Effects of Early-Life Stress on the Brain and Behaviors: Implications of Early Maternal Separation in Rodents
Abstract
:1. Introduction
2. The MS Model
3. The HPA Axis
4. Brain Regions Activated during MS
5. Alterations in Behaviors Induced by MS
5.1. Depression and Anxiety Disorders
5.2. Fear Response
5.3. Aggressive Behaviors
5.4. Reward-Seeking Behaviors
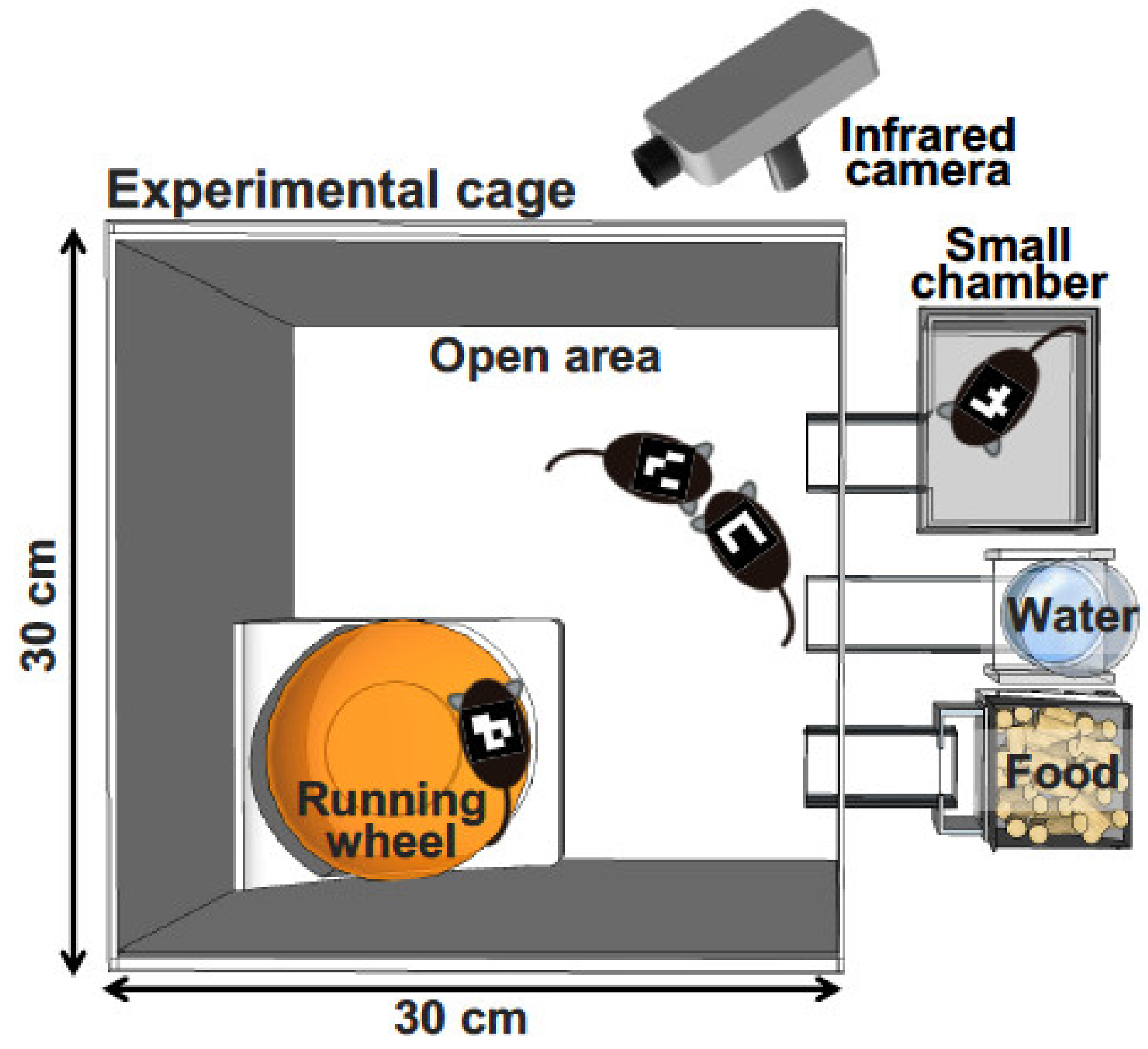
5.5. Behavioral Characteristics under Group-Housing Conditions
6. Gene Expression Alterations Induced by MS
7. Transgenerational Effects of MS
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HPA | Hypothalamic–pituitary–adrenal |
| MS | Maternal separation |
| PND | Postnatal day |
| CRH | Corticotropin-releasing hormone |
| AVP | Arginine vasopressin |
| PVN | Paraventricular nucleus of the hypothalamus |
| ACTH | Adrenocorticotropic hormone |
| CORT | Corticosterone |
| SHRP | Stress hyporesponsive period |
| GR | Glucocorticoid receptor |
| Arc | Arcuate nucleus |
| BST | Bed nucleus of the stria terminalis |
| NAc | Nucleus accumbens |
| DG | Dentate gyrus |
| CeA | Central nucleus of the amygdala |
| MePD | Medial nucleus of the amygdala |
| VTA | Ventral tegmental area |
| RFID | Radiofrequency identification |
| MAPS | Multiple Animal Positioning System |
| MeCP2 | Methyl-CpG binding protein 2 |
References
- Benmhammed, H.; El Hayek, S.; Berkik, I.; Elmostafi, H.; Bousalham, R.; Mesfioui, A.; Ouichou, A.; El Hessni, A. Animal Models of Early-Life Adversity. Psychiatric Disorders; Humana: New York, NY, USA, 2019; Volume 2011, pp. 143–161. [Google Scholar]
- Van Bodegom, M.; Homberg, J.R.; Henckens, M.J.A.G. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchecki, D. Maternal regulation of the infant’s hypothalamic-pituitary-adrenal axis stress response: Seymour ‘Gig’ Levine’s legacy to neuroendocrinology. J. Neuroendocrinol. 2018, 30, e12610. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 2016, 89, 892–909. [Google Scholar] [CrossRef] [Green Version]
- Levine, S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 2005, 30, 939–946. [Google Scholar] [CrossRef]
- Ivy, A.S.; Brunson, K.L.; Sandman, C.; Baram, T.Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience 2008, 154, 1132–1142. [Google Scholar] [CrossRef] [Green Version]
- Raineki, C.; Moriceau, S.; Sullivan, R.M. Developing a Neurobehavioral Animal Model of Infant Attachment to an Abusive Caregiver. Biol. Psychiatry 2010, 67, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Plotsky, P.M.; Meaney, M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993, 18, 195–200. [Google Scholar] [CrossRef]
- A Ellenbroek, B.; Kroonenberg, P.T.V.D.; Cools, A.R. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr. Res. 1998, 30, 251–260. [Google Scholar] [CrossRef]
- Enthoven, L.; Oitzl, M.S.; Koning, N.; Van Der Mark, M.; De Kloet, E.R. Hypothalamic-Pituitary-Adrenal Axis Activity of Newborn Mice Rapidly Desensitizes to Repeated Maternal Absence but Becomes Highly Responsive to Novelty. Endocrinology 2008, 149, 6366–6377. [Google Scholar] [CrossRef] [Green Version]
