Red Blood Cells and Hemoglobin in Human Atherosclerosis and Related Arterial Diseases
Abstract
:1. Introduction
2. How Phylogeny and Ontogeny Define Red Blood Cells (RBCs) in the Human Circulation
3. Frictional Forces Involve RBCs and Interact with Nitric Oxide (NO)
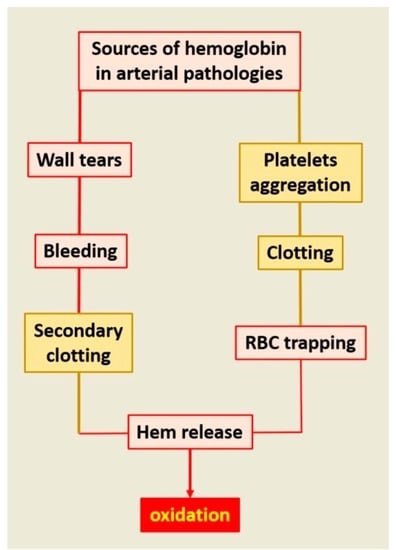
4. RBCs Colliding with the Arterial Wall
5. Presence of RBCs and Redox-Active Ferrous Iron in Different Human Arterial Pathologies
5.1. Methods for Ionized iron Detection in CV Tissues
5.2. Early Stages of Atheroma
5.3. Neo-Angiogenesis and Intraplaque Hemorrhages
5.4. In-Stent Neoatherosclerosis
5.5. Consequences of Clot Integration
5.6. RBC Clotting in Aneurysms of the Abdominal Aorta
5.7. RBCs and Vascular Calcifications
5.8. Myocardial Infarction (MI)
5.9. Stroke
6. Protection from RBC Injury: From Cells, Plasma and Antioxidant Molecules
6.1. Clearance of Senescent RBC Physiology/Pathology
6.2. Haptoglobin, CD 163, Hemopexin, Deferoxamine
6.3. Anti-Oxidants: Glutathione, Thioredoxin, Peroxiredoxin, SOD, Glutathione Peroxidases, Catalase, Paraoxonase
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Michel, J.B. Phylogenic determinants of cardiovascular frailty, focus on hemodynamics and arterial smooth muscle cells. Physiol. Rev. 2020, 100, 1779–1837. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K. Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef] [Green Version]
- Iwanaga, S. Biochemical principle of Limulus test for detecting bacterial endotoxins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 83, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chico, V.; Salvador-Mira, M.E.; Nombela, I.; Puente-Marin, S.; Ciordia, S.; Mena, M.C.; Perez, L.; Coll, J.; Guzman, F.; Encinar, J.A.; et al. IFIT5 Participates in the Antiviral Mechanisms of Rainbow Trout Red Blood Cells. Front. Immunol. 2019, 10, 613. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M. Hematopoiesis. J. Allergy Clin. Immunol. 1994, 94, 645–650. [Google Scholar] [CrossRef]
- Aarts, P.A.; van den Broek, S.A.; Prins, G.W.; Kuiken, G.D.; Sixma, J.J.; Heethaar, R.M. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis 1988, 8, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Uijttewaal, W.S.; Nijhof, E.J.; Bronkhorst, P.J.; Den Hartog, E.; Heethaar, R.M. Near-wall excess of platelets induced by lateral migration of erythrocytes in flowing blood. Am. J. Physiol. 1993, 264, H1239–H1244. [Google Scholar] [CrossRef] [PubMed]
- Viallat, A.; Abkarian, M. Red blood cell: From its mechanics to its motion in shear flow. Int. J. Lab. Hematol. 2014, 36, 237–243. [Google Scholar] [CrossRef]
- Delbosc, S.; Bayles, R.G.; Laschet, J.; Ollivier, V.; Ho-Tin-Noe, B.; Touat, Z.; Deschildre, C.; Morvan, M.; Louedec, L.; Gouya, L.; et al. Erythrocyte efferocytosis by the arterial wall promotes oxidation in early-stage atheroma in humans. Front. Cardiovasc. Med. 2017, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Lutz, H.U.; Bogdanova, A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front. Physiol. 2013, 4, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuilten, Z.K.; French, C.J.; Nichol, A.; Higgins, A.; Cooper, D.J. Effect of age of red cells for transfusion on patient outcomes: A systematic review and meta-analysis. Transfus. Med. Rev. 2018, 32, 77–88. [Google Scholar] [CrossRef]
- Baek, J.H.; D’Agnillo, F.; Vallelian, F.; Pereira, C.P.; Williams, M.C.; Jia, Y.; Schaer, D.J.; Buehler, P.W. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J. Clin. Invest. 2012, 122, 1444–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woollard, K.J.; Sturgeon, S.; Chin-Dusting, J.P.; Salem, H.H.; Jackson, S.P. Erythrocyte hemolysis and hemoglobin oxidation promote ferric chloride-induced vascular injury. J. Biol. Chem. 2009, 284, 13110–13118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, J.D.; Chauhan, A.K.; Schaeffer, G.V.; Hansen, J.K.; Motto, D.G. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood 2013, 121, 3733–3741. [Google Scholar] [CrossRef] [PubMed]
