The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition
Abstract
:1. Introduction
2. Results
2.1. Transfection of SW480 and SW620 with FUT6 and B4GALNT2 cDNAs
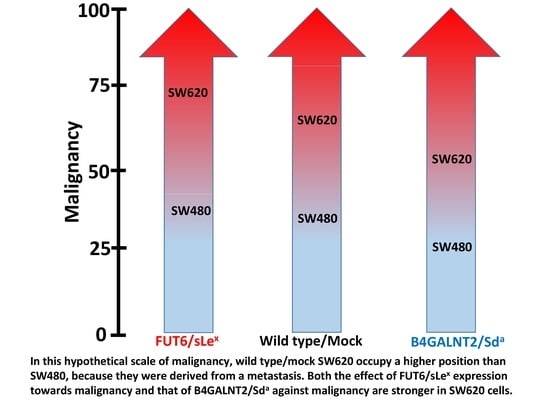
2.2. Phenotypic Changes Induced by B4GALNT2 and FUT6 Expression
- Doubling time. Expression of B4GALNT2 reduced the speed of growth of SW620, increasing the doubling time from 23 ± 3 to 28 ± 4 h (Figure 2A). Although a tendency to increased speed of growth in FUT6-expressing SW620 cells was observed, it did not reach statistical significance. On the other hand, no effect of either glycosyltransferase was observed on the doubling time of SW480.
- Clonogenic ability. The number of cells able to form colonies was affected by both glycosyltransferases in both cell lines (Figure 2B). The cell line SW620 was provided with a higher capability to form colonies, compared to SW480. However, in both cell lines FUT6 expression induced a small but significant increase of the clonogenic ability which, by contrast, was strongly impaired by B4GALNT2.
- Soft agar growth. In both cell lines, B4GALNT2 expression significantly reduced the formation of clones, while FUT6 induced a slight increase of clone formation only in SW620 cells (Figure 2C).
- Spheroid formation. This assay measures the ability of the cells to survive and proliferate in liquid medium, a condition associated with stemness, even more drastic than soft agar. SW480 cells formed mainly rounded spheroids with regular edges, whereas SW620 cells formed spheroids with irregular shape and many cells were found as single. B4GALNT2 expression reduced the formation of spheroids in both cell lines while FUT6 had no effect (Figure 2D). Owing to the fact that floating particles were nearly impossible to quantify, the growth of spheroids was indirectly quantified by collecting all the floating particles, preparing a homogenate and calculating the total amount of protein of the homogenate.
- Wound healing assay. The ability to heal a wound in a layer of confluent cells provided an example of the differential response of the two cell lines to glycosyltransferase expression. In fact, in the cell line SW480 the expression of either FUT6 or B4GALNT2 left unaltered the ability to heal a wound. On the contrary, in the cell line SW620 the healing capability was greatly enhanced by FUT6 but reduced by B4GALNT2 (Figure 2E).
- ALDEFLUOR assay. This assay for stemness revealed that B4GALNT2 induced a marked down-regulation of the number of stem cells in both cell lines (Figure 3). Unexpectedly, FUT6 induced a slight reduction of aldheyde dehydrogenase 1 (ALDH)-positive cells, which reached statistical significance only in SW480.
