Sauchinone Protects Renal Mesangial Cell Dysfunction against Angiotensin II by Improving Renal Fibrosis and Inflammation
Abstract
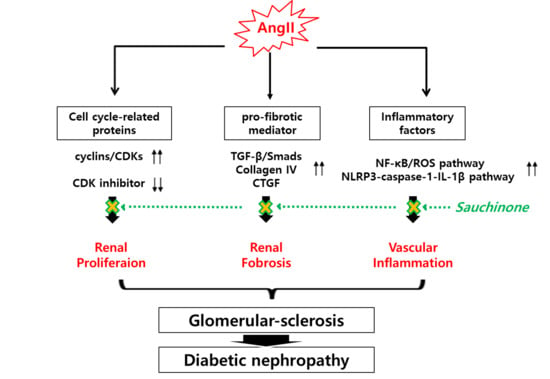
:1. Introduction
2. Results
2.1. Effect of Sauchinone on Mesangial Cell Proliferation
2.2. Effect of Sauchinone on Mesangial Cell Fibrosis
2.3. Effect of Sauchinone on Mesangial Cell Inflammation
2.4. Effect of Sauchinone on Inflammatory Responses by NF-κB/ROS Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Cultures
4.3. Measurement of Cell Proliferation
4.4. Wound Healing Assay, Migration Assay
4.5. Western Blot Analysis
4.6. Preparation of Cytoplasmic and Nuclear Extracts
4.7. RNA Isolation and Real-Time qRT-PCR
4.8. Immunofluorescence Microscopy
4.9. Measurement of ROS
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AngII | Angiotensin II |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| ColIV | Type IV collagen |
| CTGF | Connective tissue growth factor |
| DN | Diabetic nephropathy |
| ECM | Extracellular matrix |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-1β | Interleukin 1β |
| MCP-1 | Monocyte chemoattractant protein-1 |
| LPS | Lipopolysaccharide |
| NFκB | Nuclear factor kappa B |
| NLRP3 | NOD-like receptor family, pyrin domain-containing-3 |
| RAS | Renin–angiotensin system |
| ROS | Reactive oxygen species |
| TGF-β1 | Transforming growth factor-β1 |
References
- Lim, A.K.H. Diabetic nephropathy complications and treatment. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolset, S.O.; Reinholt, F.P.; Jenssen, T. Diabetic Nephropathy and Extracellular Matrix. J. Histochem. Cytochem. 2012, 60, 976–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Declèves, A.E.; Sharma, K. New pharmacological treatments for improving renal outcomes in diabetes. Nat. Rev. Nephrol. 2010, 6, 371–380. [Google Scholar] [CrossRef]
- Ying, Q.; Eva, F.; Subramanian, P.; Matthias, K.; Frank, C.B.I. Mechanisms of Glomerulosclerosis in Diabetic Nephropathy. Diabetes 2008, 57, 1439–1445. [Google Scholar]
- Sakharova, O.V.; Taal, M.W.; Brenner, B.M. Pathogenesis of diabetic nephropathy: Focus on transforming growth factor-beta and connective tissue growth factor. Curr. Opin. Nephrol. Hypertens. 2001, 10, 727–738. [Google Scholar] [CrossRef]
- Bai, J.; Geng, W.; Mei, Y.; Wu, L.; Duan, S.; Dong, Z.; Dong, Z.; Fu, B.; Wang, Y.; Zhu, F.; et al. Effect of huaier on the proliferation of mesangial cells in anti-thy-1 nephritis. Cell. Physiol. Biochem. 2017, 42, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- David, J.L.; Ashok, K.S.; Nahid, A.; Rekha, S. Role of angiotensin II in diabetic nephropathy. Kidney Int. 2000, 58, S93–S98. [Google Scholar]
- Kobori, H.; Mori, H.; Masaki, T.; Nishiyama, A. Angiotensin II blockade and renal protection. Curr. Pharm. Des. 2013, 19, 3033–3042. [Google Scholar] [CrossRef] [Green Version]
- Alan, P.; Loredo, M.L.; Padmaja, K.; Motoyasu, S.; Jack, M.J.; Mathew, D.R. Angiotensin II Regulation of TGF-β in Murine Mesangial Cells Involves Both PI3 Kinase and MAP Kinase. Ann. Clin. Lab. Sci. 2004, 34, 277–286. [Google Scholar]
- Dai, C.; Liu, Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J. Am. Soc. Nephrol. 2004, 15, 1402–1412. [Google Scholar] [CrossRef] [Green Version]