- Helms, C.C.; Gladwin, M.T.; Kim-Shapiro, D.B. Erythrocytes and vascular function: Oxygen and nitric oxide. Front. Physiol. 2018, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Gladwin, M.T.; Ognibene, F.P.; Pannell, L.K.; Nichols, J.S.; Pease-Fye, M.E.; Shelhamer, J.H.; Schechter, A.N. Relative role of heme nitrosylation and beta-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proc. Natl. Acad. Sci. USA 2000, 97, 9943–9948. [Google Scholar] [CrossRef] [Green Version]
- van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef] [Green Version]
- Dungel, P.; Penzenstadler, C.; Ashmwe, M.; Dumitrescu, S.; Stoegerer, T.; Redl, H.; Bahrami, S.; Kozlov, A.V. Impact of mitochondrial nitrite reductase on hemodynamics and myocardial contractility. Sci. Rep. 2017, 7, 12092. [Google Scholar] [CrossRef]
- Mathai, C.; Jourd’heuil, F.L.; Lopez-Soler, R.I.; Jourd’heuil, D. Emerging perspectives on cytoglobin, beyond NO dioxygenase and peroxidase. Redox Biol. 2020, 32, 101468. [Google Scholar] [CrossRef]
- Zweier, J.L.; Ilangovan, G. Regulation of nitric oxide metabolism and vascular tone by cytoglobin. Antioxid. Redox Signal. 2020, 32, 1172–1187. [Google Scholar] [CrossRef]
- Jourd’heuil, F.L.; Xu, H.; Reilly, T.; McKellar, K.; El Alaoui, C.; Steppich, J.; Liu, Y.F.; Zhao, W.; Ginnan, R.; Conti, D.; et al. The hemoglobin homolog cytoglobin in smooth muscle inhibits apoptosis and regulates vascular remodeling. Arter. Thromb. Vasc. Biol. 2017, 37, 1944–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frimat, M.; Boudhabhay, I.; Roumenina, L.T. Hemolysis derived products toxicity and endothelium: Model of the second hit. Toxins 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Weber, D.K.; Kutys, R.; Wight, T.N.; Virmani, R. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: Insights into plaque erosion. Arter. Thromb. Vasc. Biol. 2002, 22, 1642–1648. [Google Scholar] [CrossRef] [Green Version]
- Kolodgie, F.D.; Burke, A.P.; Wight, T.N.; Virmani, R. The accumulation of specific types of proteoglycans in eroded plaques: A role in coronary thrombosis in the absence of rupture. Curr. Opin. Lipidol. 2004, 15, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Davis, H.R.; Arbustini, E.; Virmani, R. Sex differences in coronary artery disease: Pathological observations. Atherosclerosis 2015, 239, 260–267. [Google Scholar] [CrossRef]
- Tarhouni, K.; Freidja, M.L.; Guihot, A.L.; Vessieres, E.; Grimaud, L.; Toutain, B.; Lenfant, F.; Arnal, J.F.; Loufrani, L.; Henrion, D. Role of estrogens and age in flow-mediated outward remodeling of rat mesenteric resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H504–H514. [Google Scholar] [CrossRef] [Green Version]
- de Jager, S.C.A.; Meeuwsen, J.A.L.; van Pijpen, F.M.; Zoet, G.A.; Barendrecht, A.D.; Franx, A.; Pasterkamp, G.; van Rijn, B.B.; Goumans, M.J.; den Ruijter, H.M. Preeclampsia and coronary plaque erosion: Manifestations of endothelial dysfunction resulting in cardiovascular events in women. Eur. J. Pharmacol. 2017, 816, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Jeney, V.; Balla, G.; Balla, J. Red blood cell, hemoglobin and heme in the progression of atherosclerosis. Front. Physiol. 2014, 5, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahid, M.; Mangin, P.; Loyau, S.; Hechler, B.; Billiald, P.; Gachet, C.; Jandrot-Perrus, M. The future of glycoprotein VI as an antithrombotic target. J. Thromb. Haemost. 2012, 10, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Mackman, N. Tissue factor and atherothrombosis. J. Atheroscler. Thromb. 