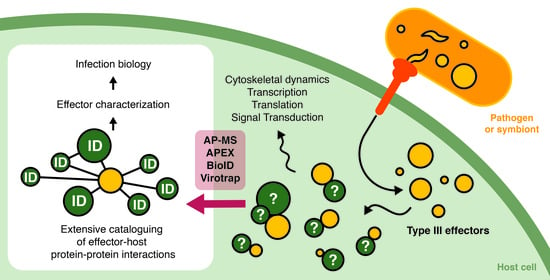
Keeping in Touch with Type-III Secretion System Effectors: Mass Spectrometry-Based Proteomics to Study Effector–Host Protein–Protein Interactions
Abstract
:1. Introduction
1.1. Effectors and Their Role in Establishing Host Interactions
1.2. Delivery of Protein Effectors by Specialized Secretion Systems
1.3. Effectors: Their Origin and Mechanisms of Action
1.4. The Past and Future Ways of Studying Effector Interactomes
2. Methods to Elucidate Effector–Host Protein–Protein Interactions
2.1. Affinity- or Immune-Based Purification of Effector Interactomes in a Host Context
2.2. Proximity-Dependent Labeling Approaches
2.3. Virotrap
3. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP | Affinity purification |
| BioID | Biotin identification |
| CBD | Chaperone-binding domain |
| EHEC | Enterohaemorrhagic Escherichia coli |
| EH-PPI | Effector–host protein–protein interaction |
| EPEC | Enteropathogenic Escherichia coli |
| ETI | Effector-triggered immunity |
| GFP | Green fluorescent protein |
| HIV | Human immunodeficiency virus |
| Hop | Hrp-dependent outer protein |
| Inc | Inclusion |
| IP | Immunopurification |
| LFQ | Label-free quantification |
| LPS | Lipopolysaccharide |
| MAMP | Microbe-associated molecular pattern |
| MS | Mass spectrometry |
| MTI | MAMP-triggered immunity |
| NGS | Next-generation sequencing |
| OSBP | Oxysterol-binding protein |
| PAI | Pathogenicity island |
| PAMP | Pathogen-associated molecular pattern |
| PBL | Protein biotin ligase |
| PDBPL | Proximity-dependent biotin ligationProximity labeling |
| PRR | Pattern recognition receptor |
| Rip | Ralstonia injected protein |
| Sip | Salmonella invasion protein |
| SNX | Sorting nexin |
| Sop | Salmonella outer protein |
| SPI | Salmonella pathogenicity island |
| STm | Salmonella enterica serovar Typhimurium |
| Sse | Type-III secretion system effector protein |
| Ssp | Stringent starvation protein |
| T3E | Type-III effector |
| T3SS | Type-III secretion system |
| T4SS | Type IV secretion system |
| T6SS | Type VI secretion system |
| TAP | Tandem affinity purification |
| Tat | Twin-arginine translocation |
| TRIM | Tripartite motif containing |
| VLP | Virus-like particle |
| Y2H | Yeast two-hybrid |
| Y2H-sequencing | Y2H-seq |
References
- Larochelle, M.; Bergeron, D.; Arcand, B.; Bachand, F. Proximity-dependent biotinylation mediated by TurboID to identify protein-protein interaction networks in yeast. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nürnberger, T.; Kemmerling, B. Pathogen-Associated Molecular Patterns (PAMP) and PAMP-Triggered Immunity. In Annual Plant Reviews; Roberts, J.A., Ed.; Wiley: Hoboken, NJ, USA, 2018; Volume 34. [Google Scholar] [CrossRef]
- Zipfel, C. Early molecular events in PAMP-triggered immunity. Curr. Opin. Plant Biol. 2009, 12, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Rajamuthiah, R.; Mylonakis, E. Effector triggered immunity activation of innate immunity in metazoans by bacterial effectors. Virulence 2014, 5, 697–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, L.M.; Paquette, N.; Boyer, L. Effector-triggered versus pattern-triggered immunity: How animals sense pathogens. Nat. Rev. Immunol. 2013, 13, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Lopes Fischer, N.; Naseer, N.; Shin, S.; Brodsky, I.E. Effector-triggered immunity and pathogen sensing in metazoans. Nat. Microbiol. 2020, 5, 14–26. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Vasse, J.; Genin, S.; Frey, P.; Boucher, C.; Brito, B. The hrpB and hrpG regulatory genes of Ralstonia solanacearum are required for different stages of the tomato root infection process. Mol. Plant. Microbe. Interact. 2000, 13, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Bugalhão, J.N.; Mota, L.J. The multiple functions of the numerous Chlamydia trachomatis secreted proteins: The tip of the iceberg. Microb. Cell (Graz Austria) 2019, 6, 414–449. [Google Scholar] [CrossRef]
- Buttner, D. Protein Export According to Schedule: Architecture, Assembly, and Regulation of Type III Secretion Systems from Plant- and Animal-Pathogenic Bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 262–310. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; McCoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.J.; Finlay, B.B. Assembly, structure, function and regulation of type III secretion systems. Nat. Rev. Microbiol. 2017, 15, 323–337. [Google Scholar] [CrossRef]
- Troisfontaines, P.; Cornelis, G.R. Type III Secretion: More systems than you think. Physiology 2005, 20, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Radics, J.; Königsmaier, L.; Marlovits, T.C. Structure of a pathogenic type 3 secretion system in action. Nat. Struct. Mol. Biol. 2014, 21, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, T.C.; Kubori, T. Structural Insights into the Assembly of the Type III Secretion Needle Complex Thomas. Science 2008, 15, 1203–1214. [Google Scholar] [CrossRef]
