Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases
Abstract
:1. Introduction
Discovery of the Endocannabinoid System
2. Cannabinoid Receptors
2.1. Molecular Architecture
2.2. Ligands
2.2.1. Endogenous Cannabinoids
2.2.2. Exogenous Cannabinoids
2.3. Tissue and Cellular Distribution
2.4. Signaling
2.4.1. Canonical
2.4.2. Non-Canonical
3. Biological Role of the Cannabinoid Receptors
3.1. Cellular Morphogenesis and Developmental Roles
3.1.1. Embryogenesis
3.1.2. Neurodevelopment
3.1.3. Implications of a Dysregulated Endocannabinoid Tone in Neurodevelopmental Disorders
3.2. Neurological
3.2.1. Neuromodulation
3.2.2. Neuroinflammation and Neuroprotection
3.2.3. Implications of Dysregulated Endocannabinoid Tone in Neurological Disorders
3.3. Metabolic
3.3.1. Hypophagia
3.3.2. Peripheral Energy Regulation
3.3.3. Implications of a Dysregulated Endocannabinoid Tone in Metabolic Disorders
3.4. Cardiovascular
3.4.1. Triple/Triphasic Response of Cannabinoids
3.4.2. Central Mechanisms of Blood Pressure Regulation
3.4.3. Peripheral Mechanisms of Blood Pressure Regulation
3.4.4. Implications of a Dysregulated Endocannabinoid Tone in Cardiovascular Disorders
3.5. Modulation of Immune System
3.5.1. Immune Cell Development and Differentiation
3.5.2. Implications of a Dysregulated Endocannabinoid Tone in Immunological Disorders
4. Therapeutic Strategies
4.1. Multi-Drug Strategy
4.2. Peripheral CB1R Antagonists
4.3. Allosteric Modulators
4.4. CB2R Modulators
4.5. Inhibitors of Degradative Enzymes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Touw, M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends Pharmacol. Sci. 2006, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Todd, A.R. Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of Cannabinol. J. Chem. Soc. 1940, 649–653. [Google Scholar] [CrossRef]
- Adams, R.; Baker, B.R.; Wearn, R.B. Structure of Cannabinol. III. Synthesis of Cannabinol, 1-Hydroxy-3-n-amyl-6,6,9-trimethyl-6-dibenzopyran. J. Am. Chem. Soc. 1940, 62, 2204–2207. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. The Isolation and Structure of Δ-Tetrahydrocannabinol and Other Neutral Cannabinoids from Hashish. J. Am. Chem. Soc. 1971, 93, 217–224. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadas, H.; Gaillet, S.; Beltramo, M.; Venance, L.; Piomelli, D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J. Neurosci. 1996, 16, 3934–3942. [Google Scholar] [CrossRef] [Green Version]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2009, 147, S163–S171. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Hoehe, M.R.; Caenazzo, L.; Martinez, M.M.; Hsieh, W.T.; Modi, W.S.; Gershon, E.S.; Bonner, T.I. Genetic and physical mapping of the human cannabinoid receptor gene to chromosome 6q14-q15. New Biol. 1991, 3, 880–885. [Google Scholar]
- Zhang, P.W.; Ishiguro, H.; Ohtsuki, T.; Hess, J.; Carillo, F.; Walther, D.; Onaivi, E.S.; Arinami, T.; Uhl, G.R. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol. Psychiatry. 2004, 9, 916–931. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, I.; Krzysik-Walker, S.M.; Doyle, M.E.; Liu, Q.R.; Cimbro, R.; Santa-Cruz Calvo, S.; Ghosh, S.; Cieala, A.; Moaddel, R.; Carlson, O.D.; et al. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.R.; Huang, N.S.; Qu, H.; O’Connell, J.F.; Gonzalez-Mariscal, I.; Santa-Cruz-Calvo, S.; Doyle, M.E.; Xi, Z.X.; Wang, Y.; Onaivi, E.S.; et al. Identification of novel mouse and rat CB1R isoforms and in silico modeling of human CB1R for peripheral cannabinoid therapeutics. Acta Pharmacol. Sin. 2019, 40, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Howlett, A.C. Rat brain cannabinoid receptors are N-linked glycosylated proteins. Life Sci. 1995, 56, 1983–1989. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, D.; Wu, M.; Guo, Y.; Guo, W.; Zhong, L.; Cai, X.; Dai, A.; Jang, W.; Shakhnovich, E.; et al. Common activation mechanism of class a GPCRs. Elife 2019, 8. [Google Scholar] [CrossRef]
- Al-Zoubi, R.; Morales, P.; Reggio, P.H. Structural Insights into CB1 Receptor Biased Signaling. Int. J. Mol. Sci. 2019, 20, 1837. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; D’Antona, A.M.; Kendall, D.A.; Von Heijne, G.; Chin, C.-N. Membrane assembly of the cannabinoid receptor 1: Impact of a long N-terminal tail. Mol. Pharmacol. 2003, 64, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Fletcher-Jones, A.; Hildick, K.L.; Evans, A.J.; Nakamura, Y.; Henley, J.M.; Wilkinson, K.A. Protein Interactors and Trafficking Pathways That Regulate the Cannabinoid Type 1 Receptor (CB1R). Front. Mol. Neurosci. 2020, 13, 108. [Google Scholar] [CrossRef]