- Pryce, C.R.; Rüedi-Bettschen, D.; Dettling, A.C.; Weston, A.; Russig, H.; Ferger, B.; Feldon, J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci. Biobehav. Rev. 2005, 29, 649–674. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, V.; Durando, P.E.; Suárez, M.M. Maternal separation in early life modifies anxious behavior and Fos and glucocorticoid receptor expression in limbic neurons after chronic stress in rats: Effects of tianeptine. Stress 2015, 19, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.E.; Soundarya, S.; Karen, C.; Shanmugapriya, V.; Radhakrishnan, K. Presence of Mother Reduces Early-Life Social Stress: Linking the Alteration in Hypothalamic-Pituitary-Adrenal Axis and Serotonergic System. Dev. Neurosci. 2019, 41, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.M.; Binder, E.B. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp. Neurol. 2012, 233, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Tomoda, A.; Andersen, S.L. Neurobiological Consequences of Early Stress and Childhood Maltreatment: Are Results from Human and Animal Studies Comparable? Ann. N. Y. Acad. Sci. 2006, 1071, 313–323. [Google Scholar] [CrossRef]
- Haller, J.; Harold, G.; Sandi, C.; Neumann, I.D. Effects of Adverse Early-Life Events on Aggression and Anti-Social Behaviours in Animals and Humans. J. Neuroendocr. 2014, 26, 724–738. [Google Scholar] [CrossRef]
- Levine, S. Infantile experience and consummatory behavior in adulthood. J. Comp. Physiol. Psychol. 1957, 50, 609–612. [Google Scholar] [CrossRef]
- Levine, S.; Alpert, M.; Lewis, G.W. Infantile Experience and the Maturation of the Pituitary Adrenal Axis. Science 1957, 126, 1347. [Google Scholar] [CrossRef]
- Arborelius, L.; Eklund, M. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience 2007, 145, 738–750. [Google Scholar] [CrossRef]
- Barreau, F.; Cartier, C.; Ferrier, L.; Fioramonti, J.; Bueno, L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology 2004, 127, 524–534. [Google Scholar] [CrossRef]
- Biagini, G.; Pich, E.M.; Carani, C.; Marrama, P.; Agnati, L.F. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int. J. Dev. Neurosci. 1998, 16, 187–197. [Google Scholar] [CrossRef]
- Caldji, C.; Diorio, J.; Meaney, M.J. Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry 2000, 48, 1164–1174. [Google Scholar] [CrossRef]
- Carrera, O.; Cerrato, M.; Sanchez, A.; Gutiérrez, E. Long maternal separation has protective effects in rats exposed to activity-based anorexia. Dev. Psychobiol. 2009, 51, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Tjong, Y.W.; Ip, S.P.; Lao, L.; Wu, J.; Fong, H.H.S.; Sung, J.J.Y.; Berman, B.; Che, C.-T. Neonatal maternal separation elevates thalamic corticotrophin releasing factor type 1 receptor expression response to colonic distension in rat. Neuro Endocrinol. Lett. 2010, 31, 215–220. [Google Scholar] [PubMed]
- Francis, D.D.; Champagne, F.A.; Liu, N.; Meaney, M.J. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann. N. Y. Acad. Sci. 1999, 896, 66–84. [Google Scholar] [CrossRef]
- Huot, R. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology 2004, 29, 279–289. [Google Scholar] [CrossRef]
- Huot, R.L.; Thrivikraman, K.; Meaney, M.J.; Plotsky, P.M. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology 2001, 158, 366–373. [Google Scholar] [CrossRef]
- Menard, J.; Champagne, D.; Meaney, M. Variations of maternal care differentially influence ‘fear’ reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience 2004, 129, 297–308. [Google Scholar] [CrossRef]
- Walker, C.-D.; Scribner, K.A.; Cascio, C.S.; Dallman, M.F. The Pituitary-Adrenocortical System of Neonatal Rats Is Responsive to Stress throughout Development in a Time-Dependent and Stressor-Specific Fashion*. Endocrinology 1991, 128, 1385–1395. [Google Scholar] [CrossRef]
- Aisa, B.; Tordera, R.M.; Lasheras, B.; Del Rio, J.; Ramírez, M.J. Effects of maternal separation on hypothalamic–pituitary–adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience 2008, 154, 1218–1226. [Google Scholar] [CrossRef]
