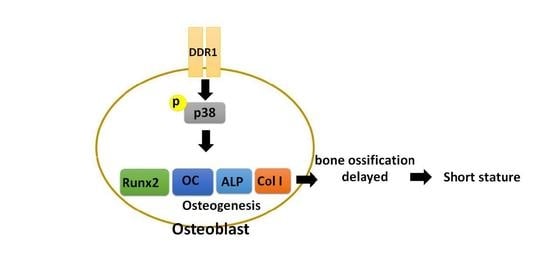
Discoidin Domain Receptor 1 Regulates Runx2 during Osteogenesis of Osteoblasts and Promotes Bone Ossification via Phosphorylation of p38
Abstract
:1. Introduction
2. Results
2.1. Generation of Osteoblast-Specific Knockout Mice (a1(I) Collagen-CreERT; Ddr1f/f; OKOΔDdr1 Mice)
2.2. Skeletal Dysplasia in OKOΔDdr1 Mice
2.3. Decreased Bone Formation in the Tibia of OKOΔDdr1 Mice
2.4. Knocking out Ddr1 in Osteoblasts Decreases Cortical Bone Thickness and Cell Number
2.5. Knocking out Ddr1 in Osteoblasts Caused Downregulation of the Osteogenesis-Related Marker Gene Expression
2.6. DDR1 Promoted Mineralization in MC3T3-E1 Cells
2.7. DDR1 Regulated Osteoblast Differentiation through Phosphorylation of p38
3. Discussion
4. Materials and Methods
4.1. Osteoblast-Specific (a1(I) Collagen-CreERT; Ddr1f/f) Ddr1-Deficient OKO Mice
4.2. Induction of 4-Hydroxytamoxifen (4-OHT)
4.3. Double Staining Analysis for Skeleton
4.4. Microcomputed Tomography (Micro-CT)
4.5. Three-Point Bending Test
4.6. Histological and Immunohistochemistry (IHC) Staining
4.7. Cell Culture and Drug Treatment
4.8. Lentivirus Constructs and Transfection
4.9. Real-Time PCR (qPCR) Analysis
4.10. Western Blot Analysis
4.11. Mineralization Assay
4.12. Alkaline Phosphatase Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Takarada, T.; Hinoi, E.; Nakazato, R.; Ochi, H.; Xu, C.; Tsuchikane, A.; Takeda, S.; Karsenty, G.; Abe, T.; Kiyonari, H.; et al. An analysis of skeletal development in osteoblast-specific and chondrocyte-specific runt-related transcription factor-2 (Runx2) knockout mice. J. Bone Miner. Res. 2013, 28, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. The complexities of skeletal biology. Nature 2003, 423, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Takarada, T.; Nakazato, R.; Tsuchikane, A.; Fujikawa, K.; Iezaki, T.; Yoneda, Y.; Hinoi, E. Genetic analysis of Runx2 function during intramembranous ossification. Development 2016, 143, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhami, M.D.; Rashid, H.; Chen, H.; Clarke, J.C.; Yang, Y.; Javed, A. Loss of Runx2 in committed osteoblasts impairs postnatal skeletogenesis. J. Bone Miner. Res. 2015, 30, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Roca, H.; Phimphilai, M.; Gopalakrishnan, R.; Xiao, G.; Franceschi, R.T. Cooperative interactions between RUNX2 and homeodomain protein-binding sites are critical for the osteoblast-specific expression of the bone sialoprotein gene. J. Biol. Chem. 2005, 280, 30845–30855. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, R.; Wakabayashi, M.; Hata, K.; Matsubara, T.; Honma, S.; Wakisaka, S.; Kiyonari, H.; Shioi, G.; Yamaguchi, A.; Tsumaki, N.; et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J. Biol. Chem. 2012, 287, 33179–33190. [Google Scholar] [CrossRef] [Green Version]
- Hauschka, P.V. Osteocalcin: The vitamin K-dependent Ca2+-binding protein of bone matrix. Haemostasis 1986, 16, 258–272. [Google Scholar] [CrossRef]
- Vogel, W.F.; Aszodi, A.; Alves, F.; Pawson, T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol. Cell Biol. 2001, 21, 2906–2917. [Google Scholar] [CrossRef] [Green Version]