- Fay, J.F.; Farrens, D.L. The membrane proximal region of the cannabinoid receptor CB1 N-terminus can allosterically modulate ligand affinity. Biochemistry 2013, 52, 8286–8294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Ulfers, A.L.; McMurry, J.L.; Miller, A.; Wang, L.; Kendall, D.A.; Mierke, D.F. Cannabinoid receptor-G protein interactions: Gαi1-bound structures of IC3 and a mutant with altered G protein specificity. Protein Sci. 2009, 11, 2526–2531. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, W.; Fan, Y.; Luo, J.; Hong, K.; Wang, Z.; Yan, J.; Chen, X.; Lu, J.; Benovic, J.; et al. Structural determinants in the second intracellular loop of the human cannabinoid CB 1 receptor mediate selective coupling to G s and G i. Br. J. Pharmacol. 2010, 161, 1817–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.; Brown, S.; Roche, J.P.; Hsieh, C.; Celver, J.P.; Kovoor, A.; Chavkin, C.; Mackie, K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 1999, 19, 3773–3780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadji, V.; Lucas-Lenard, J.M.; Chin, C.; Kendall, D.A. Involvement of the Carboxyl Terminus of the Third Intracellular Loop of the Cannabinoid CB1 Receptor in Constitutive Activation of Gs. J. Neurochem. 2008, 72, 2032–2038. [Google Scholar] [CrossRef]
- Niehaus, J.L.; Liu, Y.; Wallis, K.T.; Egertová, M.; Bhartur, S.G.; Mukhopadhyay, S.; Shi, S.; He, H.; Selley, D.E.; Howlett, A.C.; et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol. Pharmacol. 2007, 72, 1557–1566. [Google Scholar] [CrossRef]
- Bakshi, K.; Mercier, R.W.; Pavlopoulos, S. Interaction of a fragment of the cannabinoid CB1 receptor C-terminus with arrestin-2. Febs Lett. 2007, 581, 5009–5016. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Lewis, D.L. The proximal and distal C-terminal tail domains of the CB1 cannabinoid receptor mediate G protein coupling. Neuroscience 2001, 107, 161–167. [Google Scholar] [CrossRef]
- Ahn, K.H.; Pellegrini, M.; Tsomaia, N.; Yatawara, A.K.; Kendall, D.A.; Mierke, D.F. Structural analysis of the human cannabinoid receptor one carboxyl-terminus identifies two amphipathic helices. Biopolymers 2009, 91, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.H.; Nishiyama, A.; Mierke, D.F.; Kendall, D.A. Hydrophobic residues in helix 8 of cannabinoid receptor 1 are critical for structural and functional Properties. Biochemistry 2010, 49, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Fletcher-Jones, A.; Hildick, K.L.; Evans, A.J.; Nakamura, Y.; Wilkinson, K.A.; Henley, J.M. The C-Terminal helix 9 motif in rat cannabinoid receptor type 1 regulates axonal trafficking and surface expression. Elife 2019, 8. [Google Scholar] [CrossRef]
- Liu, Q.R.; Pan, C.H.; Hishimoto, A.; Li, C.Y.; Xi, Z.X.; Llorente-Berzal, A.; Viveros, M.P.; Ishiguro, H.; Arinami, T.; Onaivi, E.S.; et al. Species differences in cannabinoid receptor 2 (CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genesbrain Behav. 2009, 8, 519–530. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Bi, G.H.; Li, X.; Li, J.; Qu, H.; Zhang, S.J.; Li, C.Y.; Onaivi, E.S.; Gardner, E.L.; Xi, Z.X.; et al. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 2015, 40, 1037–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.-Q.; Chen, J.-Z.; Billings, E.M. 3D structural model of the G-protein-coupled cannabinoid CB2 receptor. Proteins Struct. Funct. Genet. 2003, 53, 307–319. [Google Scholar] [CrossRef]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Montero, C.; Campillo, N.E.; Goya, P.; Ṕez, J.A. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur. J. Med. Chem. 2005, 40, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, L.; Chen, X.; He, X.; Yang, J.; Shi, Y.; Zhou, N. The Second Intracellular Loop of the Human Cannabinoid CB2 Receptor Governs G Protein Coupling in Coordination with the Carboxyl Terminal Domain. PLoS ONE 2013, 8, e63262. [Google Scholar] [CrossRef] [Green Version]
- Basith, S.; Cui, M.; Macalino, S.J.Y.; Park, J.; Clavio, N.A.B.; Kang, S.; Choi, S. Exploring G protein-coupled receptors (GPCRs) ligand space via cheminformatics approaches: Impact on rational drug design. Front. Pharmacol. 2018, 9, 128. [Google Scholar] [CrossRef]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Melck, D.; Bobrov, M.Y.; Gretskaya, N.M.; Bezuglov, V.V.; De Petrocellis, L.; Di Marzo, V. N-acyl-dopamines: Novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity In Vitro and In Vivo. Biochem. J. 2000, 351, 817–824. [Google Scholar] [CrossRef]
- Hanus, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, A.C.; Sauer, J.M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamplona, F.A.; Ferreira, J.; De Lima, O.M.; Duarte, F.S.; Bento, A.F.; Forner, S.; Villarinho, J.G.; Bellochio, L.; Wotjak, C.T.; Lerner, R.; et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 21134–21139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrucci, V.; Chicca, A.; Glasmacher, S.; Paloczi, J.; Cao, Z.; Pacher, P.; Gertsch, J. Pepcan-12 (RVD-hemopressin) is a CB2 receptor positive allosteric modulator constitutively secreted by adrenals and in liver upon tissue damage. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Reggio, P.H. An Update on Non-CB 1, Non-CB 2 Cannabinoid Related G-Protein-Coupled Receptors. Cannabis Cannabinoid Res. 2017, 2, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci. 1991, 11, 563–583. [Google Scholar] [CrossRef]