- Holmes, A.; Le Guisquet, A.M.; Vogel, E.; Millstein, R.A.; Leman, S.; Belzung, C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci. Biobehav. Rev. 2005, 29, 1335–1346. [Google Scholar] [CrossRef]
- Lippmann, M.; Bress, A.; Nemeroff, C.B.; Plotsky, P.M.; Monteggia, L.M. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur. J. Neurosci. 2007, 25, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Marais, L.; Van Rensburg, S.J.; Van Zyl, J.M.; Stein, D.J.; Daniels, W.M. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci. Res. 2008, 61, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Plotsky, P.M.; Thrivikraman, K.V.; Nemeroff, C.B.; Caldji, C.; Sharma, S.; Meaney, M.J. Long-Term Consequences of Neonatal Rearing on Central Corticotropin-Releasing Factor Systems in Adult Male Rat Offspring. Neuropsychopharmacology 2005, 30, 2192–2204. [Google Scholar] [CrossRef]
- Miyazaki, T.; Takase, K.; Nakajima, W.; Tada, H.; Ohya, D.; Sano, A.; Goto, T.; Hirase, H.; Malinow, R.; Takahashi, T. Disrupted cortical function underlies behavior dysfunction due to social isolation. J. Clin. Investig. 2012, 122, 2690–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kloet, E.R. Hormones, brain and stress. Endocr. Regul. 2003, 37, 51–68. [Google Scholar]
- Normann, C.; Buttenschøn, H.N. Gene–environment interactions between HPA-axis genes and childhood maltreatment in depression: A systematic review. Acta Neuropsychiatr. 2020, 32, 111–121. [Google Scholar] [CrossRef]
- Rothe, N.; Steffen, J.; Penz, M.; Kirschbaum, C.; Walther, A. Examination of peripheral basal and reactive cortisol levels in major depressive disorder and the burnout syndrome: A systematic review. Neurosci. Biobehav. Rev. 2020, 114, 232–270. [Google Scholar] [CrossRef]
- Levine, S. Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiol. Behav. 2001, 73, 255–260. [Google Scholar] [CrossRef]
- Rosenfeld, P.; Gutierrez, Y.A.; Martin, A.M.; Mallett, H.A.; Alleva, E.; Levine, S. Maternal regulation of the adrenocortical response in preweanling rats. Physiol. Behav. 1991, 50, 661–671. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress 1996, 1, 1–19. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Meaney, M.J. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986, 396, 64–76. [Google Scholar] [CrossRef]
- Borges-Aguiar, A.C.; Schauffer, L.Z.; De Kloet, E.R.; Schenberg, L.C. Daily maternal separations during stress hyporesponsive period decrease the thresholds of panic-like behaviors to electrical stimulation of the dorsal periaqueductal gray of the adult rat. Behav. Brain Res. 2018, 344, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Kutty, B.M.; Laxmi, T.R. The impact of maternal separation and isolation stress during stress hyporesponsive period on fear retention and extinction recall memory from 5-week- to 1-year-old rats. Exp. Brain Res. 2018, 237, 181–190. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.M.; Kehoe, P.; Kovacs, S. Corticosterone release in response to repeated, short episodes of neonatal isolation: Evidence of sensitization. Int. J. Dev. Neurosci. 1998, 16, 175–185. [Google Scholar] [CrossRef]