- Valiathan, R.R.; Marco, M.; Leitinger, B.; Kleer, C.G.; Fridman, R. Discoidin domain receptor tyrosine kinases: New players in cancer progression. Cancer Metastasis Rev. 2012, 31, 295–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitinger, B.; Steplewski, A.; Fertala, A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J. Mol. Biol. 2004, 344, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B.; Kwan, A.P. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 2006, 25, 355–364. [Google Scholar] [CrossRef]
- Chen, H.; Ghori-Javed, F.Y.; Rashid, H.; Adhami, M.D.; Serra, R.; Gutierrez, S.E.; Javed, A. Runx2 regulates endochondral ossification through control of chondrocyte proliferation and differentiation. J. Bone Miner. Res. 2014, 29, 2653–2665. [Google Scholar] [CrossRef] [Green Version]
- Karp, S.J.; Schipani, E.; St-Jacques, B.; Hunzelman, J.; Kronenberg, H.; McMahon, A.P. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 2000, 127, 543–548. [Google Scholar]
- Zernik, J.; Twarog, K.; Upholt, W.B. Regulation of alkaline phosphatase and alpha 2(I) procollagen synthesis during early intramembranous bone formation in the rat mandible. Differentiation 1990, 44, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Wians, F.H., Jr.; Hauschka, P.V. The effect of osteocalcin on in vitro lipid-induced hydroxyapatite formation and seeded hydroxyapatite growth. Calcif. Tissue Int. 1985, 37, 57–62. [Google Scholar] [CrossRef]
- Ge, C.; Yang, Q.; Zhao, G.; Yu, H.; Kirkwood, K.L.; Franceschi, R.T. Interactions between extracellular signal-regulated kinase 1/2 and p38 MAP kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. J. Bone Miner. Res. 2012, 27, 538–551. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Guicheux, J.; Palmer, G.; Miura, Y.; Oiso, Y.; Bonjour, J.P.; Caverzasio, J. Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone 2002, 30, 91–98. [Google Scholar] [CrossRef]
- Lin, K.L.; Chou, C.H.; Hsieh, S.C.; Hwa, S.Y.; Lee, M.T.; Wang, F.F. Transcriptional upregulation of DDR2 by ATF4 facilitates osteoblastic differentiation through p38 MAPK-mediated Runx2 activation. J. Bone Miner. Res. 2010, 25, 2489–2503. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S. Runx2/Cbfa1: A multifunctional regulator of bone formation. Curr. Pharm. Des. 2003, 9, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Pratap, J.; Galindo, M.; Zaidi, S.K.; Vradii, D.; Bhat, B.M.; Robinson, J.A.; Choi, J.Y.; Komori, T.; Stein, J.L.; Lian, J.B.; et al. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003, 63, 5357–5362. [Google Scholar]
- Adhami, M.D.; Rashid, H.; Chen, H.; Javed, A. Runx2 activity in committed osteoblasts is not essential for embryonic skeletogenesis. Connect. Tissue Res. 2014, 55 (Suppl. 1), 102–106. [Google Scholar] [CrossRef] [Green Version]
- Lino, M.; Wan, M.H.; Rocca, A.S.; Ngai, D.; Shobeiri, N.; Hou, G.; Ge, C.; Franceschi, R.T.; Bendeck, M.P. Diabetic Vascular Calcification Mediated by the Collagen Receptor Discoidin Domain Receptor 1 via the Phosphoinositide 3-Kinase/Akt/Runt-Related Transcription Factor 2 Signaling Axis. Arter. Thromb Vasc Biol. 2018, 38, 1878–1889. [Google Scholar] [CrossRef] [Green Version]