2.3. Effect of B4GALNT2 or FUT6 Expression on the Transcriptome
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Slot Blot Analysis of Carbohydrate Antigens
4.3. Doubling Time Assay
4.4. Clonogenic Assay
4.5. Soft Agar Assay
4.6. Spheroid Assay
4.7. Wound Healing Assay
4.8. ALDEFLUOR Assay
4.9. Transcriptomic Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDH | aldheyde dehydrogenase 1 |
| ANOVA | Analysis of variance |
| CRC | Colorectal cancer |
| DEAB | N,N-diethylaminobenzaldehyde |
| DT | Doubling time |
| PBS | Phosphate buffered saline |
| TCGA | The Cancer Genome Atlas |
References
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. 2012, 17, 670–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Olio, F.; Malagolini, N.; Chiricolo, M. Glycosylation in cancer. Spec. Period. Rep. Carbohydr. Chem. 2012, 37, 21–56. [Google Scholar]
- Gomes Ferreira, I.; Pucci, M.; Venturi, G.; Malagolini, N.; Chiricolo, M.; Dall’Olio, F. Glycosylation as a Main Regulator of Growth and Death Factor Receptors Signaling. Int. J. Mol. Sci. 2018, 19, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Nakamori, S.; Kameyama, M.; Imaoka, S.; Furukawa, H.; Ishikawa, O.; Sasaki, Y.; Kabuto, T.; Iwanaga, T.; Matsushita, Y.; Irimura, T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: Clinicopathological and immunohistochemical study. Cancer Res. 1993, 53, 3632–3637. [Google Scholar]
- Nakamori, S.; Kameyama, M.; Imaoka, S.; Furukawa, H.; Ishikawa, O.; Sasaki, Y.; Izumi, Y.; Irimura, T. Involvement of carbohydrate antigen sialyl Lewisx in colorectal cancer metastasis. Dis. Colon Rectum 1997, 40, 420–431. [Google Scholar] [CrossRef]
- Trinchera, M.; Aronica, A.; Dall’Olio, F. Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology 2017, 6, 16. [Google Scholar] [CrossRef]
- Yamada, N.; Chung, Y.S.; Maeda, K.; Sawada, T.; Ikehara, T.; Nishino, H.; Okuno, M.; Sowa, M. Increased expression of sialyl Lewis A and sialyl Lewis X in liver metastases of human colorectal carcinoma. Invasion Metastasis 1995, 15, 95–102. [Google Scholar]
- Carvalho, A.S.; Harduin-Lepers, A.; Magalhaes, A.; Machado, E.; Mendes, N.; Costa, L.T.; Matthiesen, R.; Almeida, R.; Costa, J.; Reis, C.A. Differential expression of a-2,3-sialyltransferases and a-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42, 80–89. [Google Scholar] [CrossRef]
- Ito, H.; Hiraiwa, N.; Sawada-Kasugai, M.; Akamatsu, S.; Tachikawa, T.; Kasai, Y.; Akiyama, S.; Ito, K.; Takagi, H.; Kannagi, R. Altered mRNA expression of specific molecular species of fucosyl- and sialyl-transferases in human colorectal cancer tissues. Int. J. Cancer 1997, 71, 556–564. [Google Scholar] [CrossRef]
- Izawa, M.; Kumamoto, K.; Mitsuoka, C.; Kanamori, C.; Kanamori, A.; Ohmori, K.; Ishida, H.; Nakamura, S.; Kurata-Miura, K.; Sasaki, K.; et al. Expression of sialyl 6-sulfo Lewis X is inversely correlated with conventional sialyl Lewis X expression in human colorectal cancer. Cancer Res. 2000, 60, 1410–1416. [Google Scholar] [PubMed]