- Horii-Hayashi, N.; Sasagawa, T.; Matsunaga, W.; Matsusue, Y.; Azuma, C.; Nishi, M. Developmental Changes in Desensitisation of c-Fos Expression Induced by Repeated Maternal Separation in Pre-Weaned Mice. J. Neuroendocr. 2013, 25, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Jahng, J.; Ryu, V.; Yoo, S.; Noh, S.; Kim, J.; Lee, J.-H. Mesolimbic dopaminergic activity responding to acute stress is blunted in adolescent rats that experienced neonatal maternal separation. Neuroscience 2010, 171, 144–152. [Google Scholar] [CrossRef]
- Ryu, V.; Lee, J.-H.; Yoo, S.B.; Gu, X.F.; Moon, Y.W.; Jahng, J. Sustained hyperphagia in adolescent rats that experienced neonatal maternal separation. Int. J. Obes. 2008, 32, 1355–1362. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, S.; Martinsson, M.; Nylander, I.; Roman, E. Altered corticosterone levels and social play behavior after prolonged maternal separation in adolescent male but not female Wistar rats. Horm. Behav. 2017, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Benito, E.; Barco, A. The Neuronal Activity-Driven Transcriptome. Mol. Neurobiol. 2014, 51, 1071–1088. [Google Scholar] [CrossRef]
- Fabianová, K.; Závodská, M.; Raček, A.; Angelidis, A.; Martončiková, M.; Račeková, E. Analysis of Fos expression in the rat olfactory neurogenic region following single exposure to maternal separation during different neonatal stages. Gen. Physiol. Biophys. 2018, 37, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Tenorio-Lopes, L.; Henry, M.S.; Marques, D.; Tremblay, M.-È.; Drolet, G.; Bretzner, F.; Kinkead, R. Neonatal maternal separation opposes the facilitatory effect of castration on the respiratory response to hypercapnia of the adult male rat: Evidence for the involvement of the medial amygdala. J. Neuroendocr. 2017, 29, e12550. [Google Scholar] [CrossRef] [PubMed]
- Enishi, M.; Ehorii-Hayashi, N.; Esasagawa, T. Effects of early life adverse experiences on the brain: Implications from maternal separation models in rodents. Front. Neurosci. 2014, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.; Walker, D.L.; Miles, L.; Grillon, C. Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology 2009, 35, 105–135. [Google Scholar] [CrossRef] [Green Version]
- Henning, S.J. Plasma concentrations of total and free corticosterone during development in the rat. Am. J. Physiol. Metab. 1978, 235, E451. [Google Scholar] [CrossRef] [Green Version]
- Moriceau, S.; Roth, T.L.; Sullivan, R.M. Rodent model of infant attachment learning and stress. Dev. Psychobiol. 2010, 52, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Herdegen, T.; Leah, J. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Rev. 1998, 28, 370–490. [Google Scholar] [CrossRef]
- Smith, P.M.; Ferguson, A.V. Circulating signals as critical regulators of autonomic state—central roles for the subfornical organ. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R405–R415. [Google Scholar] [CrossRef]
- Glaser, J.-P.; Van Os, J.; Portegijs, P.J.; Myin-Germeys, I. Childhood trauma and emotional reactivity to daily life stress in adult frequent attenders of general practitioners. J. Psychosom. Res. 2006, 61, 229–236. [Google Scholar] [CrossRef]
- Macmillan, H.L.; Fleming, J.E.; Streiner, D.L.; Duku, E.K.; Wong, M.Y.-Y.; Lin, E.; Boyle, M.H.; Jamieson, E.; Walsh, C.A.; Beardslee, W.R. Childhood Abuse and Lifetime Psychopathology in a Community Sample. Am. J. Psychiatry 2001, 158, 1878–1883. [Google Scholar] [CrossRef]
- Kendler, K.S.; Neale, M.C.; Kessler, R.C.; Heath, A.C.; Eaves, L.J. Childhood Parental Loss and Adult Psychopathology in Women. Arch. Gen. Psychiatry 1992, 49, 109–116. [Google Scholar] [CrossRef]