- Tsou, K.; Brown, S.; Sañudo-Peña, M.C.; Mackie, K.; Walker, J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998, 83, 393–411. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Egertová, M.; Bradbury, E.J.; McMahon, S.B.; Rice, A.S.C.; Elphick, M.R. Cannabinoid CB1 Receptor Expression in Rat Spinal Cord. Mol. Cell. Neurosci. 2000, 15, 510–521. [Google Scholar] [CrossRef]
- Freundt-Revilla, J.; Kegler, K.; Baumgärtner, W.; Tipold, A. Spatial distribution of cannabinoid receptor type 1 (CB1) in normal canine central and peripheral nervous system. PLoS ONE 2017, 12, e0181064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishac, E.J.N.; Jiang, L.; Lake, K.D.; Varga, K.; Abood, M.E.; Kunos, G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996, 118, 2023–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutts, A.A.; Pertwee, R.G. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br. J. Pharmacol. 1997, 121, 1557–1566. [Google Scholar] [CrossRef] [Green Version]
- Croci, T.; Manara, L.; Aureggi, G.; Guagnini, F.; Rinaldi-Carmona, M.; Maffrand, J.-P.; Fur, G.; Mukenge, S.; Ferla, G. In Vitro functional evidence of neuronal cannabinoid CB 1 receptors in human ileum. Br. J. Pharmacol. 1998, 125, 1393–1395. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, A.G.; Herkenham, M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience 1999, 92, 1171–1175. [Google Scholar] [CrossRef]
- Kulkarni-Narla, A.; Brown, D.R. Localization of CB1 -cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res. 2000, 302, 73–80. [Google Scholar] [CrossRef]
- Coutts, A.A.; Irving, A.J.; Mackie, K.; Pertwee, R.G.; Anavi-Goffer, S. Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J. Comp. Neurol. 2002, 448, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Navarro, M.; Ferrer, B.; Trigo, J.M.; Bilbao, A.; Del Arco, I.; Cippitelli, A.; Nava, F.; Piomelli, D.; Rodríguez de Fonseca, F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J. Neurosci. 2002, 22, 9612–9617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutts, A.A.; Izzo, A.A. The gastrointestinal pharmacology of cannabinoids: An update. Curr. Opin. Pharmacol. 2004, 4, 572–579. [Google Scholar] [CrossRef]
- Vianna, C.R.; Donato, J.; Rossi, J.; Scott, M.; Economides, K.; Gautron, L.; Pierpont, S.; Elias, C.F.; Elmquist, J.K. Cannabinoid Receptor 1 in the Vagus Nerve Is Dispensable for Body Weight Homeostasis But Required for Normal Gastrointestinal Motility. J. Neurosci. 2012, 32, 10331–10337. [Google Scholar] [CrossRef] [Green Version]
- Ong, W.Y.; Mackie, K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience 1999, 92, 1177–1191. [Google Scholar] [CrossRef]
- Rodriguez, J.J.; Mackie, K.; Pickel, V.M. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J. Neurosci. 2001, 21, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Fukaya, M.; Maejima, T.; Yoshida, T.; Miura, E.; Watanabe, M.; Ohno-Shosaku, T.; Kano, M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 2006, 26, 2991–3001. [Google Scholar] [CrossRef] [Green Version]
- Tsou, K.; Mackie, K.; Sañudo-Peña, M.C.; Walker, J.M. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience 1999, 93, 969–975. [Google Scholar] [CrossRef]
- Katona, I.; Sperlágh, B.; Sík, A.; Käfalvi, A.; Vizi, E.S.; Mackie, K.; Freund, T.F. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 1999, 19, 4544–4558. [Google Scholar] [CrossRef] [PubMed]
- Gessa, G.L.; Casu, M.A.; Carta, G.; Mascia, M.S. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. Eur. J. Pharmacol. 1998, 355, 119–124. [Google Scholar] [CrossRef]
- Oropeza, V.C.; Mackie, K.; Van Bockstaele, E.J. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007, 1127, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Hermann, H.; Marsicano, G.; Lutz, B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 2002, 109, 451–460. [Google Scholar] [CrossRef]
- Nyilas, R.; Gregg, L.C.; Mackie, K.; Watanabe, M.; Zimmer, A.; Hohmann, A.G.; Katona, I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur. J. Neurosci. 2009, 29, 1964–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldrich, G.; Wenger, T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides 2000, 21, 1735–1742. [Google Scholar] [CrossRef]
- Molina-Holgado, E.; Vela, J.M.; Arévalo-Martín, A.; Almazán, G.; Molina-Holgado, F.; Borrell, J.; Guaza, C. Cannabinoids promote oligodendrocyte progenitor survival: Involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J. Neurosci. 2002, 22, 9742–9753. [Google Scholar] [CrossRef] [PubMed]
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef]