- Miyazaki, K.; Ohmori, K.; Izawa, M.; Koike, T.; Kumamoto, K.; Furukawa, K.; Ando, T.; Kiso, M.; Yamaji, T.; Hashimoto, Y.; et al. Loss of disialyl Lewisa the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis a expression on human colon cancers. Cancer Res. 2004, 64, 4498–4505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchera, M.; Malagolini, N.; Chiricolo, M.; Santini, D.; Minni, F.; Caretti, A.; Dall’Olio, F. The biosynthesis of the selectin-ligand sialyl Lewis x in colorectal cancer tissues is regulated by fucosyltransferase VI and can be inhibited by an RNA interference-based approach. Int. J. Biochem. Cell Biol. 2011, 43, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Serafini-Cessi, F.; Dall’Olio, F. Guinea-pig kidney b-N-acetylgalactosaminyltransferase towards Tamm-Horsfall glycoprotein. Requirement of sialic acid in the acceptor for transferase activity. Biochem. J. 1983, 215, 483–489. [Google Scholar] [CrossRef]
- Lo Presti, L.; Cabuy, E.; Chiricolo, M.; Dall’Olio, F. Molecular Cloning of the Human b1,4 N-acetylgalactosaminyltransferase responsible for the biosynthesis of the Sda histo-blood group antigen: The sequence predicts a very long cytoplasmic domain. J. Biochem. 2003, 134, 675–682. [Google Scholar] [CrossRef]
- Montiel, M.D.; Krzewinski-Recchi, M.A.; Delannoy, P.; Harduin-Lepers, A. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Aca2-3Galb-R b1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: Evidence for an unusual extended cytoplasmic domain. Biochem. J. 2003, 373, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.L.; Lowe, J.B. Molecular cloning of a murine N-acetylgalactosamine transferase cDNA that determines expression of the T lymphocyte-specific CT oligosaccharide differentiation antigen. J. Biol. Chem. 1994, 269, 15162–15171. [Google Scholar]
- Dall’Olio, F.; Malagolini, N.; Chiricolo, M.; Trinchera, M.; Harduin-Lepers, A. The expanding roles of the Sda/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim. Biophys. Acta 2014, 1840, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Stenfelt, L.; Hellberg, A.; Moller, M.; Thornton, N.; Larson, G.; Olsson, M.L. Missense mutations in the C-terminal portion of the B4GALNT2-encoded glycosyltransferase underlying the Sd(a-) phenotype. Biochem. Biophys. Rep. 2019, 19, 100659. [Google Scholar] [CrossRef]
- Groux-Degroote, S.; Schulz, C.; Cogez, V.; Noel, M.; Portier, L.; Vicogne, D.; Solorzano, C.; Dall’Olio, F.; Steenackers, A.; Mortuaire, M.; et al. The extended cytoplasmic tail of the human B4GALNT2 is critical for its Golgi targeting and post-Golgi sorting. FEBS J. 2018, 285, 3442–3463. [Google Scholar] [CrossRef] [Green Version]
- Dohi, T.; Yuyama, Y.; Natori, Y.; Smith, P.L.; Lowe, J.B.; Oshima, M. Detection of N-acetylgalactosaminyltransferase mRNA which determines expression of Sda blood group carbohydrate structure in human gastrointestinal mucosa and cancer. Int. J. Cancer 1996, 67, 626–631. [Google Scholar] [CrossRef]