- Agid, O.; Shapira, B.; Zislin, J.; Ritsner, M.; Hanin, B.; Murad, H.; Troudart, T.; Bloch, M.; Heresco-Levy, U.; Lerer, B. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol. Psychiatry 1999, 4, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, T.A.; Ogura, A.; Hirai, T.; Fujihara, S.; Kitamura, T.; Takahashi, K. Early parental separation experiences among patients with bipolar disorder and major depression: A case-control study. J. Affect. Disord. 1999, 52, 85–91. [Google Scholar] [CrossRef]
- Heim, C.; Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Boil. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef] [Green Version]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Stein, M.B.; Walker, J.R.; Anderson, G.; Hazen, A.L.; A Ross, C.; Eldridge, G.; Forde, D.R. Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. Am. J. Psychiatry 1996, 153, 275–277. [Google Scholar] [CrossRef]
- Ladd, C.O.; Owens, M.J.; Nemeroff, C.B. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology 1996, 137, 1212–1218. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, D.M.; López, J.F.; Van Hoers, H.; Watson, S.J.; Levine, S. Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 2000, 855, 76–82. [Google Scholar] [CrossRef]
- Daniels, W.M.U.; Pietersen, C.Y.; Carstens, M.E.; Stein, D.J. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab. Brain Dis. 2004, 19, 3–14. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.J.; Kim, J.G.; Ryu, V.; Kim, B.-T.; Kang, D.-W.; Jahng, J.W. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci. Res. 2007, 58, 32–39. [Google Scholar] [CrossRef]
- Newport, D.J.; Stowe, Z.N.; Nemeroff, C.B. Parental Depression: Animal Models of an Adverse Life Event. Am. J. Psychiatry 2002, 159, 1265–1283. [Google Scholar] [CrossRef]
- Ryu, V.; Yoo, S.B.; Kang, D.-W.; Lee, J.-H.; Jahng, J.W. Post-weaning isolation promotes food intake and body weight gain in rats that experienced neonatal maternal separation. Brain Res. 2009, 1295, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Zlotnik, A.; Kofman, O.; Grinshpun, J.; Severynovska, O.; Brotfain, E.; Kuts, R.; Natanel, D.; Melamed, I.; Boyko, M. Early life stress induces submissive behavior in adult rats. Behav. Brain Res. 2019, 372, 112025. [Google Scholar] [CrossRef] [PubMed]
- Kaffman, A.; White, J.D.; Wei, L.; Johnson, F.K.; Krystal, J.H. Enhancing the Utility of Preclinical Research in Neuropsychiatry Drug Development. Methods Mol. Biol. 2019, 2011, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Levine, J.L.S.; Avila-Quintero, V.; Bloch, M.; Kaffman, A. Systematic review and meta-analysis: Effects of maternal separation on anxiety-like behavior in rodents. Transl. Psychiatry 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Callaghan, B.L.; Graham, B.M.; Li, S.; Richardson, R. From Resilience to Vulnerability: Mechanistic Insights into the Effects of Stress on Transitions in Critical Period Plasticity. Front. Psychiatry 2013, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Chocyk, A.; Przyborowska, A.; Makuch, W.; Majcher-Maślanka, I.; Dudys, D.; Wedzony, K. The effects of early-life adversity on fear memories in adolescent rats and their persistence into adulthood. Behav. Brain Res. 2014, 264, 161–172. [Google Scholar] [CrossRef]
- Veenema, A.H.; Blume, A.; Niederle, D.; Buwalda, B.; Neumann, I.D. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur. J. Neurosci. 2006, 24, 1711–1720. [Google Scholar] [CrossRef] [Green Version]
