Ameliorative Properties of Boronic Compounds in In Vitro and In Vivo Models of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
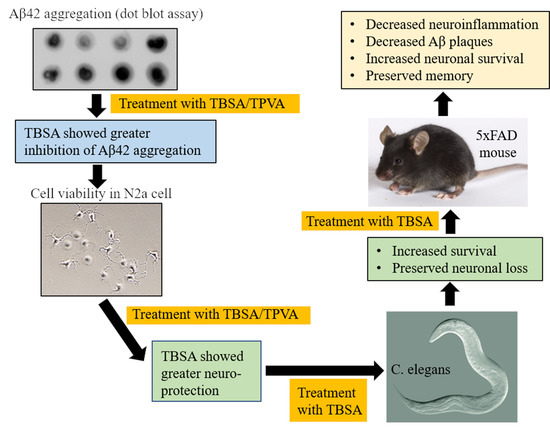
2.1. Trans-Beta-Styryl-Boronic Acid (TBSA), But Not Trans 2-Phenyl Vinylboronic Acid (TPVA) MIDA Ester Inhibited Aβ42 Aggregation and Toxicity In Vitro
2.2. Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment Was Tolerated In Vivo and Ameliorated Neurodegeneration in “AD” Aβ-Expressing C. elegans
2.3. Body Weights of Mice
2.4. Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment Moderately Decreased Aβ Plaque Load in 5xFAD Mice
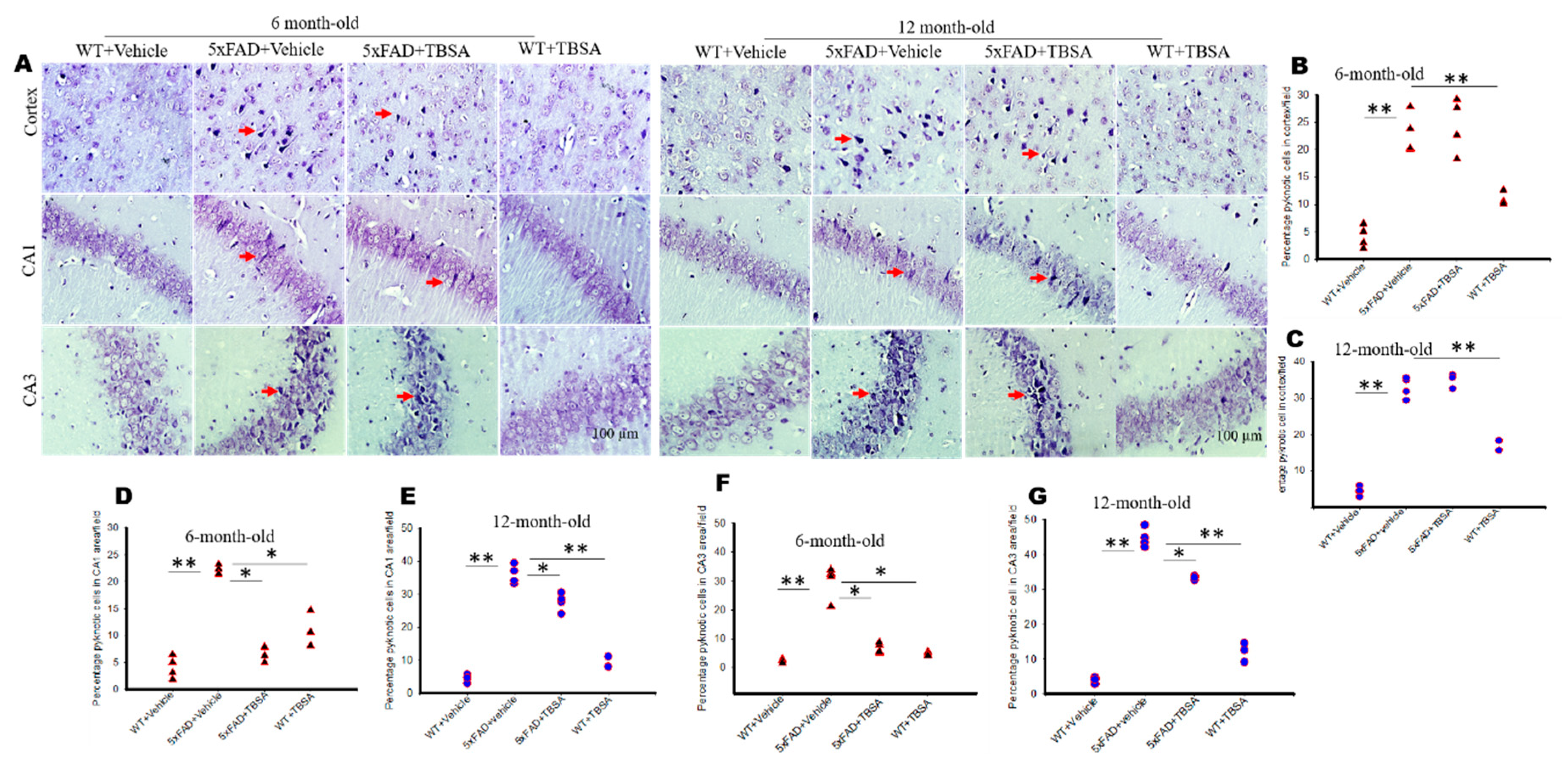
2.5. Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment Reduced Pyknotic Cells in Different Brain Areas of 5xFAD Mice
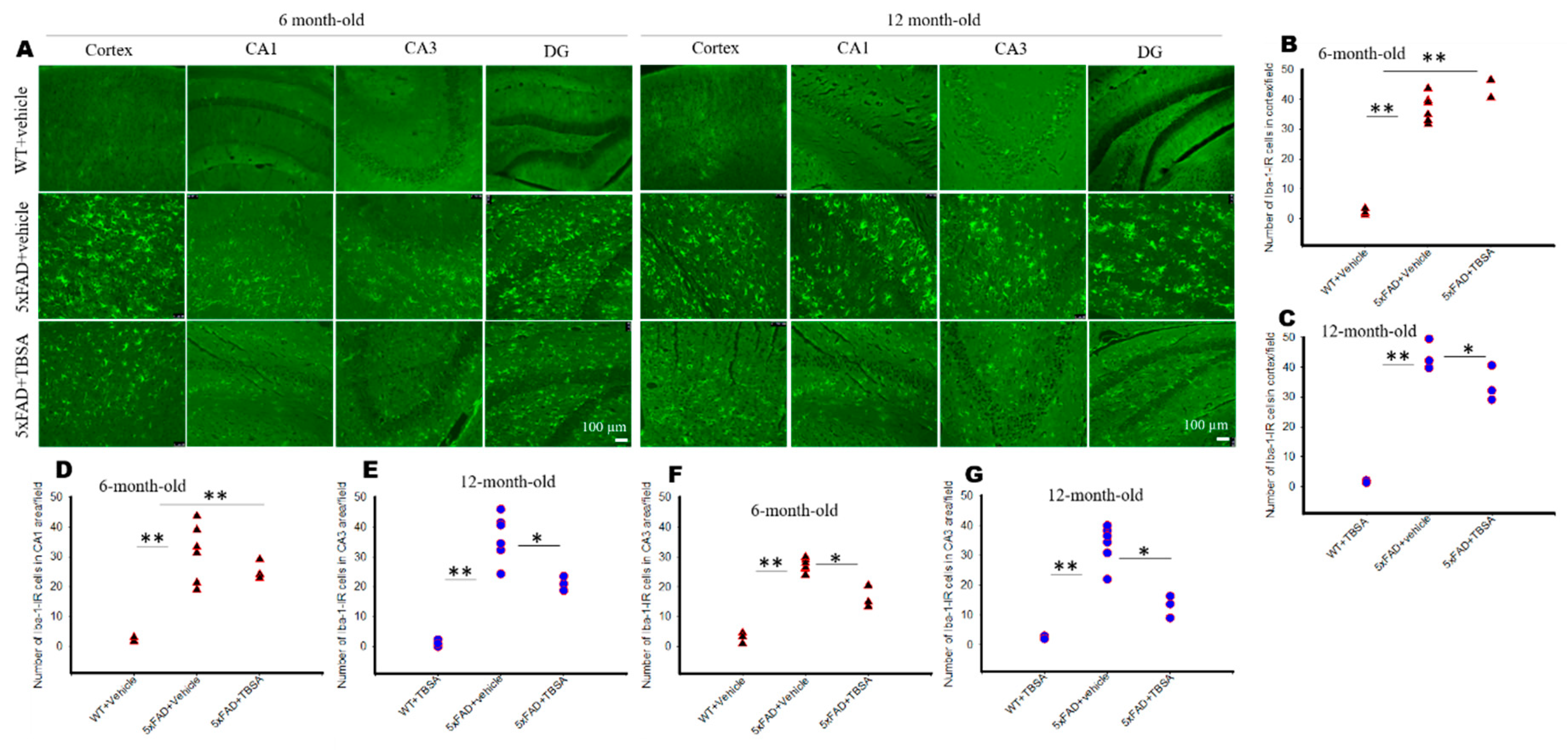
2.6. Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment Reduced Astrocyte Activation in Different Brain Areas of 5xFAD Mice
2.7. Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment Reduced Microglial Activation in Different Brain Areas of 5xFAD Mice
2.8. Effect of Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment on Open Field Test in 5xFAD Mice
2.9. Effect of Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment on Novel Objective Recognition Tasks in 5xFAD Mice
2.10. Effect of Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment on Morris Water Maze (MWM) Performance in 5xFAD Mice
2.11. Effect of Trans-Beta-Styryl-Boronic Acid (TBSA) Treatment on Spatial Memory in 5xFAD Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Inhibition of Aβ-Aggregation by Boronic Compounds Using Dot-Blot Assays
4.3. Cell Culture
4.4. Treatment of Different Boronic Compounds
4.5. Cell Viability by MTT Assay
4.6. Animals
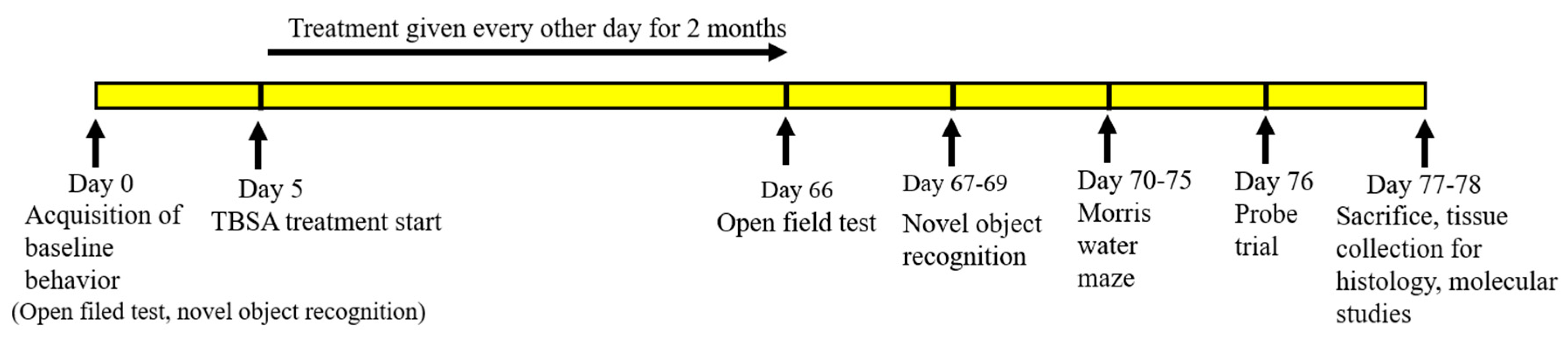
4.7. Treatment of Trans-Beta-Styryl-Boronic Acid (TBSA) Compound
4.7.1. Treatment of TBSA in C. elegans
4.7.2. Treatment of TBSA in 5xFAD Mice
5. Behavioral Assays
5.1. Open Field Test
5.2. Novel Object Recognition
5.3. Morris Water Maze (MWM)
Probe Trial
5.4. Tissue Processing
5.4.1. Amyloid β-Plaques Count
5.4.2. Neuronal Morphology by Cresyl Violet Staining
5.4.3. Neurodegeneration in C elegans
5.4.4. Immunohistochemistry of GFAP and Iba-1
5.5. Statistical Analyses
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | amyloid beta protein |