2015, 22, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, J.; Davies, M.J. Mechanisms of progression in native coronary artery disease: Role of healed plaque disruption. Heart 1999, 82, 265–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virmani, R.; Burke, A.P.; Farb, A. Plaque rupture and plaque erosion. Thromb. Haemost. 1999, 82, 1–3. [Google Scholar] [PubMed] [Green Version]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Morbini, P.; D’Armini, A.M.; Repetto, A.; Minzioni, G.; Piovella, F.; Vigano, M.; Tavazzi, L. Plaque composition in plexogenic and thromboembolic pulmonary hypertension: The critical role of thrombotic material in pultaceous core formation. Heart 2002, 88, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Michel, J.B.; Martin-Ventura, J.L.; Nicoletti, A.; Ho-Tin-Noe, B. Pathology of human plaque vulnerability: Mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 2014, 234, 311–319. [Google Scholar] [CrossRef]
- Michel, J.B.; Virmani, R.; Arbustini, E.; Pasterkamp, G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur. Heart J. 2011, 32, 1977–1985, 1985a, 1985b, 1985c. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.L.; Rawla, P. Hemochromatosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Diez-Lopez, C.; Comin-Colet, J.; Gonzalez-Costello, J. Iron overload cardiomyopathy: From diagnosis to management. Curr. Opin. Cardiol. 2018, 33, 334–340. [Google Scholar] [CrossRef]
- Ellervik, C.; Tybjaerg-Hansen, A.; Grande, P.; Appleyard, M.; Nordestgaard, B.G. Hereditary hemochromatosis and risk of ischemic heart disease: A prospective study and a case-control study. Circulation 2005, 112, 185–193. [Google Scholar] [CrossRef]
- van der, A.D.; Rovers, M.M.; Grobbee, D.E.; Marx, J.J.; Waalen, J.; Ellervik, C.; Nordestgaard, B.G.; Olynyk, J.K.; Mills, P.R.; Shepherd, J.; et al. Mutations in the HFE gene and cardiovascular disease risk: An individual patient data meta-analysis of 53 880 subjects. Circ. Cardiovasc. Genet. 2008, 1, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, J.L. Do hemochromatosis mutations protect against iron-mediated atherogenesis? Circ. Cardiovasc. Genet. 2009, 2, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Meguro, R.; Asano, Y.; Odagiri, S.; Li, C.; Iwatsuki, H.; Shoumura, K. Nonheme-iron histochemistry for light and electron microscopy: A historical, theoretical and technical review. Arch. Histol. Cytol. 2007, 70, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell. Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Anitschkow, N.; Chalatow, S. Ueber experimentelle cholesterinsteatose und ihre bedeutungfur die entstchung einiger pathologischer prozesse. Zentralbl. Allg. Pathol. 1313, 24, 1–9. [Google Scholar]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercellotti, G.M. Hemin: A possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arter. Thromb. 1991, 11, 1700–1711. [Google Scholar] [CrossRef] [Green Version]
- Balla, G.; Vercellotti, G.M.; Muller-Eberhard, U.; Eaton, J.; Jacob, H.S. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab. Invest. 1991, 64, 648–655. [Google Scholar]
- Ho-Tin-Noe, B.; Vo, S.; Bayles, R.; Ferriere, S.; Ladjal, H.; Toumi, S.; Deschildre, C.; Ollivier, V.; Michel, J.B. Cholesterol crystallization in human atherosclerosis is triggered in smooth muscle cells during the transition from fatty streak to fibroatheroma. J. Pathol. 2017, 241, 671–682. [Google Scholar] [CrossRef]
- Stadler, N.; Lindner, R.A.; Davies, M.J. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: Evidence for the presence of elevated levels of iron and copper. Arter. Thromb. Vasc. Biol. 2004, 24, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Kisugi, R. Mechanisms of LDL oxidation. Clin. Chim. Acta 2010, 411, 1875–1882. [Google Scholar] [CrossRef]
- Gall, T.; Balla, G.; Balla, J. Heme, heme oxygenase, and endoplasmic reticulum stress-a new insight into the pathophysiology of vascular diseases. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.; Pratt, D.A. The chemical basis of ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef]