- Malagolini, N.; Dall’Olio, F.; Di Stefano, G.; Minni, F.; Marrano, D.; Serafini-Cessi, F. Expression of UDP-GalNAc:NeuAc a2,3Gal b-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989, 49, 6466–6470. [Google Scholar] [PubMed]
- Malagolini, N.; Santini, D.; Chiricolo, M.; Dall’Olio, F. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology 2007, 17, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Groux-Degroote, S.; Wavelet, C.; Krzewinski-Recchi, M.A.; Portier, L.; Mortuaire, M.; Mihalache, A.; Trinchera, M.; Delannoy, P.; Malagolini, N.; Chiricolo, M.; et al. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int. J. Biochem. Cell Biol. 2014, 53, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.I.; Toyota, M.; Kawashima, R.; Hagiwara, T.; Suzuki, H.; Imai, K.; Shinomura, Y.; Tokino, T.; Kannagi, R.; Dohi, T. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology 2008, 135, 142–151. [Google Scholar] [CrossRef]
- Wang, H.R.; Hsieh, C.Y.; Twu, Y.C.; Yu, L.C. Expression of the human Sda b-1,4-N-acetylgalactosaminyltransferase II gene is dependent on the promoter methylation status. Glycobiology 2008, 18, 104–113. [Google Scholar] [CrossRef]
- Pucci, M.; Gomes Ferreira, I.; Orlandani, M.; Malagolini, N.; Ferracin, M.; Dall’Olio, F. High expression of the Sda synthase B4GALNT2 associates with good prognosis and attenuates stemness in colon cancer. Cells 2020, 9, 948. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.I.; Kawashima, R.; Fukunaga, R.; Hirai, K.; Toyama-Sorimachi, N.; Tokuhara, M.; Shimizu, T.; Dohi, T. Introduction of Sda carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005, 65, 6220–6227. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.I.; Adachi, Y.; Curiel, D.T.; Kawashima, R.; Kannagi, R.; Nishimoto, N.; Dohi, T. Therapeutic adenoviral gene transfer of a glycosyltransferase for prevention of peritoneal dissemination and metastasis of gastric cancer. Cancer Gene Ther. 2014, 21, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Leibovitz, A.; Stinson, J.C.; McCombs, W.B., III; McCoy, C.E.; Mazur, K.C.; Mabry, N.D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976, 36, 4562–4569. [Google Scholar]
- Hiller, K.M.; Mayben, J.P.; Bendt, K.M.; Manousos, G.A.; Senger, K.; Cameron, H.S.; Weston, B.W. Transfection of a1,3 fucosyltransferase antisense sequences impairs the proliferative and tumorigenic ability of human colon carcinoma cells. Mol. Carcinog. 2000, 27, 280–288. [Google Scholar] [CrossRef]
- Hirakawa, M.; Takimoto, R.; Tamura, F.; Yoshida, M.; Ono, M.; Murase, K.; Sato, Y.; Osuga, T.; Sato, T.; Iyama, S.; et al. Fucosylated TGF-b receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br. J. Cancer 2014, 110, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Gao, C.; Li, Y.; Sun, M.; Xu, J.; Li, H.; Jia, L.; Zhao, Y. miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death Dis. 2017, 8, e2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.; Liu, Y.; Liu, Q.; Xiao, Y.; Liu, B.; Ren, X.; Qi, X.; Zhou, H.; Zeng, C.; Jia, L. HOTAIR/miR-326/FUT6 axis facilitates colorectal cancer progression through regulating fucosylation of CD44 via PI3K/AKT/mTOR pathway. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Hanley, W.D.; Burdick, M.M.; Konstantopoulos, K.; Sackstein, R. CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res. 2005, 65, 5812–5817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, F.; Wang, Q.; Huska, M.R.; Andrade-Navarro, M.A.; Lemm, M.; Fichtner, I.; Dahlmann, M.; Kobelt, D.; Walther, W.; Smith, J.; et al. SPON2, a newly identified target gene of MACC1, drives colorectal cancer metastasis in mice and is prognostic for colorectal cancer patient survival. Oncogene 2016, 35, 5942–5952. [Google Scholar] [CrossRef]
- Venturi, G.; Gomes, F.I.; Pucci, M.; Ferracin, M.; Malagolini, N.; Chiricolo, M.; Dall’Olio, F. Impact of sialyltransferase ST6GAL1 overexpression on different colon cancer cell types. Glycobiology 2019, 29, 684–695. [Google Scholar] [CrossRef]
- Ferracin, M.; Bassi, C.; Pedriali, M.; Pagotto, S.; D’Abundo, L.; Zagatti, B.; Corra, F.; Musa, G.; Callegari, E.; Lupini, L.; et al. miR-125b targets erythropoietin and its receptor and their expression correlates with metastatic potential and ERBB2/HER2 expression. Mol. Cancer 2013, 12, 130. [Google Scholar] [CrossRef] [Green Version]




| Network | p-Value | Network Objects |
|---|---|---|
| Cell cycle-Mitosis | 4 × 10−7 | Cyclin B1, Cyclin B, Cyclin B2, Histone H3, PBK, Cyclin A, PLK1, Securin, CENP-H, SIL, Separase, HZwint-1, CENP-F, CAP-G/G2, Aurora-A, CDC25, CDC25C, Tubulin-β, KNSL1, CAP-E, AF15q14, HEC, CENP-E, TPX2, SPBC25, ASPM, MAD2a, Survivin, BUB1, CAP-C, Actin, Histone H1, CENP-A |
| Cell cycle-Core | 7 × 10−7 | CDC45L, Cyclin B1, Cyclin B, Cyclin B2, Cyclin A, PLK1, Securin, RPA3, CENP-H, Separase, CAP-G, Aurora-A, CDC25C, CAP-E, p18, HEC, p21, CENP-E, ORC6L, CKS2, MAD2a, Survivin, BUB1, CAP-C, CENP-A |
| Cytoskeleton-Spindle microtubules | 2 × 10−6 | Cyclin B1, Cyclin B, Cyclin B2, KIF4A, DEEPEST, PLK1, Securin, CENP-H, GTSE1, Separase, HZwint-1, CENP-F, Aurora-A, Tubulin-β, KNSL1, Tau (MAPT), HEC, MKLP2, CENP-E, CKS2, MAD2a, BUB1, CENP-A |
| Development-Regulation of angiogenesis | 2.5 × 10−6 | MMP-9, FOXM1, IL-8, PKC, CD13, Oct-3/4, TRIP6, TrkB, GLI-1, WT1, DBH, Cathepsin B, Ephrin-B, Ephrin-A, PLC-β, Gα(i)-specific peptide GPCRs, c-Myc, IL8RB, Gα(q)-specific peptide GPCRs, PI3K reg class IA, STAT5, IL-15, Ephrin-A receptors, p21, Plasminogen, Angiostatin, Plasmin, Ephrin-B receptor 4, Ephrin-B receptors, IP3 receptor, IL-1RI, Ihh, Hedgehog, EGFR, PLAUR (uPAR), EDNRB |
| Cell cycle-G2-M | 1 × 10−5 | FOXM1, Cyclin B1, Cyclin B, Cyclin B2, Histone H3, MYRL2, MRLC, Cyclin A, Cyclin A2, PLK1, Securin, GTSE1, Claspin, CAP-G, CAP-G/G2, Aurora-A, CDC25, CDC25C, RGC32, KNSL1, CAP-E, c-Myc, p21, Rad51, BLM, CKS2, MAD2a, BUB1, EGFR, CAP-C, Histone H1.5, Histone H1, FANCD2 |
| Cell cycle-S phase | 5 × 10−5 | CDC45L, Cyclin B1, Cyclin B, Cyclin B2, Histone H3, Cyclin A, Cyclin A2, Histone H4, PLK1, Securin, RPA3, Separase, DRF1, PDS5, RGC32, PRIM2A, p21, ORC6L, Rad51, AHR, DDX11, BUB1, Histone H1.5, Histone H1, Sgo1 |