- Stavrinides, J.; Ma, W.; Guttman, D.S. Terminal Reassortment Drives the Quantum Evolution of Type III Effectors in Bacterial Pathogens. PLoS Pathog. 2006, 2, e104. [Google Scholar] [CrossRef] [PubMed]
- Hensel, M. Salmonella Pathogenicity Island 2. Mol. Microbiol. 2000, 36, 1015–1023. [Google Scholar] [CrossRef]
- Cho, H.; Song, E.-S.; Heu, S.; Baek, J.; Lee, Y.K.; Lee, S.; Lee, S.-W.; Park, D.S.; Lee, T.-H.; Kim, J.-G.; et al. Prediction of Host-Specific Genes by Pan-Genome Analyses of the Korean Ralstonia solanacearum Species Complex. Front. Microbiol. 2019, 10, 506. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Shirota, M.; Zhang, Y.; Kiba, A.; Hikichi, Y.; Ohnishi, K. Involvement of HLK effectors in Ralstonia solanacearum disease development in tomato. J. Gen. Plant. Pathol. 2014, 80, 79–84. [Google Scholar] [CrossRef]
- Hueck, C.J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 1998, 62, 379–433. [Google Scholar] [CrossRef] [Green Version]
- McDermott, J.E.; Corrigan, A.; Peterson, E.; Oehmen, C.; Niemann, G.; Cambronne, E.D.; Sharp, D.; Adkins, J.N.; Samudrala, R.; Heffron, F. Minireview: Computational prediction of type III and IV secreted effectors in gram-negative bacteria. Infect. Immun. 2011, 79, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Samudrala, R.; Heffron, F.; McDermott, J.E. Accurate prediction of secreted substrates and identification of a conserved putative secretion signal for type iii secretion systems. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef] [Green Version]
- Arnold, R.; Brandmaier, S.; Kleine, F.; Tischler, P.; Heinz, E.; Behrens, S.; Niinikoski, A.; Mewes, H.W.; Horn, M.; Rattei, T. Sequence-Based Prediction of Type III Secreted Proteins. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef]
- Birtalan, S.C.; Phillips, R.M.; Ghosh, P. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol. Cell 2002, 9, 971–980. [Google Scholar] [CrossRef]
- Feldman, M.F.; Cornelis, G.R. The multitalented type III chaperones: All you can do with 15 kDa. FEMS Microbiol. Lett. 2003, 219, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Wagner, S.; Grin, I.; Malmsheimer, S.; Singh, N.; Torres-Vargas, C.E.; Westerhausen, S. Bacterial type III secretion systems: A complex device for the delivery of bacterial effector proteins into eukaryotic host cells. FEMS Microbiol. Lett. 2018, 365, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Qaidi, S.; Scott, N.E.; Hays, M.P.; Geisbrecht, B.V.; Watkins, S.; Hardwidge, P.R. An intra-bacterial activity for a T3SS effector. Sci. Rep. 2020, 10, 1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niebuhr, K.; Giuriato, S.; Pedron, T.; Philpott, D.J.; Gaits, F.; Sable, J.; Sheetz, M.P.; Parsot, C.; Sansonetti, P.J.; Payrastre, B. Conversion of PtdIns(4, 5)P2 into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J. 2002, 21, 5069–5078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, L.D.; Hueffer, K.; Wenk, M.R.; Galán, J.E. Salmonella Modulates Vesicular Traffic by Altering Phosphoinositide Metabolism. Science 2004, 304, 1805–1807. [Google Scholar] [CrossRef] [Green Version]
- Terebiznik, M.R.; Vieira, O.V.; Marcus, S.L.; Slade, A.; Yips, C.M.; Trimble, W.S.; Meyer, T.; Finlay, B.B.; Grinstein, S. Elimination of host cell Ptdlns(4, 5)P2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 2002, 4, 766–773. [Google Scholar] [CrossRef]
- Friebel, A.; Ilchmann, H.; Aepfelbacher, M.; Ehrbar, K.; Machleidt, W.; Hardt, W.D. SopE and SopE2 from Salmonella typhimurium Activate Different Sets of RhoGTPases of the Host Cell. J. Biol. Chem. 2001, 276, 34035–34040. [Google Scholar] [CrossRef] [Green Version]
- Stender, S.; Friebel, A.; Linder, S.; Rohde, M.; Mirold, S.; Hardt, W.D. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 2000, 36, 1206–1221. [Google Scholar] [CrossRef]
- Angot, A.; Peeters, N.; Lechner, E.; Vailleau, F.; Baud, C.; Gentzbittel, L.; Sartorel, E.; Genschik, P.; Boucher, C.; Genin, S. Ralstonia solanacearum requires F-box-like domain-containing type III effectors to promote disease on several host plants. Proc. Natl. Acad. Sci. USA 2006, 103, 14620–14625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teulet, A.; Busset, N.; Fardoux, J.; Gully, D.; Chaintreuil, C.; Cartieaux, F.; Jauneau, A.; Comorge, V.; Okazaki, S.; Kaneko, T.; et al. The rhizobial type III effector ErnA confers the ability to form nodules in legumes. Proc. Natl. Acad. Sci. USA 2019, 116, 21758–21768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, N.S.; Burgas, M.T. Bacteria Use Structural Imperfect Mimicry To Hijack The Host Interactome. BioRxiv 2020, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Fields, S.; Song, O. A novel genetic system to detect protein–protein interactions. Nature 1989, 340, 245–246. [Google Scholar] [CrossRef]