- Hirschhorn, T.; Stockwell, B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Sampilvanjil, A.; Karasawa, T.; Yamada, N.; Komada, T.; Higashi, T.; Baatarjav, C.; Watanabe, S.; Kamata, R.; Ohno, N.; Takahashi, M. Cigarette smoke extract induces ferroptosis in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H508–H518. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.J.; Zhang, D.; Wu, Y.; Jia, Q.H.; Zhang, L.; Li, Y.X.; Yang, Y.F.; Wang, H.; Wu, C.T.; Wang, L.S. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem. Biophys. Res. Commun. 2019, 515, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Patsch, C.; Challet-Meylan, L.; Thoma, E.C.; Urich, E.; Heckel, T.; O’Sullivan, J.F.; Grainger, S.J.; Kapp, F.G.; Sun, L.; Christensen, K.; et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell. Biol. 2015, 17, 994–1003. [Google Scholar] [CrossRef]
- Ho-Tin-Noe, B.; Michel, J.B. Initiation of angiogenesis in atherosclerosis: Smooth muscle cells as mediators of the angiogenic response to atheroma formation. Trends Cardiovasc. Med. 2011, 21, 183–187. [Google Scholar] [CrossRef]
- Le Dall, J.; Ho-Tin-Noe, B.; Louedec, L.; Meilhac, O.; Roncal, C.; Carmeliet, P.; Germain, S.; Michel, J.B.; Houard, X. Immaturity of microvessels in haemorrhagic plaques is associated with proteolytic degradation of angiogenic factors. Cardiovasc. Res. 2009. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, A.; Houard, X.; Loyau, S.; Philippe, M.; Sebbag, U.; Meilhac, O.; Michel, J.B. Topology of protease activities reflects atherothrombotic plaque complexity. Atherosclerosis 2007, 191, 1–10. [Google Scholar] [CrossRef]
- Xiao, X.; Saha, P.; Yeoh, B.S.; Hipp, J.A.; Singh, V.; Vijay-Kumar, M. Myeloperoxidase deficiency attenuates systemic and dietary iron-induced adverse effects. J. Nutr. Biochem. 2018, 62, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Zarins, C.; Giddens, D.P.; Ku, D.N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch. Pathol. Lab. Med. 1988, 112, 1018–1031. [Google Scholar] [PubMed]
- Bassiouny, H.S.; Zarins, C.K.; Kadowaki, M.H.; Glagov, S. Hemodynamic stress and experimental aortoiliac atherosclerosis. J. Vasc. Surg. 1994, 19, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, A.; Houard, X.; Philippe, M.; Ollivier, V.; Sebbag, U.; Meilhac, O.; Michel, J.B. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J. Leukoc. Biol. 2007, 82, 1420–1429. [Google Scholar] [CrossRef]
- Nakazawa, G.; Otsuka, F.; Nakano, M.; Vorpahl, M.; Yazdani, S.K.; Ladich, E.; Kolodgie, F.D.; Finn, A.V.; Virmani, R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. 2011, 57, 1314–1322. [Google Scholar] [CrossRef] [Green Version]
- Terzian, Z.; Gasser, T.C.; Blackwell, F.; Hyafil, F.; Louedec, L.; Deschildre, C.; Ghodbane, W.; Dorent, R.; Nicoletti, A.; Morvan, M.; et al. Peristrut microhemorrhages: A possible cause of in-stent neoatherosclerosis? Cardiovasc. Pathol. 2017, 26, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, F.; Nakano, M.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathologic etiologies of late and very late stent thrombosis following first-generation drug-eluting stent placement. Thrombosis 2012, 2012, 608593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfonso, F.; Fernandez-Vina, F.; Medina, M.; Hernandez, R. Neoatherosclerosis: The missing link between very late stent thrombosis and very late in-stent restenosis. J. Am. Coll. Cardiol. 2013, 61, e155. [Google Scholar] [CrossRef]
- Zhang, Y.; Cliff, W.J.; Schoefl, G.I.; Higgins, G. Plasma protein insudation as an index of early coronary atherogenesis. Am. J. Pathol. 1993, 143, 496–506. [Google Scholar]
- Rokitansky, C.V. A Manual of Pathological Anatomy; Sydenham Society: London, UK, 1855. [Google Scholar]
