Weak Ultraviolet B Enhances the Mislocalization of Claudin-1 Mediated by Nitric Oxide and Peroxynitrite Production in Human Keratinocyte-Derived HaCaT Cells
Abstract
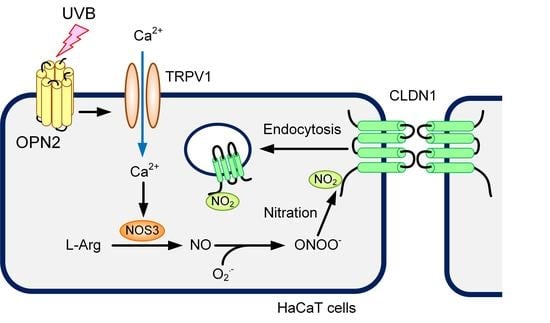
:1. Introduction
2. Results
2.1. Effect of UVB Irradiation on Cell Viability and the Cellular Localization of CLDN
2.2. Effect of UVB Irradiation on Barrier Function
2.3. Rescue of TJ Barrier Function by a Clathrin-Dependent Endocytosis Inhibitor
2.4. Increase in Tyrosine-Nitrated and Ubiquitinated CLDN1 by UVB
2.5. Involvement of RNS in UVB-Induced Mislocalization of CLDN1
2.6. Destruction of TJ Barrier Function by RNS
2.7. Inhibition of UVB-Induced Responses by TRPV1 Antagonist
2.8. Inhibition of UVB-Induced Elevation of NOS3 and Mislocalization of CLDN1 by AMG9810
2.9. Inhibition of UVB-Induced Responses by Opsin 2 (OPN2) siRNA
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. UVB Irradiation
4.4. Confocal Microscopy
4.5. Paracellular Permeability
4.6. SDS-Polyacrylamide Gel Electrophoresis and Immunoblotting
4.7. Intracellular RNS and Ca2+ Contents
4.8. Isolation of Total RNA and PCR
4.9. Transfection of Plasmid DNA and siRNA
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLDN | Claudin |
| DAF-2DA | 4,5-Diaminofluorescein-2 diacetate |
| EGTA | Glycoletherdiaminetetraacetic acid |
| FBS | Fetal bovine serum |
| FeTPPS | Fe(III)5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin |
| H2DCFDA | 2′,7′-Dichlorofluorescein diacetate |
| LY | Lucifer yellow |
| MβCD | Methyl-β-cyclodextrin |
| MDC | Monodansylcadaverine |
| L-NAME | NG-nitro-L-arginine methyl ester |
| NiSPY | 1,3,5,7-Tetramethyl-2,6-dicyano-4,4-difluoro-8-[2-(2-hydroxy-2-oxoethoxy)-4-hydroxyphenyl]-3a,4-dihydro-3a-aza-4a-azonia-4-bora(IV)-s-indacene |
| NOC12 | 1-Hydroxy-2-oxo-3-(N-ethyl-2-aminoethyl)-3-ethyl-1-triazene |
| cNOS | Constitutive NO synthase |
| iNOS | Inducible NO synthase |
| nNOS | Neuronal NO synthase |
| NO | Nitric oxide |
| NOS | NO synthase |
| NS | Not significant |
| OPN | Opsin |
| PCR | Polymerase chain reaction |
| PDZ | Postsynaptic density-95/discs large/zonula occludens-1 |
| p-Ser | Anti-phosphoserine |
| p-Thr | Anti-phosphothreonine |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
| SIN-1 | 3-(4-Morpholinyl)sydnonimine, hydrochloride |
| TER | Transepidermal electrical resistance |
| TJ | Tight junction |
| TRPV | Transient receptor potential vanilloid |
| UV | Ultraviolet |
| WST-1 | 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium |
| ZO-1 | Zonula occludens-1 |
References
- Coolen, N.A.; Schouten, K.C.; Middelkoop, E.; Ulrich, M.M. Comparison between human fetal and adult skin. Arch. Dermatol. Res. 2010, 302, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Brandner, J.M.; Kief, S.; Wladykowski, E.; Houdek, P.; Moll, I. Tight junction proteins in the skin. Skin Pharmacol. Physiol. 2006, 19, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Basler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflugers Arch. 2017, 469, 3–14. [Google Scholar] [CrossRef]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turksen, K.; Troy, T.C. Barriers built on claudins. J. Cell Sci. 2004, 117, 2435–2447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, M.; Furuse, M.; Morita, K.; Kubota, K.; Saitou, M.; Tsukita, S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999, 147, 1351–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The role of tight junctions in skin barrier function and dermal absorption. J. Control. Release Off. J. Control. Release Soc. 2016, 242, 105–118. [Google Scholar] [CrossRef]
- Sugawara, T.; Iwamoto, N.; Akashi, M.; Kojima, T.; Hisatsune, J.; Sugai, M.; Furuse, M. Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J. Dermatol. Sci. 2013, 70, 12–18. [Google Scholar] [CrossRef]
- Hadj-Rabia, S.; Baala, L.; Vabres, P.; Hamel-Teillac, D.; Jacquemin, E.; Fabre, M.; Lyonnet, S.; De Prost, Y.; Munnich, A.; Hadchouel, M.; et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterology 2004, 127, 1386–1390. [Google Scholar] [CrossRef]