- Groppe, J.; Hinck, C.S.; Samavarchi, T.P.; Zubieta, C.; Schuermann, J.P.; Taylor, A.B.; Schwarz, P.M.; Wrana, J.L.; Hinck, A.P. Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell 2008, 29, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Diverse Roles of TGF-β/Smads in Renal Fibrosis and Inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, F.Q.; Li, H.; Cai, W.M.; Tao, J.; Li, Q.; Ruan, Y.; Zheng, F.P.; Zhang, Z. Effects of pioglitazone on expressions of matrix metalloproteinases 2 and 9 in kidneys of diabetic rats. Chin. Med. J. 2004, 117, 1040–1044. [Google Scholar]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Klein, J.; Chauhan, S.D.; Neau, E.; Calise, D.; Nevoit, C.; Chaaya, R.; Miravete, M.; Delage, C.; Bascands, J.L.; et al. Delayed treatment with plasminogen activator inhibitor-1 decoys reduces tubulointerstitial fibrosis. Exp. Biol. Med. 2009, 234, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shao, X.; Tian, L.; Gu, L.; Zhang, M.; Wang, Q.; Wu, B.; Wang, L.; Yao, J.; Xu, X.; et al. Astragaloside IV ameliorates renal fibrosis via the inhibition of mitogen-activated protein kinases and antiapoptosis in vivo and in vitro. J. Pharmacol. Exp. Ther. 2014, 350, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Mansour, S.G.; Puthumana, J.; Coca, S.G.; Gentry, M.; Parikh, C.R. Biomarkers for the detection of renal fibrosis and prediction of renal outcomes: A systematic review. BMC Nephrol. 2017, 18, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barutta, F.; Bruno, G.; Grimaldi, S.; Gruden, G. Inflammation in diabetic nephropathy: Moving toward clinical biomarkers and targets for treatment. Endocrine 2015, 48, 730–742. [Google Scholar] [CrossRef]
- Sassy-Prigent, C.; Heudes, D.; Mandet, C. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 2000, 49, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Segerer, S.; Nelson, P.J.; Schlöndorff, D. Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J. Am. Soc. Nephrol. 2000, 11, 152–176. [Google Scholar]
- Massy, Z.A.; Guijarro, C.; O’Donnell, M.P.; Kim, Y.; Kashtan, C.E.; Egido, J.; Kasiske, B.L.; Keane, W.F. The central role of nuclear factor-κB in mesangial cell activation. Kidney Int. 1999, 56, S76–S79. [Google Scholar]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [PubMed]
- Manickam, N.; Patel, M.; Griendling, K.K.; Gorin, Y.; Barnes, J.L. RhoA/Rho kinase mediates TGF-beta1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am. J. Physiol. Ren. Physiol. 2014, 307, F159–F171. [Google Scholar]
- Yaribeygi, H.; Katsiki, N.; Butler, A.E.; Sahebkar, A. Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys. Drug Discov. Today 2019, 24, 256–262. [Google Scholar] [PubMed]
- Li, L.; Tang, W.; Yi, F. Role of inflammasome in chronic kidney disease. Adv. Exp. Med. Biol. 2019, 1165, 407–421. [Google Scholar] [PubMed]
- Mulay, S.R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 2019, 96, 58–66. [Google Scholar] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [PubMed] [Green Version]
- Park, H.J.; Kim, R.G.; Seo, B.R.; Ha, J.; Ahn, B.T.; Bok, S.H.; Lee, Y.S.; Kim, H.J.; Lee, K.T. Saucernetin-7 and saucernetin-8 isolated from Saururus chinensis inhibit the LPS-induced production of nitric oxide and prostaglandin E2 in macrophage RAW264.7 cells. Planta Med. 2003, 69, 947–950. [Google Scholar]
- Cui, H.; Xu, B.; Wu, T.; Xu, J.; Yuan, Y.; Gu, Q. Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein-Barr virus lytic replication. J. Nat. Prod. 2014, 77, 100–110. [Google Scholar] [PubMed]
- Ryu, S.Y.; Oh, K.S.; Kim, Y.S.; Lee, B.H. Antihypertensive, vasorelaxant and inotropic effects of an ethanolic extract of the roots of Saururus chinensis. J. Ethnopharmacol. 2008, 118, 284–289. [Google Scholar] [PubMed]
