pfaff, D.W.; McEwen, B.S. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc. Natl. Acad. Sci. USA 2009, 106, 20912–20917. [Google Scholar] [CrossRef] [Green Version]
- Rusconi, F.; Battaglioli, E.; Venturin, M. Psychiatric disorders and lncRNAs: A synaptic match. Int. J. Mol. Sci. 2020, 21, 3030. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusconi, F.; Rubino, T.; Battaglioli, E. Endocannabinoid-Epigenetic Cross-Talk: A Bridge toward Stress Coping. Int. J. Mol. Sci. 2020, 21, 6252. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176252
Rusconi F, Rubino T, Battaglioli E. Endocannabinoid-Epigenetic Cross-Talk: A Bridge toward Stress Coping. International Journal of Molecular Sciences. 2020; 21(17):6252. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176252
Chicago/Turabian StyleRusconi, Francesco, Tiziana Rubino, and Elena Battaglioli. 2020. "Endocannabinoid-Epigenetic Cross-Talk: A Bridge toward Stress Coping" International Journal of Molecular Sciences 21, no. 17: 6252. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176252