- Mukhtar, M.S.; Carvunis, A.; Dreze, M.; Epple, P.; Steinbrenner, J.; Moore, J.; Tasan, M.; Galli, M.; Hao, T.; Nishimura, M.T.; et al. Plant Immune System Network. Science 2011, 333, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Blasche, S.; Arens, S.; Ceol, A.; Siszler, G.; Schmid, M.A.; Häuser, R.; Schwarz, F.; Wuchty, S.; Aloy, P.; Uetz, P.; et al. The EHEC-host interactome reveals novel targets for the translocated intimin receptor. Sci. Rep. 2014, 4, 22–26. [Google Scholar] [CrossRef]
- Weßling, R.; Epple, P.; Altmann, S.; He, Y.; Yang, L.; Henz, R.; Mcdonald, N.; Wiley, K.; Bader, K.C.; Gläßer, C.; et al. Convergent targeting of a common host protein-network bypathogen effectors from three kingdoms of life. Cell Host Microbe 2014, 16, 364–375. [Google Scholar] [CrossRef] [Green Version]
- Erffelinck, M.L.; Ribeiro, B.; Perassolo, M.; Pauwels, L.; Pollier, J.; Storme, V.; Goossens, A. A user-friendly platform for yeast two-hybrid library screening using next generation sequencing. PLoS ONE 2018, 13, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Wan, J.; Ford, R.; Gong, Y.; Fung, P.; Nahal, H.; Wang, P.W.; Desveaux, D.; Guttman, D.S. Quantitative Interactor Screening with next-generation Sequencing (QIS-Seq) identifies Arabidopsis thaliana MLO2 as a target of the Pseudomonas syringae type III effector HopZ2. BMC Genom. 2012, 13, 8. [Google Scholar] [CrossRef] [Green Version]
- González-Fuente, M.; Carrère, S.; Monachello, D.; Marsella, B.G.; Cazalé, A.-C.; Zischek, C.; Mitra, R.M.; Rezé, N.; Cottret, L.; Mukhtar, M.S.; et al. EffectorK, a comprehensive resource to mine for Ralstonia, Xanthomonas, and other published effector interactors in the Arabidopsis proteome. Mol. Plant. Pathol. 2020. [Google Scholar] [CrossRef]
- Brückner, A.; Polge, C.; Lentze, N.; Auerbach, D.; Schlattner, U. Yeast two-hybrid, a powerful tool for systems biology. Int. J. Mol. Sci. 2009, 10, 2763–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gingras, A.-C.; Gstaiger, M.; Raught, B.; Aebersold, R. Analysis of protein complexes using mass spectrometry. Nat. Rev. Mol. Cell Biol. 2007, 8, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; Ho, Y.; Gruhler, A.; Gruhler, A.; Heilbut, A.; Heilbut, A.; Bader, G.D.; Moore, L.; Moore, L.; Adams, S.L.; et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 2002, 415, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Huang, L. Cross-Linking Mass Spectrometry: An Emerging Technology for Interactomics and Structural Biology. Anal. Chem. 2018, 90, 144–165. [Google Scholar] [CrossRef]
- Ong, S.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable Isotope Labeling by Amino Acids in Cell Culture, SILAC, as a Simple and Accurate Approach to Expression Proteomics. Mol. Cell. Proteom. 2002, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Matthes, A.; Köhl, K.; Schulze, W.X. SILAC and Alternatives in Studying Cellular Proteomes of Plants. In Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC): Methods and Protocols; Warscheid, B., Ed.; Springer: New York, NY, USA, 2014; pp. 65–83. ISBN 978-1-4939-1142-4. [Google Scholar]
- Krogan, N.J.; Cagney, G.; Yu, H.; Zhong, G.; Guo, X.; Ignatchenko, A.; Peregrı, M.; Li, J.; Pu, S.; Datta, N.; et al. Global landscape of protein complexes in the yeast Saccharomyces Cerevisiae. Nature 2006, 440, 637–643. [Google Scholar] [CrossRef]
- Mirrashidi, K.M.; Elwell, C.A.; Verschueren, E.; Johnson, J.R.; Frando, A.; Von Dollen, J.; Rosenberg, O.; Gulbahce, N.; Jang, G.; Johnson, T.; et al. Global mapping of the inc-human interactome reveals that retromer restricts chlamydia infection. Cell Host Microbe 2015, 18, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Medina-Puche, L.; Tan, H.; Dogra, V.; Wu, M.; Rosas-Diaz, T.; Wang, L.; Ding, X.; Zhang, D.; Fu, X.; Kim, C.; et al. A Defense Pathway Linking Plasma Membrane and Chloroplasts and Co-opted by Pathogens. Cell 2020. [Google Scholar] [CrossRef]
- Elwell, C.A.; Czudnochowski, N.; von Dollen, J.; Johnson, J.R.; Nakagawa, R.; Mirrashidi, K.; Krogan, N.J.; Engel, J.N.; Rosenberg, O.S. Chlamydia interfere with an interaction between the mannose-6-phosphate receptor and sorting nexins to counteract host restriction. elife 2017, 6, e22709. [Google Scholar] [CrossRef]