- Duguid, J.B. The thrombogenic hypothesis and its implications. Postgrad. Med. J. 1960, 36, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Chandler, A.B.; Hand, R.A. Phagocytized platelets: A source of lipids in human thrombi and atherosclerotic plaques. Science 1961, 134, 946–947. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; DiDonato, J.A.; Levison, B.S.; Schmitt, D.; Li, L.; Wu, Y.; Buffa, J.; Kim, T.; Gerstenecker, G.S.; Gu, X.; et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014, 20, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiDonato, J.A.; Huang, Y.; Aulak, K.S.; Even-Or, O.; Gerstenecker, G.; Gogonea, V.; Wu, Y.; Fox, P.L.; Tang, W.H.; Plow, E.F.; et al. Function and distribution of apolipoprotein A1 in the artery wall are markedly distinct from those in plasma. Circulation 2013, 128, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.B.; Martin-Ventura, J.L.; Egido, J.; Sakalihasan, N.; Treska, V.; Lindholt, J.; Allaire, E.; Thorsteinsdottir, U.; Cockerill, G.; Swedenborg, J.; et al. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc. Res. 2011, 90, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Houard, X.; Leclercq, A.; Fontaine, V.; Coutard, M.; Martin-Ventura, J.L.; Ho-Tin-Noe, B.; Touat, Z.; Meilhac, O.; Michel, J.B. Retention and activation of blood-borne proteases in the arterial wall implications for atherothrombosis. J. Am. Coll. Cardiol. 2006, 48, A3–A9. [Google Scholar] [CrossRef] [Green Version]
- Delbosc, S.; Diallo, D.; Dejouvencel, T.; Lamiral, Z.; Louedec, L.; Martin-Ventura, J.L.; Rossignol, P.; Leseche, G.; Michel, J.B.; Meilhac, O. Impaired high-density lipoprotein anti-oxidant capacity in human abdominal aortic aneurysm. Cardiovasc. Res. 2013, 100, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Dutertre, C.A.; Clement, M.; Morvan, M.; Schakel, K.; Castier, Y.; Alsac, J.M.; Michel, J.B.; Nicoletti, A. Deciphering the stromal and hematopoietic cell network of the adventitia from non-aneurysmal and aneurysmal human aorta. PLoS ONE 2014, 9, e89983. [Google Scholar] [CrossRef]
- Clement, M.; Guedj, K.; Andreata, F.; Morvan, M.; Bey, L.; Khallou-Laschet, J.; Gaston, A.T.; Delbosc, S.; Alsac, J.M.; Bruneval, P.; et al. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 2015, 131, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Pinna, R.; Lindholt, J.S.; Madrigal-Matute, J.; Blanco-Colio, L.M.; Esteban-Salan, M.; Torres-Fonseca, M.M.; Lefebvre, T.; Delbosc, S.; Laustsen, J.; Driss, F.; et al. From tissue iron retention to low systemic haemoglobin levels, new pathophysiological biomarkers of human abdominal aortic aneurysm. Thromb. Haemost. 2014, 112, 87–95. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Michel, J.B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Primers 2018, 4, 34. [Google Scholar] [CrossRef]
- Martinez-Lopez, D.; Camafeita, E.; Cedo, L.; Roldan-Montero, R.; Jorge, I.; Garcia-Marques, F.; Gomez-Serrano, M.; Bonzon-Kulichenko, E.; Blanco-Vaca, F.; Blanco-Colio, L.M.; et al. APOA1 oxidation is associated to dysfunctional high-density lipoproteins in human abdominal aortic aneurysm. EBioMedicine 2019, 43, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Burillo, E.; Lindholt, J.S.; Molina-Sanchez, P.; Jorge, I.; Martinez-Pinna, R.; Blanco-Colio, L.M.; Tarin, C.; Torres-Fonseca, M.M.; Esteban, M.; Laustsen, J.; et al. ApoA-I/HDL-C levels are inversely associated with abdominal aortic aneurysm progression. Thromb. Haemost. 2015, 113, 1335–1346. [Google Scholar] [CrossRef]