| AD | Alzheimer’s disease |
| 5xFAD | five times familial Alzheimer’s disease |
| TPVA | trans 2-phenyl vinyl-boronic acid |
| TBSA | trans-beta-styryl-boronic acid |
| GFAP | glial fibrillary acidic protein |
| Iba-1 | ionized calcium-binding adapter molecule 1 |
| MWM | Morris water maze |
| FDA | Food and Drug Administration |
| PDE4 | phosphodiesterase enzyme 4 |
| MMP+ | 1-methyl-4-phenylpyridinium |
| HFIP | 1,1,1,3,3,3-hexafluoroisopropanol |
| MEM | Minimum essential medium |
| FBS | fetal bovine serum |
| TBS | Tris-buffer saline |
| TBS-T | Tris-buffer saline-Tween-20 |
| HRP | horseradish-peroxidase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| SEM | standard error of mean |
| IACUC | Institutional Animal Care and Use Committee |
| WT | wild-type |
| OFT | open field test |
| NOR | novel object recognition |
| FO | familiar object |
| TN | time of the novel object |
| TF | time of the familiar object |
| DI | discrimination index |
| MWM | Morris water maze |
| DPX | distyrene plasticizer xylene |
| IHC | immunoreactivity |
| NGS | normal goat serum |
| DAPI | 6-diamidino-2-phenylindole |
| IR | immunoreactivity |
| ANOVA | analysis of variance |
| HSD | honestly significant difference |
| µM | micromolar |
| µg | microgram |
| CV | cresyl violet |
| TNF-α | tumor necrosis factor-α |
| GSK-3β | glycogen synthase kinase-3β |
| PBS | phosphate buffer saline |
| NGM | nematode growth medium |
| MIDA | methyliminodiacetic acid |
References
- Cummings, J.L. Alzheimer’s disease. N. Engl. J. Med. 2004, 351, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A.; Kwon, Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015, 47, e147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucukdogru, R.; Turkez, H.; Arslan, M.E.; Tozlu, O.O.; Sonmez, E.; Mardinoglu, A.; Cacciatore, I.; Di Stefano, A. Neuroprotective effects of boron nitride nanoparticles in the experimental Parkinson’s disease model against MPP+ induced apoptosis. Metab. Brain. Dis. 2020, 35, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.J.; Hu, J.; Wang, Z.; Xie, S.; Pan, T.; Huang, L.; Li, X. Discovery of boron-containing compounds as Abeta aggregation inhibitors and antioxidants for the treatment of Alzheimer’s disease. MedChemComm 2018, 9, 1862–1870. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Penland, J.G. Boron deprivation alters rat behaviour and brain mineral composition differently when fish oil instead of safflower oil is the diet fat source. Nutr. Neurosci. 2006, 9, 105–112. [Google Scholar] [CrossRef]