- Greenblatt, M.B.; Shim, J.H.; Zou, W.; Sitara, D.; Schweitzer, M.; Hu, D.; Lotinun, S.; Sano, Y.; Baron, R.; Park, J.M.; et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Investig. 2010, 120, 2457–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, M.; Svensson, L.; Roach, M.; Hambor, J.; McNeish, J.; Gabel, C.A. Deficiency of the stress kinase p38alpha results in embryonic lethality: Characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J. Exp. Med. 2000, 191, 859–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curat, C.A.; Vogel, W.F. Discoidin domain receptor 1 controls growth and adhesion of mesangial cells. J. Am. Soc. Nephrol. 2002, 13, 2648–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, A.B.; Slayden, A.V.; Kumpati, J.; Perry, C.D.; Osuna, M.A.L.; Arroyo, S.R.; Miranda-Carboni, G.A.; Krum, S.A. GATA4 Directly Regulates Runx2 Expression and Osteoblast Differentiation. JBMR Plus 2018, 2, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Guo, S.; Zhang, Y.; Weng, Y.; Wang, L.; Ma, J. GATA4 regulates osteoblastic differentiation and bone remodeling via p38-mediated signaling. J. Mol. Histol. 2017, 48, 187–197. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell Biol. 1995, 15, 1858–1869. [Google Scholar] [CrossRef] [Green Version]
- Rossert, J.; Eberspaecher, H.; de Crombrugghe, B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J. Cell Biol. 1995, 129, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Nakashima, K.; de Crombrugghe, B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: A new tool to examine physiology and disease of postnatal bone and tooth. Am. J. Pathol. 2004, 165, 1875–1882. [Google Scholar] [CrossRef]
- Chou, L.Y.; Chen, C.H.; Lin, Y.H.; Chuang, S.C.; Chou, H.C.; Lin, S.Y.; Fu, Y.C.; Chang, J.K.; Ho, M.L.; Wang, C.Z. Discoidin domain receptor 1 regulates endochondral ossification through terminal differentiation of chondrocytes. FASEB J. 2020, 34, 5767–5781. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Group | Ddr1f/f-4OHT | (Mean ± SE) | OKO△Ddr1 | (Mean ± SE) | p Value | ||
|---|---|---|---|---|---|---|---|
| MaxLoad | N | 10.68 | ±3.55 | 4.26 | ±0 | 0.001 | ** |
| Break point | N | 8.31 | ±3.42 | 4.04 | ±0.01 | 0.01 | * |
| stiffness(S) | N/mm^2 | 70.69 | ±31.13 | 33.51 | ±0.01 | 0.006 | ** |
| area under the Max curve(AUC1) | J | 0.003 | ±0.0017 | 0.0012 | ±0.0099 | 0.01 | * |
| area under the Break curve(AUC2) | J | 0.0059 | ±0.0031 | 0.0028 | ±0.0134 | 0.013 | * |
| UStress | MPa | 0.53 | ±0.23 | 0.37 | ±0.19 | 0.188 | |
| Modulus | GPa | 3.23 | ±0.91 | 1.83 | ±0.14 | 0.048 | * |
| Toughness | MPa | 1.77 | ±0.73 | 0.94 | ±0.059 | 0.04 | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, L.-Y.; Chen, C.-H.; Chuang, S.-C.; Cheng, T.-L.; Lin, Y.-H.; Chou, H.-C.; Fu, Y.-C.; Wang, Y.-H.; Wang, C.-Z. Discoidin Domain Receptor 1 Regulates Runx2 during Osteogenesis of Osteoblasts and Promotes Bone Ossification via Phosphorylation of p38. Int. J. Mol. Sci. 2020, 21, 7210. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197210
Chou L-Y, Chen C-H, Chuang S-C, Cheng T-L, Lin Y-H, Chou H-C, Fu Y-C, Wang Y-H, Wang C-Z. Discoidin Domain Receptor 1 Regulates Runx2 during Osteogenesis of Osteoblasts and Promotes Bone Ossification via Phosphorylation of p38. International Journal of Molecular Sciences. 2020; 21(19):7210. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197210
Chicago/Turabian StyleChou, Liang-Yin, Chung-Hwan Chen, Shu-Chun Chuang, Tsung-Lin Cheng, Yi-Hsiung Lin, Hsin-Chiao Chou, Yin-Chih Fu, Yan-Hsiung Wang, and Chau-Zen Wang. 2020. "Discoidin Domain Receptor 1 Regulates Runx2 during Osteogenesis of Osteoblasts and Promotes Bone Ossification via Phosphorylation of p38" International Journal of Molecular Sciences 21, no. 19: 7210. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197210