- Sontag, R.L.; Nakayasu, E.S.; Brown, R.N.; Niemann, G.S.; Sydor, M.A.; Sanchez, O.; Ansong, C.; Lu, S.-Y.; Choi, H.; Valleau, D.; et al. Identification of Novel Host Interactors of Effectors Secreted by Salmonella and Citrobacter. mSystems 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- D’Costa, V.M.; Coyaud, E.; Boddy, K.C.; Laurent, E.M.N.; St-Germain, J.; Li, T.; Grinstein, S.; Raught, B.; Brumell, J.H. BioID screen of Salmonella type 3 secreted effectors reveals host factors involved in vacuole positioning and stability during infection. Nat. Microbiol. 2019, 4, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Reinicke, A.T.; Hutchinson, J.L.; Magee, A.I.; Mastroeni, P.; Trowsdale, J.; Kelly, A.P. A Salmonella typhimurium effector Protein SifA is modified by host cell prenylation and S-acylation machinery. J. Biol. Chem. 2005, 280, 14620–14627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knodler, L.A.; Vallance, B.A.; Hensel, M.; Jäckel, D.; Finlay, B.B.; Steele-Mortimer, O. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes. Mol. Microbiol. 2003, 49, 685–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walch, P.; Selkrig, J.; Knodler, L.A.; Rettel, M.; Stein, F.; Scholzen, K.; Geyer, M.; Rottner, K.; Steele-mortimer, O.; Savitski, M.M.; et al. Global mapping of Salmonella enterica-host protein-protein interactions during infection. bioRxiv 2020, 1–59. [Google Scholar] [CrossRef]
- Hurley, B.; Lee, D.; Mott, A.; Wilton, M.; Liu, J.; Liu, Y.C.; Angers, S.; Coaker, G.; Guttman, D.S.; Desveaux, D. The Pseudomonas syringae Type III Effector HopF2 Suppresses Arabidopsis Stomatal Immunity. PLoS ONE 2014, 9, e114921. [Google Scholar] [CrossRef] [Green Version]
- Üstün, S.; Sheikh, A.; Gimenez-Ibanez, S.; Jones, A.; Ntoukakis, V.; Börnke, F. The Proteasome Acts as a Hub for Plant Immunity and Is Targeted by Pseudomonas Type III Effectors. Plant. Physiol. 2016, 172, 1941–1958. [Google Scholar] [CrossRef] [Green Version]
- Sang, Y.; Wang, Y.; Ni, H.; Cazalé, A.-C.; She, Y.-M.; Peeters, N.; Macho, A.P. The Ralstonia solanacearum type III effector RipAY targets plant redox regulators to suppress immune responses. Mol. Plant. Pathol. 2018, 19, 129–142. [Google Scholar] [CrossRef]
- Li, W.; Yadeta, K.A.; Elmore, J.M.; Coaker, G. The Pseudomonas syringae effector HopQ1 promotes bacterial virulence and interacts with tomato 14-3-3 proteins in a phosphorylation-dependent manner. Plant. Physiol. 2013, 161, 2062–2074. [Google Scholar] [CrossRef] [Green Version]
- De La Mota-Peynado, A.; Lee, S.Y.-C.; Pierce, B.M.; Wani, P.; Singh, C.R.; Roelofs, J. The proteasome-associated protein Ecm29 inhibits proteasomal ATPase activity and in vivo protein degradation by the proteasome. J. Biol. Chem. 2013, 288, 29467–29481. [Google Scholar] [CrossRef] [Green Version]
- Auweter, S.D.; Bhavsar, A.P.; de Hoog, C.L.; Li, Y.; Chan, Y.A.; van der Heijden, J.; Lowden, M.J.; Coombes, B.K.; Rogers, L.D.; Stoynov, N.; et al. Quantitative Mass Spectrometry Catalogues Salmonella Pathogenicity Island-2 Effectors and Identifies Their Cognate Host Binding Partners. J. Biol. Chem. 2011, 286, 24023–24035. [Google Scholar] [CrossRef] [Green Version]
- Vogels, M.W.; van Balkom, B.W.M.; Heck, A.J.R.; de Haan, C.A.M.; Peter, J.M. Quantitative proteomic identification of host factors involved in the Salmonella typhimurium infection cycle. Proteomics 2011, 1–39. [Google Scholar] [CrossRef]
- Fiskin, E.; Bhogaraju, S.; Herhaus, L.; Kalayil, S.; Hahn, M.; Dikic, I. Structural basis for the recognition and degradation of host TRIM proteins by Salmonella effector SopA. Nat. Commun. 2017, 8, 14004. [Google Scholar] [CrossRef] [PubMed]
- Law, R.J.; Law, H.T.; Scurll, J.M.; Scholz, R.; Santos, A.S.; Shames, S.R.; Deng, W.; Croxen, M.A.; Li, Y.; de Hoog, C.L.; et al. Quantitative Mass Spectrometry Identifies Novel Host Binding Partners for Pathogenic Escherichia coli Type III Secretion System Effectors. J. Proteome Res. 2016, 15, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Shames, S.R.; Deng, W.; Guttman, J.A.; De Hoog, C.L.; Li, Y.; Hardwidge, P.R.; Sham, H.P.; Vallance, B.A.; Foster, L.J.; Finlay, B.B. The pathogenic E. coli type III effector EspZ interacts with host CD98 and facilitates host cell prosurvival signalling. Cell. Microbiol. 2010, 12, 1322–1339. [Google Scholar] [CrossRef] [PubMed]
- Shames, S.R.; Bhavsar, A.P.; Croxen, M.A.; Law, R.J.; Mak, S.H.C.; Deng, W.; Li, Y.; Bidshari, R.; de Hoog, C.L.; Foster, L.J.; et al. The pathogenic Escherichia coli type III secreted protease NleC degrades the host acetyltransferase p300. Cell. Microbiol. 2011, 13, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Zhang, K.; Andres, S.N.; Fang, Y.; Kaniuk, N.A.; Hannemann, M.; Brumell, J.H.; Foster, L.J.; Junop, M.S.; Coombes, B.K. Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Salmonella Required for Pathogenic Association with a Host. PLoS Pathog. 2010, 6, e1000751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi-Rhee, E.; Schulman, H.; Cronan, J.E. Promiscuous protein biotinylation by Escherichia coli biotin protein ligase. Protein Sci. 2004, 13, 3043–3050. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.I.; KC, B.; Zhu, W.; Motamedchaboki, K.; Doye, V.; Roux, K.J. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc. Natl. Acad. Sci. USA 2014, 111, E2453–E2461. [Google Scholar] [CrossRef] [Green Version]