- Penland, J.G. Quantitative analysis of EEG effects following experimental marginal magnesium and boron deprivation. Magnes. Res. 1995, 8, 341–358. [Google Scholar]
- Penland, J.G. The importance of boron nutrition for brain and psychological function. Biol. Trace Elem. Res. 1998, 66, 299–317. [Google Scholar] [CrossRef]
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.; Dou, Q.P. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr. Cancer Drug Targets 2011, 11, 239–253. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.J.; Zhang, Y.-K.; Akama, T.; Lau, A.; Zhou, H.; Hernandez, V.; Mao, W.; Alley, M.R.K.; Sanders, V.; Plattner, J.J. Discovery of a New Boron-Containing Antifungal Agent, 5-Fluoro-1,3-dihydro-1-hydroxy-2,1- benzoxaborole (AN2690), for the Potential Treatment of Onychomycosis. J. Med. Chem. 2006, 49, 4447–4450. [Google Scholar] [CrossRef]
- Das, B.C.; Thapa, P.; Karki, R.; Schinke, C.; Das, S.; Kambhampati, S.; Banerjee, S.K.; Van Veldhuizen, P.; Verma, A.; Weiss, L.M.; et al. Boron chemicals in diagnosis and therapeutics. Future Med. Chem. 2013, 5, 653–676. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, R.T.; Nare, B.; Wring, S.A.; Orr, M.D.; Chen, D.; Sligar, J.M.; Jenks, M.X.; Noe, R.A.; Bowling, T.S.; Mercer, L.T.; et al. SCYX-7158, an Orally-Active Benzoxaborole for the Treatment of Stage 2 Human African Trypanosomiasis. PLoS Neglected Trop. Dis. 2011, 5, e1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, Y.R.; Akama, T.; Alley, M.; Antunes, J.; Dong, C.; Jarnagin, K.; Kimura, R.; Nieman, J.A.; Maples, K.R.; Plattner, J.J.; et al. Boron-based phosphodiesterase inhibitors show novel binding of boron to PDE4 bimetal center. FEBS Lett. 2012, 586, 3410–3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routray, I.; Ali, S. Boron Induces Lymphocyte Proliferation and Modulates the Priming Effects of Lipopolysaccharide on Macrophages. PLoS ONE 2016, 11, e0150607. [Google Scholar] [CrossRef] [Green Version]