| Cell cycle-Meiosis | 7 × 10−5 | Cyclin B1, HSP70, Cyclin A, GCNF, PARD3, PLK1, Securin, SMC1L2, FANCG, RAD54L, Separase, CDC25C, Tubulin-β, c-Myc, PP2A regulatory, PI3K reg class IA, Rad51, BLM, RAD54B, EGFR |
| Development-Neurogenesis-Synaptogenesis | 5 × 10−4 | FGF7, APOE, Syntaxin 1A, TrkB, ErbB3, nAChR alpha, WNT, Ephrin-B3, Ephrin-B, Neurexin beta, Ionotropic glutamate receptor, Kainate receptor, NT-4/5, Neuregulin 2, NMDA receptor, Frizzled, MAGI-1(BAIAP1), FGFR2, Synaptotagmin VII, Synaptotagmin, Ephrin-B receptors, X11, FGFR4, Endophilin A3, Actin, NR1 |
| Cell adhesion-Attractive and repulsive receptors | 7 × 10−4 | 5T4, Semaphorin 3A, MENA, SLIT1, c-Fes, UNC5B, AF-6, Ephrin-B3, Ephrin-B, Ephrin-A, Ephrin-A3, Ephexin, Tau (MAPT), L1CAM, PI3K reg class IA (p55-gamma), PI3K reg class IA, Ephrin-A receptors, Ephrin-A receptor 3, Collagen XIII, Ephrin-B receptor 4, Ephrin-B receptors, RHO6, Actin, Integrin, Intersectin |
| Development-Neurogenesis-Axonal guidance | 1 × 10−3 | AHNAK, Syntenin 2, APOE, Semaphorin 3A, PKA-reg (cAMP-dependent), PARD3, CRMP4, TrkB, MENA, SLIT1, c-Fes, Ryanodine receptor 1, UNC5B, Ephrin-B3, Ephrin-B, Ephrin-A, Ephrin-A3, PLC-β, NT-4/5, L1CAM, PI3K reg class IA, Ephrin-A receptors, Ephrin-A receptor 3, Guanine deaminase, Ephrin-B receptor 4, Ephrin-B receptors, RHO6, IP3 receptor, Actin, Integrin |
| Network | p-Value | Network Objects |
|---|---|---|
| Cell adhesion- Cell matrix interactions | 0.002 | Mindin, Galectin-7, CD44 (EXT), CD44 (ICD), CD44 soluble, CD44 |
| Cell cycle-S phase | 0.012 | Rad51, MCM10, ORC6L, RGC32 |
| Development-Neurogenesis-Axon guidance | 0.012 | DISC1, Mindin, Semaphorin 3B, PLC-β, Netrin-1 |
| Cytoskeleton-Intermediate filaments | 0.013 | Tubulin-β2, Tubulin-β, Kinesin heavy chain |
| Reproduction-GnRH signaling pathway | 0.017 | mGluR8, Gα(i)-specific metabotropic glutamate GPCRs, PLC-β, PLC-β1 |
| Cytoskeleton-Regulation of cytoskeleton rearrangement | 0.024 | SPTBN(spectrin1-4), Tubulin-β2, Tubulin-β, CD44 |
| Reproduction-Gonadotropin regulation | 0.031 | mGluR8, Gα(i)-specific metabotropic glutamate GPCRs, PLC-β, PLC-β1 |
| Cytoskeleton-Cytoplasmic microtubules | 0.032 | Tubulin-β, Kinesin heavy chain, KIF5A |
| Reproduction-Feeding and Neurohormone signaling | 0.037 | CD44, PLC-β, PLC-β1, AKR1C1 |
| Functional Class | Both in SW480 and SW620 | Only in SW480 | Only in SW620 |
|---|---|---|---|
| Apoptosis | BEX2; PTPN13; HRK | RSL1D1; BIRC3; PPM1K | |
| Ca binding | CALB2 | CAB39L | |
| Cell adhesion | MCAM; GPR126; ANTXR2; SLIT1; PEAR1; NTN4 | ITGB7; NEBL | CDH16;DOCK4; AGR2 |
| Cell cycle | BRSK2; BEX2 | CCNI | TERT; ORC6; CDKN2C; TYMS; POLE4 |
| Chromatin remodelling | HIST1H2AI; HIST1H2BE; HIST1H2AG; HIST1H1B; HIST1H4L | ||
| Cytoskeleton-cytokinesis | MYH7B; CENPI; BRSK2; TUBB2; FILIP1 | LLGL2; FGFR1OP; WDR1;RGCC; CEP95; TUBB2A | TRIM58; FRMD4A; ANLN; TNNC1; KIF18A; DNM3; MICAL3;APC2; MARCKS; KIF19; TUBB2B |