- Khosh-Naucke, M.; Becker, J.; Mesén-Ramírez, P.; Kiani, P.; Birnbaum, J.; Fröhlke, U.; Jonscher, E.; Schlüter, H.; Spielmann, T. Identification of novel parasitophorous vacuole proteins in P. falciparum parasites using BioID. Int. J. Med. Microbiol. 2018, 308, 13–24. [Google Scholar] [CrossRef]
- Lambert, J.-P.; Tucholska, M.; Go, C.; Knight, J.D.R.; Gingras, A.-C. Proximity biotinylation and affinity purification are complementary approaches for the interactome mapping of chromatin-associated protein complexes. J. Proteom. 2015, 118, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.I.; Jensen, S.C.; Noble, K.A.; Kc, B.; Roux, K.H.; Motamedchaboki, K.; Roux, K.J. An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 2016, 27, 1188–1196. [Google Scholar] [CrossRef]
- Ramanathan, M.; Majzoub, K.; Rao, D.S.; Neela, P.H.; Zarnegar, B.J.; Mondal, S.; Roth, J.G.; Gai, H.; Kovalski, J.R.; Siprashvili, Z.; et al. RNA-protein interaction detection in living cells. Nat. Methods 2018, 15, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Branon, T.C.; Bosch, J.A.; Sanchez, A.D.; Udeshi, N.D.; Svinkina, T.; Carr, S.A.; Feldman, J.L.; Perrimon, N.; Ting, A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018, 36, 880–898. [Google Scholar] [CrossRef] [PubMed]
- Remnant, L.; Booth, D.G.; Vargiu, G.; Spanos, C.; Kerr, A.R.W.; Earnshaw, W.C. In vitro BioID: Mapping the CENP-A microenvironment with high temporal and spatial resolution. Mol. Biol. Cell 2019, 30, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhou, Z.; Luo, W.; Fang, M.; Li, M.; Li, H. Screening of Proximal and Interacting Proteins in Rice Protoplasts by Proximity-Dependent Biotinylation. Front. Plant. Sci. 2017, 8, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Youn, J.-Y.; Gingras, A.-C.; Subramaniam, R.; Desveaux, D. In planta proximity dependent biotin identification (BioID). Sci. Rep. 2018, 8, 9212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, K.J. Marked by association: Techniques for proximity-dependent labeling of proteins in eukaryotic cells. Cell. Mol. Life Sci. 2013, 70, 3657–3664. [Google Scholar] [CrossRef]
- Conlan, B.; Stoll, T.; Gorman, J.J.; Saur, I.; Rathjen, J.P. Development of a Rapid in planta BioID System as a Probe for Plasma Membrane-Associated Immunity Proteins. Front. Plant. Sci. 2018, 9, 1882. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Song, G.; Lal, N.K.; Nagalakshmi, U.; Li, Y.; Zheng, W.; Huang, P.-J.; Branon, T.C.; Ting, A.Y.; Walley, J.W.; et al. TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nat. Commun. 2019, 10, 3252. [Google Scholar] [CrossRef] [Green Version]
- Mair, A.; Xu, S.-L.; Branon, T.C.; Ting, A.Y.; Bergmann, D.C. Proximity labeling of protein complexes and cell-type-specific organellar proteomes in Arabidopsis enabled by TurboID. elife 2019, 8, e47864. [Google Scholar] [CrossRef]
- Arora, D.; Abel, N.B.; Liu, C.; Van Damme, P.; Yperman, K.; Vu, L.D.; Wang, J.; Tornkvist, A.; Impens, F.; Korbei, B.; et al. Establishment of Proximity-dependent Biotinylation Approaches in Different Plant Model Systems. Plant. Cell Accept. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mojica, S.A.; Hovis, K.M.; Frieman, M.B.; Tran, B.; Hsia, R.C.; Ravel, J.; Jenkins-Houk, C.; Wilson, K.L.; Bavoil, P.M. SINC, a type III secreted protein of Chlamydia psittaci, targets the inner nuclear membrane of infected cells and uninfected neighbors. Mol. Biol. Cell 2015, 26, 1918–1934. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, S.P.; Rucks, E.A. Proximity Labeling To Map Host-Pathogen Interactions at the Membrane of a Bacterium-Containing Vacuole in Chlamydia trachomatis-Infected Human Cells. Infect. Immun. 2019, 87, e00537. [Google Scholar] [CrossRef] [Green Version]
- Bunney, P.E.; Zink, A.N.; Holm, A.A.; Billington, C.J.; Kotz, C.M. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Udeshi, N.D.; Pedram, K.; Svinkina, T.; Fereshetian, S.; Myers, S.A.; Aygun, O.; Krug, K.; Clauser, K.; Ryan, D.; Ast, T.; et al. Antibodies to biotin enable large-scale detection of biotinylation sites on proteins. Nat. Methods 2017, 14, 1167–1170. [Google Scholar] [CrossRef]
- Lam, S.S.; Martell, J.D.; Kamer, K.J.; Deerinck, T.J.; Ellisman, M.H.; Mootha, V.K.; Ting, A.Y. Directed evolution of APEX2 for electron microscopy and proteomics. Nat. Methods 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Lobingier, B.T.; Hüttenhain, R.; Eichel, K.; Miller, K.B.; Ting, A.Y.; von Zastrow, M.; Krogan, N.J. An Approach to Spatiotemporally Resolve Protein Interaction Networks in Living Cells. Cell 2017, 169, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paek, J.; Kalocsay, M.; Staus, D.P.; Wingler, L.; Pascolutti, R.; Paulo, J.A.; Gygi, S.P.; Kruse, A.C. Multidimensional Tracking of GPCR Signaling via Peroxidase-Catalyzed Proximity Labeling. Cell 2017, 169, 338–349. [Google Scholar] [CrossRef] [Green Version]