- Romero-Aguilar, K.S.; Arciniega-Martínez, I.M.; Farfán-García, E.D.; Campos-Rodríguez, R.; Reséndiz-Albor, A.A.; Soriano-Ursúa, M.A. Effects of boron-containing compounds on immune responses: Review and patenting trends. Expert Opin. Ther. Pat. 2019, 29, 339–351. [Google Scholar] [CrossRef]
- Treusch, S.; Hamamichi, S.; Goodman, J.L.; Matlack, K.E.S.; Chung, C.Y.; Baru, V.; Shulman, J.M.; Parrado, A.; Bevis, B.J.; Valastyan, J.S.; et al. Functional Links Between A Toxicity, Endocytic Trafficking, and Alzheimer’s Disease Risk Factors in Yeast. Science 2011, 334, 1241–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, P.; Hall, T.C.; Paladugu, L.; Kolli, N.; Learman, C.; Rossignol, J.; Dunbar, G.L. A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5x-familial Alzheimer’s disease mice. Histochem. Cell Biol. 2016, 146, 609–625. [Google Scholar] [CrossRef]
- Maiti, P.; Paladugu, L.; Dunbar, G.L. Solid lipid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects than curcumin in the 5xFAD mouse model of Alzheimer’s disease. BMC Neurosci 2018, 19, 7. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Li, X.; Cheng, J.; Hou, L. Drug Development for Alzheimer’s Disease: Microglia Induced Neuroinflammation as a Target? Int. J. Mol. Sci. 2019, 20, 558. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.-K.; Chao, S.-P.; Hu, C.-J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 13–18. [Google Scholar] [CrossRef]
- Mendiola-Precoma, J.; Berumen, L.C.; Padilla, K.; García-Alcocer, G. Therapies for Prevention and Treatment of Alzheimer’s Disease. BioMed Res. Int. 2016, 2016, 2589276. [Google Scholar] [CrossRef] [Green Version]
- Necula, M.; Kayed, R.; Milton, S.; Glabe, C.G. Small Molecule Inhibitors of Aggregation Indicate That Amyloid β Oligomerization and Fibrillization Pathways Are Independent and Distinct. J. Boil. Chem. 2007, 282, 10311–10324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoiu, I.; Militaru, C.; Obleagă, O.; Hunter, J.M.; Neamţu, J.; Bita, A.; Scorei, R.I.; Rogoveanu, O.C. Effects of boron-containing compounds on cardiovascular disease risk factors—A review. J. Trace Elements Med. Boil. 2018, 50, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Zhanel, M.; Lagace-Wiens, P.R.S.; Walkty, A.; Denisuik, A.; Golden, A. Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-beta-Lactamase Inhibitor Combinations. Drugs 2018, 78, 65–98. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.C.; Camargo, C.H.R.; Carvalho, C.A.T.; De Oliveira, L.D.; Camargo, S.E.A.; Rodrigues, C.M. Effectiveness of carbamide peroxide and sodium perborate in non-vital discolored teeth. J. Appl. Oral Sci. 2009, 17, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Ono, M.; Matsumura, K.; Yoshimura, M.; Kimura, H.; Saji, H. Molecular imaging of beta-amyloid plaques with near-infrared boron dipyrromethane (BODIPY)-based fluorescent probes. Mol. Imaging 2013, 12, 338–347. [Google Scholar] [CrossRef]
- Kumar, S.K.; Hager, E.; Pettit, C.; Gurulingappa, H.; Davidson, N.E.; Khan, S.R. Design, Synthesis, and Evaluation of Novel Boronic-Chalcone Derivatives as Antitumor Agents. J. Med. Chem. 2003, 46, 2813–2815. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Li, L.; Wang, D.-Q. Lifespan extension in Caenorhabditis elegans by DMSO is dependent on sir-2.1 and daf-16. Biochem. Biophys. Res. Commun. 2010, 400, 613–618. [Google Scholar] [CrossRef]
- Wong, H.E.; Qi, W.; Choi, H.-M.; Fernandez, E.J.; Kwon, I. A Safe, Blood-Brain Barrier Permeable Triphenylmethane Dye Inhibits Amyloid-β Neurotoxicity by Generating Nontoxic Aggregates. ACS Chem. Neurosci. 2011, 2, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Irwin, J.A.; Wong, H.E.; Kwon, I. Different Fates of Alzheimer’s Disease Amyloid-β Fibrils Remodeled by Biocompatible Small Molecules. Biomacromolecules 2012, 14, 264–274. [Google Scholar] [CrossRef]
- Oh, S.B.; Kim, J.A.; Park, S.; Lee, J.-Y. Associative Interactions among Zinc, Apolipoprotein E, and Amyloid-β in the Amyloid Pathology. Int. J. Mol. Sci. 2020, 21, 802. [Google Scholar] [CrossRef] [Green Version]
- Hatami, A.; Albay, R.; Monjazeb, S.; Milton, S.; Glabe, C. Monoclonal Antibodies against A42 Fibrils Distinguish Multiple Aggregation State Polymorphisms in Vitro and in Alzheimer Disease Brain. JBC 2014, 289, 32131–32143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, P.M.; Jelliss, P.A.; Nonaka, N.; Shi, X.; Banks, W.A. Permeability of the blood-brain barrier to a rhenacarborane. J. Pharmacol. Exp. Ther. 2009, 329, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Hall, I.H.; Burnham, B.S.; Chen, S.Y.; Sood, A.; Spielvogel, B.F.; Morse, K.W. The Anti-Inflammatory Activity of Boron Derivatives in Rodents. Met. Drugs 1995, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benderdour, M.; Hess, K.; Dzondo-Gadet, M.; Nabet, P.; Belleville, F.; Dousset, B. Boron Modulates Extracellular Matrix and TNFα Synthesis in Human Fibroblasts. Biochem. Biophys. Res. Commun. 