| DNA damage response | RAD51AP1 | ||
| Drug metabolism | CYB5R2 | CYB5R2;CYP2J2; ADH1C | |
| Energy production | DNAJC15 | DNAJC15 | |
| Extracellular matrix | SCEL; HS3ST1 | COL9A3; COL6A1 | FMOD; SDC4 |
| Glycosylation | GALNT18 | ||
| Growth factors | MIA | IGFBP2;MDK | IHH; KITLG;WLS |
| Growth factors receptors | GRB10; GPR126; FZD6; CALCA | NTRK2 | GFRA3;EFNB3; GPR160; NOTCH2; RAMP1; SMO |
| Hypoxia response | HEPAS1 | HIF1A | |
| Inflammation and immunity | CD55; CXCL8; NCF2 | ANXA1 | RARRES2 |
| Intracellular transport | CPLX2; CPLX1; CAPN8 | MVB12B; SEZ6L2; GOLGA8A | RAB36; HIP1; MVB12B; BLOC1S4; HTT; CPE; AGR2; SPIRE2 |
| Ion transport | KCNJ2; AHNAK2; TRPV6; BEST1; FXYD4 | TMC4; AKAP7; PIEZO1; MFSD10; BSPRY; MTL5; CACNA2D4; ATP6V0A4 | |
| Lipid metabolism | LIPC; CYB5R2 | PLIN4;LIPC; CYB5R2; CYP2J2 | |
| Mucosa protection | TFF1; TFF3; SLPI | AGR2 | |
| Nuclear structure and function | NPIPB5 | NOP14 | |
| Phosphatases | SGPP2 | DUSP23; PPM1K | |
| Proteolysis | SERPINE2; TIMP3; TPSAB1; TFPI | MME; WFDC2 | |
| RNA maturation | GPAT2; SNORA30; SNORA62 | SNORA75; SNORA2B; SNORA13; SFPQ; CLK1 | TRA2A |
| Signal transduction | RASGEF1A; CAPN5; AKAP12; GBP3; ADRBK2; REPS2 | AFAP1L2; SGPP2; NGEF; PLCB1; CRABP2; PIM1; PTPRM; PTPRS | CHN2; SHCBP1; GPER1; CNPY1; APC2; DGKQ; MYZAP; NOTCH2NL; SQSTM1; LMTK3; ARHGEF4; PROM1; PKIB; TSPAN5; RRAGD; PTPN13; GRB10; AKT3 |
| Stress response | OXR1 | ||
| Transcription | CAPN15; BEX2; BHLHE41; DIP2C; HEPAS1; CREB5; ZNF462; HES7; ZIC5 | LEF1; PAX6; HIF1A; RNF187; TCEA3; TCEA2 | PRRX1; KLF17; NELFA; ZNF581; MXD4; RCOR2; MXI1; ESSRA; MYCL; RELB; FOXD1; BCL3; ZNF22 |
| Translation | EEF1A2 | ||
| Transporters | SLC43A3; ABCC3 | SLC39A11; SLC29A2 | |
| Ubiquitin proteasome pathway | UBASH3B; OTUD1 | NEURL3 |
| Functional Class | Both in SW480 and SW620 | Only in SW480 | Only in SW620 |
|---|---|---|---|
| Apoptosis | G0S2; CDIP1; LGALS7; RNF157; FILIP1L; SEMA3B | RNF130 | EVA1A; PTPN13; CDH13 |
| Cell adhesion | CD44; CDHR2; DDR1 | ELSPBP1; PCDH9 | NEBL; SERPINB8 |
| Cell cycle | ORC6; DISC1; RGCC | CDK14; BRSK2 | |
| Chromatin remodelling | MTA2 | ING4; BAZ2B | ATRX; ZNF462 |
| Cytoskeleton-cytokinesis | ORC6; CDCA5; MCM10; DISC1; SPIRE2; PTPRN2; SEMA3B; TUBB2A; KRT15; SPTBN5 | DLC1; SHROOM2 | CENPI; ASPM; ERCC6L; NUF2; ANLN; CEP152; KIFC3; MAP1LC3B; BRSK2; SPEG2; KRT14; ARC |
| DNA damage response | MCM10; RAD51; ANKRD32 (SLF1) | RAD51AP1; ERCC6L; DNA2; ARHGAP11A | |
| Drug metabolism | CYB5R2; SLC47A1; FMO3 | ||
| Extracellular matrix | COL7A1; KRTAP3-2 | ECM2; COL9A3 | |
| Glycosylation | GALNT18; GALC; AMY1C | FUT3 | |
| Growth factors | IGFBP2; ANGPTL2; ISM1; EDA; IGFALS | AREG | |
| Growth factors receptors | RHBDF1 | NOTCH2; ROR1; EFNA1 | |
| Hypoxia response | EGLN3 | ||
| Inflammation and immunity | IL18; SPON2 | CD8B; NCF2; SLAMF7 | |
| Intracellular transport | SPIRE2; BAIAP3; TUBB2A; SYT13 | BET1L; C16orf62; LMF1; MYRIP | LPHN2; KIFC3; RAB37; GOLGA7B |
| Ion transport | PLLP; MAGED2; SLC4A11 | KCNS3; CNKSR3; STAC3; ATP6AP1L; | STOM |