- Varnaitė, R.; MacNeill, S.A. Meet the neighbors: Mapping local protein interactomes by proximity-dependent labeling with BioID. Proteomics 2016, 16, 2503–2518. [Google Scholar] [CrossRef] [Green Version]
- Gingras, A.C.; Abe, K.T.; Raught, B. Getting to know the neighborhood: Using proximity-dependent biotinylation to characterize protein complexes and map organelles. Curr. Opin. Chem. Biol. 2019, 48, 44–54. [Google Scholar] [CrossRef]
- Go, C.D.; Knight, J.D.R.; Rajasekharan, A.; Rathod, B.; Hesketh, G.G.; Abe, K.T.; Youn, J.-Y.; Samavarchi-Tehrani, P.; Zhang, H.; Zhu, L.Y.; et al. A proximity biotinylation map of a human cell. bioRxiv 2019, 796391. [Google Scholar] [CrossRef] [Green Version]
- Santin, Y.G.; Doan, T.; Lebrun, R.; Espinosa, L.; Journet, L.; Cascales, E. In vivo TssA proximity labelling during type VI secretion biogenesis reveals TagA as a protein that stops and holds the sheath. Nat. Microbiol. 2018, 3, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Weigele, B.A.; Orchard, R.C.; Jimenez, A.; Cox, G.W.; Alto, N.M. A systematic exploration of the interactions between bacterial effector proteins and host cell membranes. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.I.; Cutler, J.A.; Na, C.H.; Reckel, S.; Renuse, S.; Madugundu, A.K.; Tahir, R.; Goldschmidt, H.L.; Reddy, K.L.; Huganir, R.L.; et al. BioSITe: A Method for Direct Detection and Quantitation of Site-Specific Biotinylation. J. Proteome Res. 2018, 17, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Eyckerman, S.; Titeca, K.; Van Quickelberghe, E.; Cloots, E.; Verhee, A.; Samyn, N.; De Ceuninck, L.; Timmerman, E.; De Sutter, D.; Lievens, S.; et al. Trapping mammalian protein complexes in viral particles. Nat. Commun. 2016, 7, 11416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titeca, K.; Van Quickelberghe, E.; Samyn, N.; De Sutter, D.; Verhee, A.; Gevaert, K.; Tavernier, J.; Eyckerman, S. Analyzing trapped protein complexes by Virotrap and SFINX. Nat. Protoc. 2017, 12, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Masschaele, D.; Wauman, J.; Vandemoortele, G.; de Sutter, D.; de Ceuninck, L.; Eyckerman, S.; Tavernier, J. High-Confidence Interactome for RNF41 Built on Multiple Orthogonal Assays. J. Proteome Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ataie Kachoie, E.; Behjatnia, S.A.A.; Kharazmi, S. Expression of Human Immunodeficiency Virus type 1 (HIV-1) coat protein genes in plants using cotton leaf curl Multan betasatellite-based vector. PLoS ONE 2018, 13, e0190403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Salokas, K.; Tamene, F.; Jiu, Y.; Weldatsadik, R.G.; Öhman, T.; Varjosalo, M. An AP-MS- and BioID-compatible MAC-tag enables comprehensive mapping of protein interactions and subcellular localizations. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Roux, K.J.; Kim, D.I.; Burke, B.; May, D.G. BioID: A Screen for Protein-Protein Interactions. Curr. Protoc. Protein Sci. 2018, 91. [Google Scholar] [CrossRef]
- Firat-karalar, E.N.; Stearns, T. Probing mammalian centrosome structure using BioID proximity-dependent biotinylation. Methods Cell Biol. 2015, 153–170. [Google Scholar] [CrossRef]
- Garnier, L.; Ratner, L.; Rovinski, B.; Cao, S.X.; Wills, J.W. Particle size determinants in the human immunodeficiency virus type 1 Gag protein. J. Virol. 1998, 72, 4667–4677. [Google Scholar] [CrossRef] [Green Version]
- Vandemoortele, G.; De Sutter, D.; Moliere, A.; Pauwels, J.; Gevaert, K.; Eyckerman, S. A Well-Controlled BioID Design for Endogenous Bait Proteins. J. Proteome Res. 2019, 18, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Titeca, K.; Lemmens, I.; Tavernier, J.; Eyckerman, S. Discovering cellular protein-protein interactions: Technological strategies and opportunities. Mass Spectrom. Rev. 2019, 38, 79–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ron, M.; Kajala, K.; Pauluzzi, G.; Wang, D.; Reynoso, M.A.; Zumstein, K.; Garcha, J.; Winte, S.; Masson, H.; Inagaki, S.; et al. Hairy Root Transformation Using Agrobacterium rhizogenes as a Tool for Exploring Cell Type-Specific Gene Expression and Function Using Tomato as a Model. Plant. Physiol. 2014, 166, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Dean, P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol. Rev. 2011, 35, 1100–1125. [Google Scholar] [CrossRef] [Green Version]
- Vanengelenburg, S.B.; Palmer, A.E. Imaging Type-III Secretion reveals dynamics and spatial segregation of Salmonella effectors. Nat. Methods 2010, 7, 325–330. [Google Scholar] [CrossRef]