1998, 246, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yan, H.; Tang, N.; Li, X.; Pang, P.; Li, H.; Chen, W.; Guo, Y.; Shu, S.; Cai, Y.; et al. Impairments of spatial memory in an Alzheimer’s disease model via degeneration of hippocampal cholinergic synapses. Nat. Commun. 2017, 8, 1676. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Comparative Neuroprotective Effects of Dietary Curcumin and Solid Lipid Curcumin Particles in Cultured Mouse Neuroblastoma Cells after Exposure to Abeta42. Int. J. Alzheimer’s Dis. 2017, 2017, 4164872. [Google Scholar]
- Maiti, P.; Al-Gharaibeh, A.; Kolli, N.; Dunbar, G.L. Solid Lipid Curcumin Particles Induce More DNA Fragmentation and Cell Death in Cultured Human Glioblastoma Cells than Does Natural Curcumin. Oxidative Med. Cell. Longev. 2017, 2017, 9656719. [Google Scholar] [CrossRef] [Green Version]
- Maiti, P.; Plemmons, A.; Bowers, Z.; Weaver, C.; Dunbar, G. Labeling and Imaging of Amyloid Plaques in Brain Tissue Using the Natural Polyphenol Curcumin. J. Vis. Exp. 2019, 153, e60377. [Google Scholar] [CrossRef]
- Griffin, E.F.; Caldwell, K.A.; Caldwell, G.A. Genetic and Pharmacological Discovery for Alzheimer’s Disease UsingCaenorhabditis elegans. ACS Chem. Neurosci. 2017, 8, 2596–2606. [Google Scholar] [CrossRef]
- Larkin, J.D.; Bhat, K.L.; Markham, G.D.; James, T.D.; Brooks, B.R.; Bock, C.W. A computational characterization of boron-oxygen multiple bonding in HN=CH-CH=CH-NH-BO. J. Phys. Chem. A 2008, 112, 8446–8454. [Google Scholar] [CrossRef] [Green Version]
- Boyd, W.A.; McBride, S.J.; Rice, J.R.; Snyder, D.W.; Freedman, J.H. A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol. Appl. Pharmacol. 2010, 245, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. 2015, 2015, 52434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lueptow, L. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, e55718. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Singh, S.B.; Mallick, B.; Muthuraju, S.; Ilavazhagan, G. High altitude memory impairment is due to neuronal apoptosis in the hippocampus, cortex and striatum. J. Chem. Neuroanat. 2008, 36, 227–238. [Google Scholar] [CrossRef]