| Lipid metabolism | CYB5R2; CROT; LMF1; FABP6 | ELOVL2; SLCO1B3 | |
| Lysosomal enzymes | IDS | ||
| Mucosa protection | MUC6 | ||
| Phosphatases | PTPRN2 | SGPP2 | PTPN13 |
| Proteolysis | TPP2 | ADAM30; SERPINA3 | |
| RNA maturation | SRPK3 | SNAR-G1; SNAR-F; SNAR-G2; SNAR-H; SNAR-D; SNAR-A3 | |
| Signal transduction | PLCB1; DDR1; NXN; RNF157 | DLC1; SGPP2; STK32C; KSR2 | ATRNL1; DKK4; ARHGAP11A; ADRB1; RAB37; ADRB2; TBC1D4 |
| Stress response | HSPA1A | ||
| Transcription | MAF; MTA2; ZNF276; NXN; ZNF83 | ZNF316; LEF1; TFAP2C; KLF12; HBP1; ZFP62 | ZSCAN20; ZNF462 |
| Transporters | SLC43A3; ABCC3; AKR1C1 | SLC47A1; XK; SLC2A8 | SLC2A6; SLC7A2 |
| Ubiquitin proteasome pathway | RNF157 | TPP2; RNF130; OTUD1 |
| Cell Line | Type of Transfection | Gene Symbol | ||||||
|---|---|---|---|---|---|---|---|---|
| MCOLN2 | PLLP | FILIP1L | FAM231A | SPON2 | COL20A1 | BCL2L10 | ||
| SW480 | Neo | 10 | 674 | 59 | 8 | 10,476 | 40 | 26 |
| B4GALNT2 | 22 | 441 | 36 | 5 | 5779 | 21 | 12 | |
| SW620 | Neo | 19 | 803 | 107 | 16 | 8579 | 54 | 33 |
| B4GALNT2 | 59 | 509 | 57 | 8 | 5447 | 28 | 12 | |
| LS174T | Neo | 4 | 104 | 20 | 24 | 338 | 14 | 8 |
| B4GALNT2 | 9 | 52 | 13 | 15 | 181 | 8 | 2 | |
| Gene name | Mucolipin 2 | Plasmolipin | Filamin A interacting protein like | Family with sequence similarity 231 member A | Spondin 2 | Collagen XX α1 | BCL2-like 10 | |
| Role in cancer | Promotes glioma progression | No information | Inhibits CRC progression | No information | Promotes malignancy of CRC | Overexpressed in glioma | Tumor suppressor | |
| PMID | 27248469 | 7750216 | 26686083 | 31556357 | 31894274 27770580 | |||
| Biological Function | SW480 | SW620 | LS174T * | ||
|---|---|---|---|---|---|
| FUT6 | B4GALNT2 | FUT6 | B4GALNT2 | B4GALNT2 | |
| Proliferation rate | Unchanged | Unchanged | Unchanged | Down | Unchanged |
| Clonogenic ability (solid) | Up | Down | Up | Down | Unchanged |
| Soft agar growth | Unchanged | Down | Up | Down | Down |
| Spheroid formation | Unchanged | Down | Unchanged | Down | Down |
| Wound healing ability | Unchanged | Unchanged | Up | Down | Unchanged |
| ALDH expression | Down | Down | Unchanged | Down | Down |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, M.; Gomes Ferreira, I.; Malagolini, N.; Ferracin, M.; Dall’Olio, F. The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition. Int. J. Mol. Sci. 2020, 21, 6558. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186558
Pucci M, Gomes Ferreira I, Malagolini N, Ferracin M, Dall’Olio F. The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition. International Journal of Molecular Sciences. 2020; 21(18):6558. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186558
Chicago/Turabian StylePucci, Michela, Inês Gomes Ferreira, Nadia Malagolini, Manuela Ferracin, and Fabio Dall’Olio. 2020. "The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition" International Journal of Molecular Sciences 21, no. 18: 6558. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186558