- Zhang, H.; Hilton, D.A.; Hanemann, C.O.; Zajicek, J. Cannabinoid Receptor and N-acyl Phosphatidylethanolamine Phospholipase D-Evidence for Altered Expression in Multiple Sclerosis. Brain Pathol. 2011, 21. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, S.J.; Marciano-Cabral, F.; Staab, A.; Ludwick, C.; Cabral, G.A. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2002, 2, 69–82. [Google Scholar] [CrossRef]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Ehrhart, J.; Obregon, D.; Mori, T.; Hou, H.; Sun, N.; Bai, Y.; Klein, T.; Fernandez, F.; Tan, J.; Shytle, D. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflammation 2005, 2. [Google Scholar] [CrossRef] [Green Version]
- Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010, 58, 1017–1030. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Bátkai, S.; Kunos, G. Cardiovascular pharmacology of cannabinoids. Handb. Exp. Pharmacol. 2005, 168, 599–625. [Google Scholar] [CrossRef]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Tóth, Z.E.; Balla, A.; Catt, K.J.; Hunyady, L. Angiotensin II induces vascular endocannabinoid release, which attenuates its vasoconstrictor effect via CB1 cannabinoid receptors. J. Biol. Chem. 2012, 287, 31540–31550. [Google Scholar] [CrossRef] [Green Version]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Bátkai, S.; Harvey-White, J.; Mackie, K.; Offertáler, L.; Wang, L.; et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005, 115, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, M.; Gary-Bobo, M.; Esclangon, A.; Maffrand, J.P.; Le Fur, G.; Oury-Donat, F.; Soubrié, P. The Cannabinoid CB 1 Receptor Antagonist SR141716 Increases Acrp30 mRNA Expression in Adipose Tissue of Obese fa/fa Rats and in Cultured Adipocyte Cells. Mol. Pharmacol. 2003, 63, 908–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebremedhin, D.; Lange, A.R.; Campbell, W.B.; Hillard, C.J.; Harder, D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999, 276, H2085–H2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Gao, B.; Mirshahi, F.; Sanyal, A.J.; Khanolkar, A.D.; Makriyannis, A.; Kunos, G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 2000, 346 Pt 3, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Bonz, A.; Laser, M.; Küllmer, S.; Kniesch, S.; Babin-Ebell, J.; Popp, V.; Ertl, G.; Wagner, J.A. Cannabinoids Acting on CB1 Receptors Decrease Contractile Performance in Human Atrial Muscle. J. Cardiovasc. Pharmacol. 2003, 41, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Bátkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertáler, L.; Mackie, K.; Rudd, M.A.; Bukoski, R.D.; et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- Nakata, M.; Yada, T. Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet β-cells via CB1 receptors. Regul. Pept. 2008, 145, 49–53. [Google Scholar] [CrossRef]
- Meccariello, R.; Battista, N.; Bradshaw, H.B.; Wang, H. Updates in reproduction coming from the endocannabinoid system. Int. J. Endocrinol. 2014, 2014, 412354. [Google Scholar] [CrossRef]
- Rice, W.; Shannon, J.M.; Burton, F.; Fiedeldey, D. Expression of a brain-type cannabinoid receptor (CB1) in alveolar Type II cells in the lung: Regulation by hydrocortisone. Eur. J. Pharmacol. 1997, 327, 227–232. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Julien, B.; Grenard, P.; Teixeira-Clerc, F.; Van Nhieu, J.T.; Li, L.; Karsak, M.; Zimmer, A.; Mallat, A.; Lotersztajn, S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, S.; Yang, P.; Tian, Y.; Feng, Z.; Xie, X.Q.; Liu, Y. Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int. 2018, 94, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J.; Duarte, J.A.; Sánchez-Navarro, A.; Remião, F.; Bastos, M.L.; Carvalho, F. Paraquat Poisonings: Mechanisms of Lung Toxicity, Clinical Features, and Treatment. Crit. Rev. Toxicol. 2008, 38, 13–71. [Google Scholar] [CrossRef] [PubMed]
- Michler, T.; Storr, M.; Kramer, J.; Ochs, S.; Malo, A.; Reu, S.; Göke, B.; Schäfer, C. Activation of cannabinoid receptor 2 reduces inflammation in acute experimental pancreatitis via intra-acinar activation of p38 and MK2-dependent mechanisms. Am. J. Physiol. Liver Physiol. 2013, 304, G181–G192. [Google Scholar] [CrossRef] [Green Version]
- Roche, R.; Hoareau, L.; Bes-Houtmann, S.; Gonthier, M.-P.; Laborde, C.; Baron, J.-F.; Haffaf, Y.; Cesari, M.; Festy, F. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem. Cell Biol. 2006, 126, 177–187. [Google Scholar] [CrossRef]
- Yu, T.S.; Cheng, Z.H.; Li, L.Q.; Zhao, R.; Fan, Y.Y.; Du, Y.; Ma, W.X.; Guan, D.W. The cannabinoid receptor type 2 is time-dependently expressed during skeletal muscle wound healing in rats. Int. J. Leg. Med. 2010, 124, 397–404. [Google Scholar] [CrossRef]
- Lépicier, P.; Lagneux, C.; Sirois, M.G.; Lamontagne, D. Endothelial CB1-receptors limit infarct size through NO formation in rat isolated hearts. Life Sci. 2007, 81, 1373–1380. [Google Scholar] [CrossRef]