- Steele-mortimer, O. The Salmonella-containing Vacuole—Moving with the Times. Curr. Opin. Microbiol. 2008, 11, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Mousnier, A.; Schroeder, G.N.; Stoneham, C.A.; So, E.C.; Garnett, J.A.; Yu, L.; Matthews, S.J.; Choudhary, J.S.; Hartland, E.L.; Frankel, G. A New Method To Determine In Vivo Interactomes Reveals Binding of the Legionella pneumophila Effector PieE to Multiple Rab GTPases. mBio 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Freeman, J.A.; Ohl, M.E.; Miller, S.I. The Salmonella enterica serovar typhimurium translocated effectors SseJ and SifB are targeted to the Salmonella-containing vacuole. Infect. Immun. 2003, 71, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Brady, M.J.; Campellone, K.G.; Ghildiyal, M.; Leong, J.M. Enterohaemorrhagic and enteropathogenic Escherichia coli Tir proteins trigger a common Nck-independent actin assembly pathway. Cell. Microbiol. 2007, 9, 2242–2253. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, M.A.; Cirulis, J.T.; Brown, N.F.; Finlay, B.B.; Brumell, J.H. SopD acts cooperatively with SopB during Salmonella enterica serovar Typhimurium invasion. Cell. Microbiol. 2007, 9, 2839–2855. [Google Scholar] [CrossRef] [PubMed]
- Westerhausen, S.; Nowak, M.; Torres-Vargas, C.E.; Bilitewski, U.; Bohn, E.; Grin, I.; Wagner, S. A NanoLuc luciferase-based assay enabling the real-time analysis of protein secretion and injection by bacterial type III secretion systems. Mol. Microbiol. 2020, 113, 1240–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechner, E.L.; Lang, S.; Schildbach, J.F. Assembly and mechanisms of bacterial type IV secretion machines. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.; Toruño, T.Y.; Jauneau, A.; Deslandes, L.; Coaker, G. Direct and Indirect Visualization of Bacterial Effector Delivery into Diverse Plant Cell Types during Infection. Plant. Cell 2017, 29, 1555–1570. [Google Scholar] [CrossRef] [Green Version]
- Breton, A.M.; Buon, I.; Guespin-Michel, J.F. Use of Tn phoA to tag exported proteins in Myxococcus xanthus. FEMS Microbiol. Lett. 1990, 67, 179–185. [Google Scholar] [CrossRef]
- Veneziano, R.; Rossi, C.; Chenal, A.; Devoisselle, J.-M.; Ladant, D.; Chopineau, J. Bordetella pertussis adenylate cyclase toxin translocation across a tethered lipid bilayer. Proc. Natl. Acad. Sci. USA 2013, 110, 20473–20478. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Mizukami, S.; Hori, Y.; Kikuchi, K. Multicolor Protein Labeling in Living Cells Using Mutant β-Lactamase-Tag Technology. Bioconjugate Chem. 2010, 21, 2320–2326. [Google Scholar] [CrossRef]
- Willems, P.; Fels, U.; Staes, A.; Gevaert, K.; Damme, P. Van The use of hybrid data-dependent and -independent acquisition spectral libraries empower dual-proteome profiling. bioRxiv 2020. [Google Scholar] [CrossRef]
- Liu, X.; Salokas, K.; Weldatsadik, R.G.; Gawriyski, L.; Varjosalo, M. Combined Proximity Labeling and Affinity Purification—Mass Spectrometry Workflow for Mapping and Visualizing Protein Interaction Networks. Nat. Protoc. 2020. [Google Scholar] [CrossRef]
- Lu, M.; Wei, W. Proximity labeling to detect RNA-protein interactions in live cells. FEBS Open Bio 2019, 9, 1860–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Bacterial Pathogen | Type-III Effector | (Candidate) Host Interactors (Gene Names) | Proteomics Approach | Species or Cell Type | Ref. |
|---|---|---|---|---|---|
| Pseudomonas syringae | HopF2 | AHA2, AHA11, PDR8/PEN3, ERD4, PIP2A, PIP3, Clathrin heavy chain, ADP/ATP Carrier protein, REM1.3, HIR2 | AP-MS | Arabidopsis | [56] |
| HopM1 | UPL1, UPL3, ECM29, proteins related to 26S proteasome non-ATPase regulatory subunits 2, 3, 6, 12, 14, BIG, orthologues of AtMIN7 and AtMIN10 | AP-MS (GFP-trap) | Tobacco | [57] | |
| HopQ1 | TFT1*, TFT2, TFT3, TFT4, TFT5*, TFT6, TFT7, TFT9, TFT10 | AP-MS | Tomato (cv. Moneymaker) | [58] | |
| Ralstonia solanacearum | RipAY | NbTRX-h11, NbTRX-h09, NbTRX-h10, NbTRX-h15* | AP-MS (GFP-trap) | Tobacco and Arabidopsis | [59] |
| Chlamydia trachomatis | 58 Inc-class effectors | 354 high-confident PPIs | AP-MS | HEK293T | [48] |
| Citrobacter rodentium | EspT | HSPD1 | AP-MS (in vitro) | RAW 264.7 and HeLa lysates | [51] |
| NleA | LDHB, PHGDH, SEC24B, DLG1, SEC23A, SLC3A2 | ||||
| NleG1 | TUFM, GAPDH, UQCRC2, PKM, MCM7, PRKDC, CPS1, SLC25A6, SLC25A5, SERPINH1, PHGDH, ACADM | ||||
| NleK | HNRNPM | ||||
| Salmonella enterica serovar Typhimurium | SopB | CDC42 | AP-MS (SILAC) | HEK293T | [61] |
| SspH2 | SUGT1*, AIP, BUB3*, YWHAG, BAG2 | ||||
| SseJ | RHOA, RHOC | ||||
| SspH1 | PKN1 | ||||
| PKN1* | AP-MS | RAW 264.7 and HeLa lysates | [51] | ||
| SseG | DSP, CAPRIN1 | AP-MS (SILAC) | HEK293T | [62] | |