- Sasagawa, T.; Horii-Hayashi, N.; Okuda, A.; Hashimoto, T.; Azuma, C.; Nishi, M. Long-term effects of maternal separation coupled with social isolation on reward seeking and changes in dopamine D1 receptor expression in the nucleus accumbens via DNA methylation in mice. Neurosci. Lett. 2017, 641, 33–39. [Google Scholar] [CrossRef]
- Hikida, T.; Kimura, K.; Wada, N.; Funabiki, K.; Nakanishi, S. Distinct Roles of Synaptic Transmission in Direct and Indirect Striatal Pathways to Reward and Aversive Behavior. Neuron 2010, 66, 896–907. [Google Scholar] [CrossRef] [Green Version]
- Hikida, T.; Yawata, S.; Yamaguchi, T.; Danjo, T.; Sasaoka, T.; Wang, Y.; Nakanishi, S. Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc. Natl. Acad. Sci. USA 2012, 110, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Der-Avakian, A.; Markou, A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience 2010, 170, 1189–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, T.D.A.; Correia, D.; De Carvalho, L.M.; Damasceno, S.; Godard, A.L.B. Maternal separation affects expression of stress response genes and increases vulnerability to ethanol consumption. Brain Behav. 2017, 8, e00841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of Addiction. Neuropsychopharmacology 2010, 35, 1051. [Google Scholar] [CrossRef] [Green Version]
- Benner, S.; Endo, T.; Endo, N.; Kakeyama, M.; Tohyama, C. Early deprivation induces competitive subordinance in C57BL/6 male mice. Physiol. Behav. 2014, 137, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Endo, N.; Ujita, W.; Fujiwara, M.; Miyauchi, H.; Mishima, H.; Makino, Y.; Hashimoto, L.; Oyama, H.; Makinodan, M.; Nishi, M.; et al. Multiple animal positioning system shows that socially-reared mice influence the social proximity of isolation-reared cagemates. Commun. Biol. 2018, 1, 225. [Google Scholar] [CrossRef] [PubMed]
- Endo, N.; Makinodan, M.; Somayama, N.; Komori, T.; Kishimoto, T.; Nishi, M. Characterization of behavioral phenotypes in the BTBR T(+) Itpr3(tf)/J mouse model of autism spectrum disorder under social housing conditions using the multiple animal positioning system. Exp. Anim. 2019, 68, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Meaney, M.J.; Szyf, M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005, 28, 456–463. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Bludau, A.; Royer, M.; Meister, G.; Neumann, I.D.; Menon, R. Epigenetic Regulation of the Social Brain. Trends Neurosci. 2019, 42, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Kuek, E.J.W.; Su, C.-L.; Gean, P.-W. MicroRNA-206 Regulates Stress-Provoked Aggressive Behaviors in Post-weaning Social Isolation Mice. Mol. Ther. Nucleic Acids 2020, 20, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Fogelman, N.; Canli, T. Early Life Stress, Physiology, and Genetics: A Review. Front. Psychol. 2019, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Talarowska, M. Epigenetic Mechanisms in the Neurodevelopmental Theory of Depression. Depress. Res. Treat. 2020, 2020, 6357873. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Shepard, R.D.; Langlois, L.D.; Authement, M.E.; Nugent, F.S. Histone deacetylase inhibition reduces ventral tegmental area dopamine neuronal hyperexcitability involving AKAP150 signaling following maternal deprivation in juvenile male rats. J. Neurosci. Res. 2020, 98, 1457–1467. [Google Scholar] [CrossRef]
- Murphy, J.G.; Crosby, K.C.; Dittmer, P.J.; Sather, W.A.; Dell’Acqua, M.L. AKAP79/150 recruits the transcription factor NFAT to regulate signaling to the nucleus by neuronal L-type Ca(2+) channels. Mol. Biol. Cell 2019, 30, 1743–1756. [Google Scholar] [CrossRef]