- Fede, C.; Albertin, G.; Petrelli, L.; Sfriso, M.M.; Biz, C.; De Caro, R.; Stecco, C. Expression of the endocannabinoid receptors in human fascial tissue. Eur. J. Histochem. 2016, 60, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Whyte, L.S.; Ford, L.; Ridge, S.A.; Cameron, G.A.; Rogers, M.J.; Ross, R.A. Cannabinoids and bone: Endocannabinoids modulate human osteoclast function In Vitro. Br. J. Pharmacol. 2012, 165, 2584–2597. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C.; Fleming, R.M. Cannabinoid Inhibition of Adenylate Cyclase. Pharmacology of the Response in Neuroblastoma Cell Membranes-PubMed. Mol Pharm. 1984, 26, 532–538. [Google Scholar]
- Howlett, A.; Qualy, J.M.; L, K.L. Involvement of Gi in the Inhibition of Adenylate Cyclase by Cannabimimetic Drugs-PubMed. Mol Pharm. 1986, 29, 307–313. [Google Scholar]
- Henry, D.J.; Chavkin, C. Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neurosci. Lett. 1995, 186, 91–94. [Google Scholar] [CrossRef]
- Mackie, K.; Lai, Y.; Westenbroek, R.; Mitchell, R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995, 15, 6552–6561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Otero, J.; Ahn, K.H.; Delgado-Peraza, F.; Mackie, K.; Kendall, D.A.; Yudowski, G.A. Ligand-specific endocytic dwell times control functional selectivity of the cannabinoid receptor 1. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Nogueras-Ortiz, C.; Yudowski, G.A. The multiple waves of cannabinoid 1 receptor signaling. Mol. Pharmacol. 2016, 90, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.T.; Schmid, C.L.; Raehal, K.M.; Selley, D.E.; Bohn, L.M.; Sim-Selley, L.J. β-Arrestin2 regulates cannabinoid CB 1 receptor signaling and adaptation in a central nervous system region-dependent manner. Biol. Psychiatry 2012, 71, 714–724. [Google Scholar] [CrossRef] [Green Version]
- Gyombolai, P.; Boros, E.; Hunyady, L.; Turu, G. Differential β-arrestin 2 requirements for constitutive and agonist-induced internalization of the CB1 cannabinoid receptor. Mol. Cell. Endocrinol. 2013, 372, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.H.; Mahmoud, M.M.; Shim, J.-Y.; Kendall, D.A. Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J. Biol. Chem. 2013, 288, 9790–9800. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Peraza, F.; Ahn, K.H.; Nogueras-Ortiz, C.; Mungrue, I.N.; Mackie, K.; Kendall, D.A.; Yudowski, G.A. Mechanisms of Biased β-Arrestin-Mediated Signaling Downstream from the Cannabinoid 1 Receptor. Mol. Pharmacol. 2016, 89, 618–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahavadi, S.; Sriwai, W.; Huang, J.; Grider, J.R.; Murthy, K.S. Inhibitory signaling by CB1 receptors in smooth muscle mediated by GRK5/β-arrestin activation of ERK1/2 and Src kinase. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G535. [Google Scholar] [CrossRef] [Green Version]
- Graham, E.S.; Ball, N.; Scotter, E.L.; Narayan, P.; Dragunow, M.; Glass, M. Induction of Krox-24 by endogenous cannabinoid type 1 receptors in neuro2a cells is mediated by the MEK-ERK MAPK pathway and is suppressed by the phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 2006, 281, 29085–29095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanda, D.; Kim, D.K.; Li, T.; Kim, Y.H.; Koo, S.H.; Lee, C.H.; Chiang, J.Y.L.; Choi, H.S. Cannabinoid Receptor Type 1 (CB1R) signaling regulates hepatic gluconeogenesis via induction of endoplasmic reticulum-bound transcription factor cAMP-responsive element-binding protein H (CREBH) in primary hepatocytes. J. Biol. Chem. 2011, 286, 27971–27979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haspula, D.; Clark, M.A. MAPK activation patterns of AT1R and CB1R in SHR versus Wistar astrocytes: Evidence of CB1R hypofunction and crosstalk between AT1R and CB1R. Cell. Signal. 2017, 40, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; García-Rincón, D.; Sendtner, M.; Timmusk, T.; Lutz, B.; Galve-Roperh, I.; et al. The CB1 cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015, 22, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Turu, G.; Hunyady, L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010, 44, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Bouaboula, M.; Poinot-Chazel, C.; Marchand, J.; Canat, X.; Bourrié, B.; Rinaldi-Carmona, M.; Calandra, B.; Le Fur, G.; Casellas, P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor: Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur. J. Biochem. 1996, 237, 704–711. [Google Scholar] [CrossRef]
- Viscomi, M.T.; Oddi, S.; Latini, L.; Pasquariello, N.; Florenzano, F.; Bernardi, G.; Molinari, M.; Maccarrone, M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J. Neurosci. 2009, 29, 4564–4570. [Google Scholar] [CrossRef] [Green Version]