- Nchimi, A.; Defawe, O.; Brisbois, D.; Broussaud, T.K.; Defraigne, J.O.; Magotteaux, P.; Massart, B.; Serfaty, J.M.; Houard, X.; Michel, J.B.; et al. MR imaging of iron phagocytosis in intraluminal thrombi of abdominal aortic aneurysms in humans. Radiology 2010, 254, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Nchimi, A.; Courtois, A.; El Hachemi, M.; Touat, Z.; Drion, P.; Withofs, N.; Warnock, G.; Bahri, M.A.; Dogne, J.M.; Cheramy-Bien, J.P.; et al. Multimodality imaging assessment of the deleterious role of the intraluminal thrombus on the growth of abdominal aortic aneurysm in a rat model. Eur. Radiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gomel, M.A.; Lee, R.; Grande-Allen, K.J. Comparing the role of mechanical forces in vascular and valvular calcification progression. Front. Cardiovasc. Med. 2018, 5, 197. [Google Scholar] [CrossRef]
- Voelkl, J.; Lang, F.; Eckardt, K.U.; Amann, K.; Kuro, O.M.; Pasch, A.; Pieske, B.; Alesutan, I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell. Mol. Life Sci. 2019, 76, 2077–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Liu, M.; Martin, C.; Sun, W. A deep learning approach to estimate stress distribution: A fast and accurate surrogate of finite-element analysis. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.Y.; Howarth, S.; Tang, T.; Graves, M.; Jean, U.K.-I.; Gillard, J.H. Does calcium deposition play a role in the stability of atheroma? Location may be the key. Cerebrovasc. Dis. 2007, 24, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Ports, T.A.; Yock, P.G. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation 1992, 86, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akahori, H.; Tsujino, T.; Masuyama, T.; Ishihara, M. Mechanisms of aortic stenosis. J. Cardiol. 2018, 71, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akahori, H.; Tsujino, T.; Naito, Y.; Matsumoto, M.; Lee-Kawabata, M.; Ohyanagi, M.; Mitsuno, M.; Miyamoto, Y.; Daimon, T.; Hao, H.; et al. Intraleaflet haemorrhage is associated with rapid progression of degenerative aortic valve stenosis. Eur. Heart J. 2011, 32, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morvan, M.; Arangalage, D.; Franck, G.; Perez, F.; Cattan-Levy, L.; Codogno, I.; Jacob-Lenet, M.P.; Deschildre, C.; Choqueux, C.; Even, G.; et al. Relationship of Iron Deposition to Calcium Deposition in Human Aortic Valve Leaflets. J. Am. Coll. Cardiol. 2019, 73, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Kawada, S.; Nagasawa, Y.; Kawabe, M.; Ohyama, H.; Kida, A.; Kato-Kogoe, N.; Nanami, M.; Hasuike, Y.; Kuragano, T.; Kishimoto, H.; et al. Iron-induced calcification in human aortic vascular smooth muscle cells through interleukin-24 (IL-24), with/without TNF-alpha. Sci. Rep. 2018, 8, 658. [Google Scholar] [CrossRef]
- Tziakas, D.N.; Chalikias, G.; Pavlaki, M.; Kareli, D.; Gogiraju, R.; Hubert, A.; Bohm, E.; Stamoulis, P.; Drosos, I.; Kikas, P.; et al. Lysed erythrocyte membranes promote vascular calcification. Circulation 2019, 139, 2032–2048. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Suhara, T.; Baba, Y.; Kawasaki, N.K.; Higa, J.K.; Matsui, T. Pathological roles of iron in cardiovascular disease. Curr. Drug. Targets 2018, 19, 1068–1076. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Malyszko, J.; Ardehali, H.; Koc-Zorawska, E.; Banasiak, W.; von Haehling, S.; Macdougall, I.C.; Weiss, G.; McMurray, J.J.; Anker, S.D.; et al. Iron status in patients with chronic heart failure. Eur. Heart J. 2013, 34, 827–834. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef]
- Grote Beverborg, N.; van Veldhuisen, D.J.; van der Meer, P. Anemia in Heart Failure: Still Relevant? JACC Heart Fail. 2018, 6, 201–208. [Google Scholar] [CrossRef]
- Carberry, J.; Carrick, D.; Haig, C.; Ahmed, N.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; et al. Persistent iron within the infarct core after ST-segment elevation myocardial infarction: Implications for left ventricular remodeling and health outcomes. JACC Cardiovasc. Imaging 2018, 11, 1248–1256. [Google Scholar] [CrossRef]
- Baba, Y.; Higa, J.K.; Shimada, B.K.; Horiuchi, K.M.; Suhara, T.; Kobayashi, M.; Woo, J.D.; Aoyagi, H.; Marh, K.S.; Kitaoka, H.; et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H659–H668. [Google Scholar] [CrossRef] [Green Version]