- Misztakk, P.; Panczyszyn-Trzewik, P.; Sowa-Kućma, M. Histone deacetylases (HDACs) as therapeutic target for depressive disorders. Pharmacol. Rep. 2018, 70, 398–408. [Google Scholar] [CrossRef]
- Murgatroyd, C.A.; Patchev, A.V.; Wu, Y.; Micale, V.; Bockmühl, Y.; Fischer, D.; Holsboer, F.; Wotjak, C.T.; Almeida, O.F.X.; Spengler, D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009, 12, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Murgatroyd, C.A.; Nephew, B.C. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology 2012, 38, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Kember, R.L.; Dempster, E.; Lee, T.H.A.; Schalkwyk, L.C.; Mill, J.; Fernandes, C. Maternal separation is associated with strain-specific responses to stress and epigenetic alterations toNr3c1,Avp, andNr4a1in mouse. Brain Behav. 2012, 2, 455–467. [Google Scholar] [CrossRef] [Green Version]
- E Helfer, R.; Pollock, C.B. The battered child syndrome. Adv. Pediatr. 1968, 15, 9–27. [Google Scholar]
- Widom, C.S.; Czaja, S.J.; Dumont, K.A. Intergenerational transmission of child abuse and neglect: Real or detection bias? Science 2015, 347, 1480–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlin, L.J.; Appleyard, K.; Dodge, K.A. Intergenerational Continuity in Child Maltreatment: Mediating Mechanisms and Implications for Prevention. Child. Dev. 2011, 82, 162–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, L.; Browne, K.D.; Hamilton-Giachritsis, C. Risk factors of parents abused as children: A mediational analysis of the intergenerational continuity of child maltreatment (Part I). J. Child. Psychol. Psychiatry 2005, 46, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Maestripieri, D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc. Natl. Acad. Sci. USA 2005, 102, 9726–9729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renner, L.M.; Slack, K.S. Intimate partner violence and child maltreatment: Understanding intra- and intergenerational connections. Child. Abus. Negl. 2006, 30, 599–617. [Google Scholar] [CrossRef]
- Sidebotham, P.; Golding, J. Child maltreatment in the “children of the nineties” a longitudinal study of parental risk factors. Child. Abus. Negl. 2001, 25, 1177–1200. [Google Scholar] [CrossRef]
- Meaney, M.J. Maternal Care, Gene Expression, and the Transmission of Individual Differences in Stress Reactivity Across Generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef]
- Franklin, T.B.; Russig, H.; Weiss, I.C.; Gräff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I. Epigenetic Transmission of the Impact of Early Stress Across Generations. Boil. Psychiatry 2010, 68, 408–415. [Google Scholar] [CrossRef]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, M. Effects of Early-Life Stress on the Brain and Behaviors: Implications of Early Maternal Separation in Rodents. Int. J. Mol. Sci. 2020, 21, 7212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197212
Nishi M. Effects of Early-Life Stress on the Brain and Behaviors: Implications of Early Maternal Separation in Rodents. International Journal of Molecular Sciences. 2020; 21(19):7212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197212
Chicago/Turabian StyleNishi, Mayumi. 2020. "Effects of Early-Life Stress on the Brain and Behaviors: Implications of Early Maternal Separation in Rodents" International Journal of Molecular Sciences 21, no. 19: 7212. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197212