- Atwood, B.K.; Wager-Miller, J.; Haskins, C.; Straiker, A.; Mackie, K. Functional selectivity in CB 2 cannabinoid receptor signaling and regulation: Implications for the therapeutic potential of CB 2 ligands. Mol. Pharmacol. 2012, 81, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Nogueras-Ortiz, C.; Roman-Vendrell, C.; Mateo-Semidey, G.E.; Liao, Y.H.; Kendall, D.A.; Yudowski, G.A. Retromer stops beta-arrestin 1-mediated signaling from internalized cannabinoid 2 receptors. Mol. Biol. Cell 2017, 28, 3554–3561. [Google Scholar] [CrossRef]
- Lauckner, J.E.; Hille, B.; Mackie, K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 19144–19149. [Google Scholar] [CrossRef] [Green Version]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Dupré, D.J.; Denovan-Wright, E.M. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J. Biol. Chem. 2014, 289, 24845–24862. [Google Scholar] [CrossRef] [Green Version]
- Asimaki, O.; Mangoura, D. Cannabinoid receptor 1 induces a biphasic ERK activation via multiprotein signaling complex formation of proximal kinases PKCε, Src, and Fyn in primary neurons. Neurochem. Int. 2011, 58, 135–144. [Google Scholar] [CrossRef]
- Navarrete, M.; Araque, A. Endocannabinoids mediate neuron-astrocyte communication. Neuron 2008, 57, 883–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Petrocellis, L.; Marini, P.; Matias, I.; Moriello, A.S.; Starowicz, K.; Cristino, L.; Nigam, S.; Di Marzo, V. Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma β-cells. Exp. Cell Res. 2007, 313, 2993–3004. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, C.; Kipmen-Korgun, D.; Osibow, K.; Malli, R.; Graier, W.F. Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 2003, 140, 1351–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brailoiu, G.C.; Deliu, E.; Marcu, J.; Hoffman, N.E.; Console-Bram, L.; Zhao, P.; Madesh, M.; Abood, M.E.; Brailoiu, E. Differential activation of intracellular versus plasmalemmal CB2 Cannabinoid receptors. Biochemistry 2014, 53, 4990–4999. [Google Scholar] [CrossRef] [Green Version]
- Kearn, C.S.; Blake-Palmer, K.; Daniel, E.; Mackie, K.; Glass, M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 2005, 67, 1697–1704. [Google Scholar] [CrossRef] [Green Version]
- Marcellino, D.; Carriba, P.; Filip, M.; Borgkvist, A.; Frankowska, M.; Bellido, I.; Tanganelli, S.; Müller, C.E.; Fisone, G.; Lluis, C.; et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology 2008, 54, 815–823. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Lo, Y.-N.; Chen, J.-C. Crosstalk between dopamine D2 receptors and cannabinoid CB1 receptors regulates CNR1 promoter activity via ERK1/2 signaling. J. Neurochem. 2013, 127, 163–176. [Google Scholar] [CrossRef]
- Carriba, P.; Ortiz, O.; Patkar, K.; Justinova, Z.; Stroik, J.; Themann, A.; Müller, C.; Woods, A.S.; Hope, B.T.; Ciruela, F.; et al. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 2007, 32, 2249–2259. [Google Scholar] [CrossRef] [Green Version]
- Navarro, G.; Carriba, P.; Gandí, J.; Ciruela, F.; Casadó, V.; Cortés, A.; Mallol, J.; Canela, E.I.; Lluis, C.; Franco, R. Detection of Heteromers Formed by Cannabinoid CB 1, Dopamine D 2, and Adenosine A 2A G-Protein-Coupled Receptors by Combining Bimolecular Fluorescence Complementation and Bioluminescence Energy Transfer. Sci. World J. 2008, 8, 1088–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilairet, S.; Bouaboula, M.; Carrière, D.; Le Fur, G.; Casellas, P. Hypersensitization of the Orexin 1 Receptor by the CB1 Receptor. J. Biol. Chem. 2003, 278, 23731–23737. [Google Scholar] [CrossRef] [Green Version]
- Rios, C.; Gomes, I.; Devi, L.A. μ opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006, 148, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinar, R.; Freund, T.F.; Katona, I.; Mackie, K.; Szucs, M. Reciprocal inhibition of G-protein signaling is induced by CB1 cannabinoid and GABAB receptor interactions in rat hippocampal membranes. Neurochem. Int. 2008, 52, 1402–1409. [Google Scholar] [CrossRef]
- Rozenfeld, R.; Gupta, A.; Gagnidze, K.; Lim, M.P.; Gomes, I.; Lee-Ramos, D.; Nieto, N.; Devi, L.A. AT1R-CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. Embo J. 2011, 30, 2350–2363. [Google Scholar] [CrossRef] [Green Version]
- Gyombolai, P.; Pap, D.; Turu, G.; Catt, K.J.; Bagdy, G.; Hunyady, L. Regulation of endocannabinoid release by G proteins: A paracrine mechanism of G protein-coupled receptor action. Mol. Cell. Endocrinol. 2012, 353, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haspula, D.; Clark, M.A. Molecular Basis of the Brain Renin Angiotensin System in Cardiovascular and Neurologic Disorders: Uncovering a Key Role for the Astroglial Angiotensin Type 1 Receptor AT1R. J. Pharmacol. Exp. Ther. 2018, 366, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Callén, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortés, A.; Mallol, J.; Casadó, V.; Lanciego, J.L.; Franco, R.; Lluis, C.; et al. Cannabinoid receptors CB 1 and CB 2 form functional heteromers in brain. J. Biol. Chem. 2012, 287, 20851–20865. [Google Scholar] [CrossRef] [Green Version]