- Maiti, P.; Singh, S.B.; Muthuraju, S.; Veleri, S.; Ilavazhagan, G. Hypobaric hypoxia damages the hippocampal pyramidal neurons in the rat brain. Brain Res. 2007, 1175, 1–9. [Google Scholar] [CrossRef]
- Maiti, P.; Singh, S.B.; Sharma, A.K.; Muthuraju, S.; Banerjee, P.K.; Ilavazhagan, G. Hypobaric hypoxia induces oxidative stress in the rat brain. Neurochem. Int. 2006, 49, 709–716. [Google Scholar] [CrossRef]









| Groups | Age Groups | Behavior | CV Stain | GFAP/Iba-1 | Aβ Plaques |
|---|---|---|---|---|---|
| WT + Vehicle | 6 months | 12 (M = 8, F = 4) | 4 (M = 2, F = 2) | 3 (M = 2, F = 1) | 3 (M = 2, F = 1) |
| 5xFAD + Vehicle | 6 months | 12 (M = 7, F = 5) | 4 (M = 3, F = 1) | 6 (M = 4, F = 2) | 6 (M = 4, F = 2) |
| 5xFAD + TBSA | 6 months | 12 (M = 6, F = 6) | 4 (M = 2, F = 2) | 3 (M = 1, F = 2) | 3 (M = 2, F = 1) |
| WT + TBSA | 6 months | 12 (M = 8, F = 4) | 3 (M = 2, F = 1) | 3 (M = 2, F = 1) | 3 (M = 2, F = 1) |
| WT + Vehicle | 12 months | 11 (M = 6, F = 5) | 4 (M = 2, F = 2) | 3 (M = 1, F = 2) | 3 (M = 1, F = 2) |
| 5xFAD + Vehicle | 12 months | 10 (M = 6, F = 4) | 4 (M = 2, F = 2) | 4 (M = 3, F = 1) | 6 (M = 4, F = 2) |
| 5xFAD + TBSA | 12 months | 11 (M = 7, F = 5) | 4 (M = 3, F = 1) | 3 (M = 2, F = 1) | 3 (M = 2, F = 1) |
| WT + TBSA | 12 months | 10 (M = 5, F = 5) | 3 (M = 2, F = 1) | 3 (M = 1, F = 2) | 3 (M = 1, F = 2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiti, P.; Manna, J.; Burch, Z.N.; Flaherty, D.B.; Larkin, J.D.; Dunbar, G.L. Ameliorative Properties of Boronic Compounds in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6664. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186664
Maiti P, Manna J, Burch ZN, Flaherty DB, Larkin JD, Dunbar GL. Ameliorative Properties of Boronic Compounds in In Vitro and In Vivo Models of Alzheimer’s Disease. International Journal of Molecular Sciences. 2020; 21(18):6664. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186664
Chicago/Turabian StyleMaiti, Panchanan, Jayeeta Manna, Zoe N. Burch, Denise B. Flaherty, Joseph D. Larkin, and Gary L. Dunbar. 2020. "Ameliorative Properties of Boronic Compounds in In Vitro and In Vivo Models of Alzheimer’s Disease" International Journal of Molecular Sciences 21, no. 18: 6664. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186664