- Hugelshofer, M.; Buzzi, R.M.; Schaer, C.A.; Richter, H.; Akeret, K.; Anagnostakou, V.; Mahmoudi, L.; Vaccani, R.; Vallelian, F.; Deuel, J.W.; et al. Haptoglobin administration into the subarachnoid space prevents hemoglobin-induced cerebral vasospasm. J. Clin. Invest. 2019, 129, 5219–5235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquez-Ropero, M.; Benito, E.; Plaza-Zabala, A.; Sierra, A. Microglial corpse clearance: Lessons from macrophages. Front. Immunol. 2020, 11, 506. [Google Scholar] [CrossRef] [Green Version]
- Van Acker, Z.P.; Luyckx, E.; Dewilde, S. Neuroglobin expression in the brain: A story of tissue homeostasis preservation. Mol. Neurobiol. 2019, 56, 2101–2122. [Google Scholar] [CrossRef] [PubMed]
- Gregson, J.M.; Freitag, D.F.; Surendran, P.; Stitziel, N.O.; Chowdhury, R.; Burgess, S.; Kaptoge, S.; Gao, P.; Staley, J.R.; Willeit, P.; et al. Genetic invalidation of Lp-PLA2 as a therapeutic target: Large-scale study of five functional Lp-PLA2-lowering alleles. Eur. J. Prev. Cardiol. 2017, 24, 492–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zille, M.; Kumar, A.; Kundu, N.; Bourassa, M.W.; Wong, V.S.C.; Willis, D.; Karuppagounder, S.S.; Ratan, R.R. Ferroptosis in neurons and cancer cells is similar but differentially regulated by histone deacetylase inhibitors. eNeuro 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Ste Marie, E.J.; et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 2019, 177, 1262–1279.e1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, S.; Vranckx, R.; Huisse, M.G.; Michel, J.B.; Meilhac, O. The phosphatidylserine receptor mediates phagocytosis by vascular smooth muscle cells. J. Pathol. 2007, 212, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Mesa, K.R.; Rompolas, P.; Zito, G.; Myung, P.; Sun, T.Y.; Brown, S.; Gonzalez, D.G.; Blagoev, K.B.; Haberman, A.M.; Greco, V. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature 2015, 522, 94–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeberg, J.C.; Loibl, M.; Moser, F.; Schwegler, M.; Buttner-Herold, M.; Daniel, C.; Engel, F.B.; Hartmann, A.; Schlotzer-Schrehardt, U.; Goppelt-Struebe, M.; et al. Non-professional phagocytosis: A general feature of normal tissue cells. Sci. Rep. 2019, 9, 11875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morioka, S.; Maueroder, C.; Ravichandran, K.S. Living on the edge: Efferocytosis at the interface of homeostasis and pathology. Immunity 2019, 50, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Gibson, D.F.; Schwartz, S.M.; Tait, J.F. Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ. Res. 1995, 77, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.F.; Stodkilde, K.; Saederup, K.L.; Kuhlee, A.; Raunser, S.; Graversen, J.H.; Moestrup, S.K. Haptoglobin. Antioxid. Redox Signal. 2017, 26, 814–831. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, L.; Pedersen, S.L.; Toe, Q.K.; West, L.E.; Mumby, S.; Casbolt, H.; Issitt, T.; Garfield, B.; Lawrie, A.; Wort, S.J.; et al. The hepcidin/ferroportin axis modulates proliferation of pulmonary artery smooth muscle cells. Sci. Rep. 2018, 8, 12972. [Google Scholar] [CrossRef]
- Vanacore, R.; Eskew, J.D.; Sung, L.; Davis, T.; Smith, A. Safe coordinated trafficking of heme and iron with copper maintain cell homeostasis: Modules from the hemopexin system. Biometals 2019, 32, 355–367. [Google Scholar] [CrossRef]
- Etique, N.; Verzeaux, L.; Dedieu, S.; Emonard, H. LRP-1: A checkpoint for the extracellular matrix proteolysis. Biomed. Res. Int. 2013, 2013, 152163. [Google Scholar] [CrossRef] [Green Version]
- Fibach, E.; Rachmilewitz, E.A. Iron overload in hematological disorders. Presse. Med. 2017, 46, e296–e305. [Google Scholar] [CrossRef]