- Bouaboula, M.; Desnoyer, N.; Carayon, P.; Combes, T.; Casellas, P. G(i) protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: Implication for intracellular signalization cross-regulation. Mol. Pharmacol. 1999, 55, 473–480. [Google Scholar]
- Coke, C.J.; Scarlett, K.A.; Chetram, M.A.; Jones, K.J.; Sandifer, B.J.; Davis, A.S.; Marcus, A.I.; Hinton, C.V. Simultaneous activation of induced heterodimerization between CXCR4 chemokine receptor and cannabinoid receptor 2 (CB2) reveals a mechanism for regulation of tumor progression. J. Biol. Chem. 2016, 291, 9991–10005. [Google Scholar] [CrossRef] [Green Version]
- Scarlett, K.A.; White, E.S.Z.; Coke, C.J.; Carter, J.R.; Bryant, L.K.; Hinton, C.V. Agonist-induced CXCR4 and CB2 heterodimerization inhibits Ga13/RhoA-mediated migration. Mol. Cancer Res. 2018, 16, 728–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turu, G.; Simon, A.; Gyombolai, P.; Szidonya, L.; Bagdy, G.; Lenkei, Z.; Hunyady, L. The Role of Diacylglycerol Lipase in Constitutive and Angiotensin AT1 Receptor-stimulated Cannabinoid CB1 Receptor Activity. J. Biol. Chem. 2007, 282, 7753–7757. [Google Scholar] [CrossRef] [Green Version]
- Turu, G.; Várnai, P.; Gyombolai, P.; Szidonya, L.; Offertaler, L.; Bagdy, G.; Kunos, G.; Hunyady, L. Paracrine transactivation of the CB1 cannabinoid receptor by AT1 angiotensin and other Gq/11 protein-coupled receptors. J. Biol. Chem. 2009, 284, 16914–16921. [Google Scholar] [CrossRef] [Green Version]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids Induce Cancer Cell Proliferation via Tumor Necrosis Factor α-Converting Enzyme (TACE/ADAM17)-Mediated Transactivation of the Epidermal Growth Factor Receptor. Cancer Res. 2004, 64, 1943–1950. [Google Scholar] [CrossRef] [Green Version]
- Berghuis, P.; Dobszay, M.B.; Wang, X.; Spano, S.; Ledda, F.; Sousa, K.M.; Schulte, G.; Ernfors, P.; Mackie, K.; Paratcha, G.; et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 19115–19120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, Z.; Capó-Aponte, J.E.; Zhang, F.; Pan, Z.; Reinach, P.S. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp. Eye Res. 2010, 91, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khajehali, E.; Malone, D.T.; Glass, M.; Sexton, P.M.; Christopoulos, A.; Leach, K. Biased Agonism and Biased Allosteric Modulation at the CB1 Cannabinoid Receptor. Mol. Pharmacol. 2015, 88, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Grabiec, U.; Dehghani, F. N-Arachidonoyl Dopamine: A Novel Endocannabinoid and Endovanilloid with Widespread Physiological and Pharmacological Activities. Cannabis Cannabinoid Res. 2017, 2, 183–196. [Google Scholar] [CrossRef] [Green Version]
- Redmond, W.J.; Cawston, E.E.; Grimsey, N.L.; Stuart, J.; Edington, A.R.; Glass, M.; Connor, M. Identification of N-arachidonoyl dopamine as a highly biased ligand at cannabinoid CB1 receptors. Br. J. Pharmacol. 2016, 173, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Biased Type 1 Cannabinoid Receptor Signaling Influences Neuronal Viability in a Cell Culture Model of Huntington Disease. Mol. Pharmacol. 2016, 89, 364–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breivogel, C.S.; Vaghela, M.S. The effects of beta-arrestin1 deletion on acute cannabinoid activity, brain cannabinoid receptors and tolerance to cannabinoids in mice. J. Recept. Signal Transduct. Res. 2015, 35, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, J.L.; Ruckle, M.B.; Mayeux, P.R.; Prather, P.L. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J. Pharmacol. Exp. Ther. 2005, 315, 828–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soethoudt, M.; Grether, U.; Fingerle, J.; Grim, T.W.; Fezza, F.; De Petrocellis, L.; Ullmer, C.; Rothenhäusler, B.; Perret, C.; Van Gils, N.; et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dhopeshwarkar, A.; Mackie, K. Functional selectivity of CB2 cannabinoid receptor ligands at a canonical and noncanonical pathways. J. Pharmacol. Exp. Ther. 2016, 358, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouaboula, M.; Perrachon, S.; Milligan, L.; Canat, X.; Rinaldi-Carmona, M.; Portier, M.; Barth, F.; Calandra, B.; Pecceu, F.; Lupker, J.; et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997, 272, 22330–22339. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Ikeda, S.R.; Lewis, D.L. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol. Pharmacol. 1998, 54, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Kearn, C.S.; Greenberg, M.J.; DiCamelli, R.; Kurzawa, K.; Hillard, C.J. Relationships between ligand affinities for the cerebellar cannabinoid receptor CB1 and the induction of GDP/GTP exchange. J. Neurochem. 1999, 72, 2379–2387. [Google Scholar] [CrossRef] [Green Version]