- Young, A.R.; Chadwick, C.A.; Harrison, G.I.; Nikaido, O.; Ramsden, J.; Potten, C.S. The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J. Investig. Dermatol. 1998, 111, 982–988. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R. Action spectrum for photocarcinogenesis. In Skin Cancer: Basic Science, Clinical Research and Treatment; Springer: Berlin/Heidelberg, Germany, 1995; Volume 139, pp. 21–30. [Google Scholar]
- Averilla, J.N.; Oh, J.; Kim, J.S. Carbon Monoxide Partially Mediates Protective Effect of Resveratrol Against UVB-Induced Oxidative Stress in Human Keratinocytes. Antioxidants 2019, 8, 432. [Google Scholar] [CrossRef] [Green Version]
- Abd Elmageed, Z.Y.; Gaur, R.L.; Williams, M.; Abdraboh, M.E.; Rao, P.N.; Raj, M.H.; Ismail, F.M.; Ouhtit, A. Characterization of coordinated immediate responses by p16INK4A and p53 pathways in UVB-irradiated human skin cells. J. Investig. Dermatol. 2009, 129, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pustisek, N.; Situm, M. UV-radiation, apoptosis and skin. Coll. Antropol. 2011, 35, 339–341. [Google Scholar]
- Suschek, C.V.; Oplander, C.; van Faassen, E.E. Non-enzymatic NO production in human skin: Effect of UVA on cutaneous NO stores. Nitric Oxide 2010, 22, 120–135. [Google Scholar] [CrossRef]
- Nakai, K.; Yoneda, K.; Moriue, T.; Igarashi, J.; Kosaka, H.; Kubota, Y. HB-EGF-induced VEGF production and eNOS activation depend on both PI3 kinase and MAP kinase in HaCaT cells. J. Dermatol. Sci. 2009, 55, 170–178. [Google Scholar] [CrossRef]
- Liu, W.; Wu, S. Differential roles of nitric oxide synthases in regulation of ultraviolet B light-induced apoptosis. Nitric Oxide 2010, 23, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef]
- Marunaka, K.; Kobayashi, M.; Shu, S.; Matsunaga, T.; Ikari, A. Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells. Int. J. Mol. Sci. 2019, 20, 3869. [Google Scholar] [CrossRef] [Green Version]
- Shigetomi, K.; Ikenouchi, J. Regulation of the epithelial barrier by post-translational modifications of tight junction membrane proteins. J. Biochem. 2018, 163, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Calee, Y.M.; Kim, Y.K.; Kim, K.H.; Park, S.J.; Kim, S.J.; Chung, J.H. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J. Cell. Physiol. 2009, 219, 766–775. [Google Scholar]
- Olivan-Viguera, A.; Garcia-Otin, A.L.; Lozano-Gerona, J.; Abarca-Lachen, E.; Garcia-Malinis, A.J.; Hamilton, K.L.; Gilaberte, Y.; Pueyo, E.; Kohler, R. Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes. PLoS ONE 2018, 13, e0190307. [Google Scholar] [CrossRef] [PubMed]
- Terakita, A. The opsins. Genome Biol. 2005, 6, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhasaniah, A.; Sherratt, M.J.; O’Neill, C.A. The Impact of Ultraviolet Radiation on Barrier Function in Human Skin: Molecular Mechanisms and Topical Therapeutics. Curr. Med. Chem. 2018, 25, 5503–5511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, C.; Yu, Z.; Wang, X.; Yan, L.; Zhang, J.; Li, H.; Wang, J.; Wen, A. Echinacoside Alleviates UVB Irradiation-Mediated Skin Damage via Inhibition of Oxidative Stress, DNA Damage, and Apoptosis. Oxidative Med. Cell. Longev. 2017, 2017, 6851464. [Google Scholar] [CrossRef]
- Krause, G.; Protze, J.; Piontek, J. Assembly and function of claudins: Structure-function relationships based on homology models and crystal structures. Semin. Cell Dev. Biol. 2015, 42, 3–12. [Google Scholar] [CrossRef]