- Seo, C.S.; Lee, Y.K.; Kim, Y.J.; Jung, J.S.; Jahng, Y.; Chang, H.W.; Song, D.K.; Son, J.K. Protective effect of lignans against sepsis from the roots of Saururus chinensis. Biol. Pharm. Bull. 2008, 31, 523–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Kim, J.; Yi, J.M.; Oh, S.M.; Kim, N.S.; Kim, H.; Oh, D.S.; Bang, O.S.; Lee, J. Anti-proliferative neolignans from Saururus chinensis against human cancer cell lines. Biol. Pharm. Bull. 2012, 35, 1361–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.K.; Sung, S.H.; Kim, Y.C.; Kim, S.G. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-α and COX-2 expression by sauchinone effects on I-κ phosphorylation, C/EBP and AP-1 activation. Br. J. Pharmacol. 2003, 139, 11–20. [Google Scholar] [PubMed] [Green Version]
- Jürgen, F.; Nicholas, T.; Klans, R. Regulation of Mesangial Cell Proliferation. Am. J. Kidney Dis. 1991, 17, 673–676. [Google Scholar]
- Qiu, G.; Ji, Z. AngII-induced glomerular mesangial cell proliferation inhibited by losartan via changes in intracellular calcium ion concentration. Clin. Exp. Med. 2014, 14, 169–176. [Google Scholar]
- Ou, Y.C.; Li, J.R.; Wang, J.D.; Chang, C.Y.; Wu, C.C.; Chen, W.Y.; Kuan, Y.H.; Liao, S.L.; Lu, H.C.; Chen, C.J. Fibronectin Promotes Cell Growth and Migration in Human Renal Cell Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2792. [Google Scholar] [CrossRef] [Green Version]
- Elizabeth, G.H.; Daniel, S.; Malgorzata, M.; Shianlen, L.; Debra, H.M.; Eddie, L.G.; Gary, G.; Maria, T. TGF-β and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am. J. Physiol. Ren. Physiol. 2000, 283, F707–F716. [Google Scholar]
- Meng, X.M.; Tang, P.M.K.; Li, J.; Lan, H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar]
- Yoon, J.J.; Park, J.H.; Kim, H.J.; Jin, H.G.; Kim, H.Y.; Ahn, Y.M.; Kim, Y.C.; Lee, H.S.; Lee, Y.J.; Kang, D.G. Dianthus superbus Improves Glomerular Fibrosis and Renal Dysfunction in Diabetic Nephropathy Model. Nutrients 2019, 11, 553. [Google Scholar] [CrossRef] [Green Version]
- Mezzano, S.; Droguett, A.; Burgos, M.E.; Ardiles, L.G.; Flores, C.A.; Aros, C.A.; Caorsi, I.; Vío, C.P.; Ruiz-Ortega, M.; Egido, J. Renin–angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int. 2003, 86, S64–S70. [Google Scholar] [CrossRef] [Green Version]
- Rivero, A.; Mora, C.; Muros, M.; Garcia, J.; Herrera, H.; Navarro-Gonzalez, J.F. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin. Sci. 2009, 116, 479–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohga, S.; Shikata, K.; Yozai, K.; Okada, S.; Ogawa, D.; Usui, H. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-κB activation. Am. J. Physiol. Ren. Physiol. 2007, 292, F1141–F1150. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.J.; Lee, H.K.; Kim, H.Y.; Han, B.H.; Lee, H.S.; Lee, Y.J.; Kang, D.G. Sauchinone Protects Renal Mesangial Cell Dysfunction against Angiotensin II by Improving Renal Fibrosis and Inflammation. Int. J. Mol. Sci. 2020, 21, 7003. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197003
Yoon JJ, Lee HK, Kim HY, Han BH, Lee HS, Lee YJ, Kang DG. Sauchinone Protects Renal Mesangial Cell Dysfunction against Angiotensin II by Improving Renal Fibrosis and Inflammation. International Journal of Molecular Sciences. 2020; 21(19):7003. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197003
Chicago/Turabian StyleYoon, Jung Joo, Hyeon Kyoung Lee, Hye Yoom Kim, Byung Hyuk Han, Ho Sub Lee, Yun Jung Lee, and Dae Gill Kang. 2020. "Sauchinone Protects Renal Mesangial Cell Dysfunction against Angiotensin II by Improving Renal Fibrosis and Inflammation" International Journal of Molecular Sciences 21, no. 19: 7003. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197003