| MYH10, IPO5, PHB2, MYL12B, EPHX1, RANBP6, EIF3B, NNT, SDHA, EIF3A, VDAC1, OCIAD1, NDUFA13, FAM162A, ARL6IP5, GK, API5, EIF3E, COX5B, VDAC2, PSMD12, RAB8A, AP3D1, AGK, CLPTM1L, CUL4B, VAMP3, BAX, CYP51A1, HMOX2, RDH11, TMEM48 | AP-MS | HEK293T | [52] | ||
| SseL | OSBPL1A*, TLN1 | AP-MS (SILAC) | HEK293T | [63] | |
| NEDD8, TXN, PSME2, S100A6, RCC2, S100A11, PRDC1, UBA52 | AP-MS | RAW 264.7 and HeLa lysates | [51] | ||
| SseF | JUP | AP-MS (SILAC) | HEK293T | [62] | |
| RBM10, THRAP3, ARGLU1 | AP-MS | HEK293T | [52] | ||
| GogA | PRPF31 | AP-MS | RAW 264.7 and HeLa lysates | [51] | |
| GtgA | MOGS, SLC25A11, PTGES2, SSR1, ATP5O, USMG5, GPNMB, BCAP29, ALDH3B1, 1700055N04RIK, RPN2, HADHA, YME1L1, ABHD12, IQGAP1, GALNT7, SGPL1, HSD17B12, CYC1, SLC25A12, SLC25A13, ACSL4, GM10250, B4GAINT1, LRRC59 | ||||
| GtgE | LYN, GOPC | ||||
| SpvC | LPXN | ||||
| SrfH | DNAJA1, ABCF2, ERK2*, UPF1, PFKI, MSH2, GM9755, SUCLG1, GALK1, GRPEL1, ACADM, PFKP, EPRS, IDH3B, SLC25A12 | ||||
| SssB | GRN | ||||
| SifA | MYH10, MYL6, MYL12B, EIF3B, RBM10, HM13, EIF3A, CDIPT, EIF5AL1, AP3D1, NDUFA13, TMEM59, ATP5D | AP-MS | HEK293T | [52] | |
| PipB2 | GCN1L1, XPO1, IRS4, MYH10, FANCI, XPO5, ATP2B1, FKBP5, SUGT1, NCAPD2, MYH9, SCRIB, KIAA0368, TNPO1, LRRC1, TELO2, DIS3, ACTA1, DDX19A, AP3D1, PDS5A, MMS19, HDDC2, MYL12B, CAND2, NTPCR, RHOG, TRMT1, CDC73, YTHDF2, RDH11, ANXA4, PELO, UMPS, PRPF6, NAA15, PSMD12, MTHFD1L, EEF1E1, ADCK3, PLAA, CALM2, UROD, ANP32E, FABP5, LTN1, MLF2, SYNE1, ATP6V1H, CUL1, NEDD8-MDP1, FBXO22, SNRPG, UBXN1, AUP1, PIN1, LYPLA2, ARIH1, PCID2, LARP4B, CELF1, ARGLU1, GOLPH3, ORC3, DDX23 | ||||
| SopD2 | MYH10, MYL6, MYL12B, MYH9, RBM10, RAB10, EIF3B, CYFIP1, PHB2, EIF3A, AP2B1, EIF3E, AP3D1, MYO1B, RAB8A, AP3B1, AP2A1 | ||||
| SopA a | TRIM56*, TRIM65*, HDAC10, GSTM3, PCMT1, MAPK3, AP2B1, XRCC5, PPP2R2A, XRCC6 | AP-MS (SILAC; GFP-trap) | HeLa | [63] | |
| TRIM56*, TRIM65*, EPS15L1, GTF2F2, PDLIM7, CSTF1, GTF2F1, RAD23B, MAPRE1, G6PD | AP-MS (SILAC) | HCT116 | |||
| 15 STm T3Es | 446 high-confident PPIs | AP-MS (delivery of chromosomally tagged T3Es in the context of infection) | HeLa and RAW 264.7 | [55] | |
| EPEC | Map | NERF2 | AP-MS (SILAC) | HEK293T | [64] |
| EspJ | WDR23 | ||||
| EspL | MAP7* | ||||
| EspX | MAP7 | ||||
| NleA | SEC23A, SEC24B, DLG1 | ||||
| NleB1 | MAP7* | ||||
| NleC | P300 | ||||
| EspZ | CD98*, RPS27A, HSP90AB1, HSP90AA1 | AP-MS (SILAC) | HEK293 | [61] |
| Bacterial Pathogen | Type-III Effector | (Candidate) Host Interactors (Gene Names) | Proteomics Approach | Species or Cell Type | Ref. |
|---|---|---|---|---|---|
| Pseudomonas syringae | HopF2 | 19 a RIN4, REM1.3, PCAP1, PHOT1, PHOT2, SYP122, PMI1, PATL1, PATL2, RBCS1A | BirA* | Arabidopsis | [77] |
| AvrPto | 25 a RIN4, APK1, APK2, TOM1, APP4* | BirA* | Tobacco | [79] | |
| Chlamydia psittaci | SINC | 22 a ELYS, laminB1*, emerin*, MAN1, LAP1, LBR | BirA* | HeLa | [83] |
| IncF | 13 a LRRF1, MAP1B, CYTB, BASP1, YWHAH, MARCKS, YWHAB, K1C20 | APEX2 | HeLa | [84] | |
| IncATM | 18 a LRRF1, MAP1B, CYTB, BASP1, MYPT1, TERA, MAP4, PUR6, SNX1, SRC8, MEP50, SNX6, PLIN3, IF4B, NDKA, NDKB | ||||
| IncA | 192 a LRRF1, MAP1B, CYTB, BASP1, MYPT1, TERA, MAP4, PUR6, SNX1, SRC8, MEP50, SNX6, PLIN3, IF4B, NDKA, NDKB, YWHAH, MARCKS, YWHAB, K1C20 | ||||
| Salmonella enterica serovarTyphimurium | SifA | 167 b BLOC-2*, IRS4, EPB41L3, EPB41L2, MLLT4, DST, LBR, YKT6, TMPO, SLC12A2 | BirA* | HEK293T | [52] |
| PipB2 | 149 b KIF5B, EPB41L3, IRS4, TMPO, LEMD3, EPB41, MLLT4, KLC1, EPB41L2, KLC2 | ||||
| SseF | 107 b TUFM, IRS4, LBR, EPHA2EPB41L2, UBB, DDX17, CKAP4, PTPLAD1, HNRNPC | ||||
| SseG | 145 b TMPO, ESYT1, LBR, PGRMC2, LEMD3, CKAP4, ABCD3, RPN1, ACSL3, LMAN1 | ||||
| SopD2 | 61 b RUVBL1, RUVBL2, HSP90AA1, CCT2, CLCN7, SUGT1, RAB7A, YKT6, ZFYVE16, HNRNPAB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, M.D.; Ryck, J.D.; Goormachtig, S.; Van Damme, P. Keeping in Touch with Type-III Secretion System Effectors: Mass Spectrometry-Based Proteomics to Study Effector–Host Protein–Protein Interactions. Int. J. Mol. Sci. 2020, 21, 6891. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186891
Meyer MD, Ryck JD, Goormachtig S, Van Damme P. Keeping in Touch with Type-III Secretion System Effectors: Mass Spectrometry-Based Proteomics to Study Effector–Host Protein–Protein Interactions. International Journal of Molecular Sciences. 2020; 21(18):6891. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186891
Chicago/Turabian StyleMeyer, Margaux De, Joren De Ryck, Sofie Goormachtig, and Petra Van Damme. 2020. "Keeping in Touch with Type-III Secretion System Effectors: Mass Spectrometry-Based Proteomics to Study Effector–Host Protein–Protein Interactions" International Journal of Molecular Sciences 21, no. 18: 6891. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186891