- El-Chami, C.; Haslam, I.S.; Steward, M.C.; O’Neill, C.A. Organic osmolytes preserve the function of the developing tight junction in ultraviolet B-irradiated rat epidermal keratinocytes. Sci. Rep. 2018, 8, 5167. [Google Scholar] [CrossRef] [Green Version]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med. 2019, 51, 370–382. [Google Scholar] [CrossRef] [Green Version]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Kojima, D.; Mori, S.; Torii, M.; Wada, A.; Morishita, R.; Fukada, Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS ONE 2011, 6, e26388. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Son, E.D.; Jung, J.Y.; Choi, H.; Lee, T.R.; Shin, D.W. Violet light down-regulates the expression of specific differentiation markers through Rhodopsin in normal human epidermal keratinocytes. PLoS ONE 2013, 8, e73678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Assis, L.V.M.; Moraes, M.N.; Magalhaes-Marques, K.K.; Castrucci, A.M.L. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: Unravelling the photosensitive system of the skin. Eur. J. Cell Biol. 2018, 97, 150–162. [Google Scholar] [CrossRef]
- Poletini, M.O.; Moraes, M.N.; Ramos, B.C.; Jeronimo, R.; Castrucci, A.M. TRP channels: A missing bond in the entrainment mechanism of peripheral clocks throughout evolution. Temperature 2015, 2, 522–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talagas, M.; Misery, L. Role of Keratinocytes in Sensitive Skin. Front. Med. 2019, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.F.; Ma, K.H.; Liu, P.S.; Chen, B.W.; Chueh, S.H. Ultraviolet B irradiation increases keratin 1 and keratin 10 expressions in HaCaT keratinocytes via TRPV1 activation and ERK phosphorylation. Exp. Dermatol. 2017, 26, 832–835. [Google Scholar] [CrossRef]
- Cai, H.; Davis, M.E.; Drummond, G.R.; Harrison, D.G. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1571–1576. [Google Scholar] [CrossRef] [Green Version]
- Fujii, N.; Matsuo, Y.; Matsunaga, T.; Endo, S.; Sakai, H.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; Ikari, A. Hypotonic stress-induced down-regulation of claudin-1 and -2 mediated by dephosphorylation and clathrin-dependent endocytosis in renal tubular epithelial cells. J. Biol. Chem. 2016, 291, 24787–24799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.E.; Yu, L.R.; Abdelmegeed, M.A.; Yoo, S.H.; Song, B.J. Apoptosis of enterocytes and nitration of junctional complex proteins promote alcohol-induced gut leakiness and liver injury. J. Hepatol. 2018, 69, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef] [PubMed]










| Name | Direction | Sequence |
|---|---|---|
| CLDN1 | Forward | 5′-ATGAGGATGGCTGTCATTGG-3′ |
| Reverse | 5′-ATTGACTGGGGTCATAGGGT-3′ | |
| CLDN4 | Forward | 5′-TTGTCACCTCGCAGACCATC-3′ |
| Reverse | 5′-GCAGCGAGTCGTACACCTTG-3′ | |
| β-Actin | Forward | 5′-CCTGAGGCACTCTTCCAGCCTT-3′ |
| Reverse | 5′-TGCGGATGTCCACGTCACACTTC-3′ |
| Name | Direction | Sequence |
|---|---|---|
| OPN1 | Forward | 5′-CTGCATCTTCTCTGTCTTCCCT-3′ |
| Reverse | 5′-CAGTGACCATCCTGTAACCAGA-3′ | |
| OPN2 | Forward | 5′-GCACAGAAGGCCCTAACTTCTA-3′ |
| Reverse | 5′-CGTAGAGCGTGAGGAAGTTGAT-3′ | |
| OPN3 | Forward | 5′-TGGTGCTCGTCCTCTACTACAA-3′ |
| Reverse | 5′-AGGACACGAAGGTAAAGGTGAC-3′ | |
| OPN5 | Forward | 5′-ACTCCATTCCCATACAGCTCTC-3′ |
| Reverse | 5′-ACTTCCTGACTGTGGTTACGGT-3′ | |
| β-Actin | Forward | 5′-CCTGAGGCACTCTTCCAGCCTT-3′ |
| Reverse | 5′-TGCGGATGTCCACGTCACACTTC-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Shu, S.; Marunaka, K.; Matsunaga, T.; Ikari, A. Weak Ultraviolet B Enhances the Mislocalization of Claudin-1 Mediated by Nitric Oxide and Peroxynitrite Production in Human Keratinocyte-Derived HaCaT Cells. Int. J. Mol. Sci. 2020, 21, 7138. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197138
Kobayashi M, Shu S, Marunaka K, Matsunaga T, Ikari A. Weak Ultraviolet B Enhances the Mislocalization of Claudin-1 Mediated by Nitric Oxide and Peroxynitrite Production in Human Keratinocyte-Derived HaCaT Cells. International Journal of Molecular Sciences. 2020; 21(19):7138. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197138
Chicago/Turabian StyleKobayashi, Mao, Shokoku Shu, Kana Marunaka, Toshiyuki Matsunaga, and Akira Ikari. 2020. "Weak Ultraviolet B Enhances the Mislocalization of Claudin-1 Mediated by Nitric Oxide and Peroxynitrite Production in Human Keratinocyte-Derived HaCaT Cells" International Journal of Molecular Sciences 21, no. 19: 7138. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197138