- Bennett, C.; Mohammed, F.; Alvarez-Ciara, A.; Nguyen, M.A.; Dietrich, W.D.; Rajguru, S.M.; Streit, W.J.; Prasad, A. Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamine as an iron chelator on acute foreign body response. Biomaterials 2019, 188, 144–159. [Google Scholar] [CrossRef]
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative stress in human atherothrombosis: Sources, markers and therapeutic targets. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [Green Version]
- Martin-Ventura, J.L.; Madrigal-Matute, J.; Martinez-Pinna, R.; Ramos-Mozo, P.; Blanco-Colio, L.M.; Moreno, J.A.; Tarin, C.; Burillo, E.; Fernandez-Garcia, C.E.; Egido, J.; et al. Erythrocytes, leukocytes and platelets as a source of oxidative stress in chronic vascular diseases: Detoxifying mechanisms and potential therapeutic options. Thromb. Haemost. 2012, 108, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagababu, E.; Mohanty, J.G.; Bhamidipaty, S.; Ostera, G.R.; Rifkind, J.M. Role of the membrane in the formation of heme degradation products in red blood cells. Life Sci. 2010, 86, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Pinna, R.; Burillo, E.; Madrigal-Matute, J.; Lopez, J.A.; Camafeita, E.; Torres-Fonseca, M.M.; Llamas-Granda, P.; Egido, J.; Michel, J.B.; Blanco-Colio, L.M.; et al. Label-free proteomic analysis of red blood cell membrane fractions from abdominal aortic aneurysm patients. Proteomics Clin. Appl. 2014, 8, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.E.; Bonovas, S.; Travlou, A.; Sitaras, N.M. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid. Redox Signal. 2006, 8, 1205–1216. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J.; et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef] [Green Version]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Schnabel, R.; Genth-Zotz, S.; Torzewski, M.; Lackner, K.; Munzel, T.; Blankenberg, S.; AtheroGene, I. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am. J. Cardiol. 2007, 99, 808–812. [Google Scholar] [CrossRef]
- Ramos-Mozo, P.; Madrigal-Matute, J.; Martinez-Pinna, R.; Blanco-Colio, L.M.; Lopez, J.A.; Camafeita, E.; Meilhac, O.; Michel, J.B.; Aparicio, C.; Vega de Ceniga, M.; et al. Proteomic analysis of polymorphonuclear neutrophils identifies catalase as a novel biomarker of abdominal aortic aneurysm: Potential implication of oxidative stress in abdominal aortic aneurysm progression. Arter. Thromb. Vasc. Biol. 2011, 31, 3011–3019. [Google Scholar] [CrossRef] [Green Version]
- Vardi, M.; Levy, N.S.; Levy, A.P. Vitamin E in the prevention of cardiovascular disease: The importance of proper patient selection. J. Lipid Res. 2013, 54, 2307–2314. [Google Scholar] [CrossRef] [Green Version]
- Bamm, V.V.; Tsemakhovich, V.A.; Shaklai, M.; Shaklai, N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry 2004, 43, 3899–3906. [Google Scholar] [CrossRef]
- Burillo, E.; Tarin, C.; Torres-Fonseca, M.M.; Fernandez-Garcia, C.E.; Martinez-Pinna, R.; Martinez-Lopez, D.; Llamas-Granda, P.; Camafeita, E.; Lopez, J.A.; Vega de Ceniga, M.; et al. Paraoxonase-1 overexpression prevents experimental abdominal aortic aneurysm progression. Clin. Sci. 2016, 130, 1027–1038. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, J.-B.; Martin-Ventura, J.L. Red Blood Cells and Hemoglobin in Human Atherosclerosis and Related Arterial Diseases. Int. J. Mol. Sci. 2020, 21, 6756. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186756
Michel J-B, Martin-Ventura JL. Red Blood Cells and Hemoglobin in Human Atherosclerosis and Related Arterial Diseases. International Journal of Molecular Sciences. 2020; 21(18):6756. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186756
Chicago/Turabian StyleMichel, Jean-Baptiste, and José Luis Martin-Ventura. 2020. "Red Blood Cells and Hemoglobin in Human Atherosclerosis and Related Arterial Diseases" International Journal of Molecular Sciences 21, no. 18: 6756. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186756