- Hillard, C.J.; Muthian, S.; Kearn, C.S. Effects of CB1 cannabinoid receptor activation on cerebellar granule cell nitric oxide synthase activity. FEBS Lett. 1999, 459, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Vásquez, C.; Lewis, D.L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 1999, 19, 9271–9280. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, H.H.; Song, C.; Howlett, A.C. CB1 cannabinoid receptor: Cellular regulation and distribution in N18TG2 neuroblastoma cells. Mol. Brain Res. 1998, 53, 163–173. [Google Scholar] [CrossRef]
- Leterrier, C.; Bonnard, D.; Carrel, D.; Rossier, J.; Lenkei, Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J. Biol. Chem. 2004, 279, 36013–36021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozenfeld, R.; Devi, L.A. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 2008, 22, 2311–2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meye, F.J.; Trezza, V.; Vanderschuren, L.J.M.J.; Ramakers, G.M.J.; Adan, R.A.H. Neutral antagonism at the cannabinoid 1 receptor: A safer treatment for obesity. Mol. Psychiatry 2013, 18, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Meye, F.J.; Ramakers, G.M.J.; Adan, R.A.H. The vital role of constitutive GPCR activity in the mesolimbic dopamine system. Transl. Psychiatry 2014, 4, e361. [Google Scholar] [CrossRef] [Green Version]
- Mancini, I.; Brusa, R.; Quadrato, G.; Foglia, C.; Scandroglio, P.; Silverman, L.; Tulshian, D.; Reggiani, A.; Beltramo, M. Constitutive activity of cannabinoid-2 (CB 2) receptors plays an essential role in the protean agonism of (+)AM1241 and L768242. Br. J. Pharmacol. 2009, 158, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Salort, G.; Álvaro-Bartolomé, M.; García-Sevilla, J.A. Regulation of cannabinoid CB2 receptor constitutive activity In Vivo: Repeated treatments with inverse agonists reverse the acute activation of JNK and associated apoptotic signaling in mouse brain. Psychopharmacol. Berl. 2017, 234, 925–941. [Google Scholar] [CrossRef]
- Den Boon, F.S.; Chameau, P.; Schaafsma-Zhao, Q.; Van Aken, W.; Bari, M.; Oddi, S.; Kruse, C.G.; Maccarrone, M.; Wadman, W.J.; Werkmana, T.R. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 3534–3539. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.E.; Brown, S.; Hille, B.; Mackie, K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J. Neurosci. 1998, 18, 2834–2841. [Google Scholar] [CrossRef] [Green Version]
- Bénard, G.; Massa, F.; Puente, N.; Lourenço, J.; Bellocchio, L.; Soria-Gómez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism-Supplementary information. Nat. Neurosci. 2012, 15, 558–564. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Liu, J.; Osei-Hyiaman, D.; Godlewski, G.; Mukhopadhyay, P.; Wang, L.; Jeong, W.-I.; Gao, B.; Duester, G.; Mackie, K.; et al. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-gamma. J. Biol. Chem. 2010, 285, 19002–19011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.K.; Devi, L.A. The highs and lows of cannabinoid receptor expression in disease: Mechanisms and their therapeutic implications. Pharmacol. Rev. 2011, 63, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Kelly, M.E.M.; Denovan-Wright, E.M. The dynamic nature of type 1 cannabinoid receptor CB1 transcription. Br. J. Pharmacol. 2012, 167, 1583–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegyi, Z.; Oláh, T.; Koszeghy, Á.; Pisticelli, F.; Holló, K.; Pál, B.; Csernoch, L.; Di Marzo, V.; Antal, M. CB1 receptor activation induces intracellular Ca2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Börner, C.; Höllt, V.; Sebald, W.; Kraus, J. Transcriptional regulation of the cannabinoid receptor type 1 gene in T cells by cannabinoids. J. Leukoc. Biol. 2007, 81, 336–343. [Google Scholar] [CrossRef]
- Lahesmaa, M.; Eriksson, O.; Gnad, T.; Oikonen, V.; Bucci, M.; Hirvonen, J.; Koskensalo, K.; Teuho, J.; Niemi, T.; Taittonen, M.; et al. Cannabinoid type 1 receptors are upregulated during acute activation of brown adipose tissue. Diabetes 2018, 67, 1226–1236. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.S.; Camilleri, M.; Eckert, D.; Carlson, P.; Ryks, M.; Burton, D.; Zinsmeister, A.R. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol. Motil. 2012, 24. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.; Mirabella, M.; De Fino, C.; Bianco, A.; Lucchini, M.; Losavio, F.; Sabino, A.; Nociti, V. Sativex® effects on promoter methylation and on CNR1/CNR2 expression in peripheral blood mononuclear cells of progressive multiple sclerosis patients. J. Neurol. Sci. 2017, 379, 298–303. [Google Scholar] [CrossRef]
- Ceccarini, J.; De Hert, M.; Van Winkel, R.; Peuskens, J.; Bormans, G.; Kranaster, L.; Enning, F.; Koethe, D.; Leweke, F.M.; Van Laere, K. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage 2013, 79, 304–312. [Google Scholar] [CrossRef]
- Ceccarini, J.; Ahmad, R.; Van De Vliet, L.; Casteels, C.; Vandenbulcke, M.; Vandenberghe, W.; Van Laere, K. Behavioral symptoms in premanifest Huntington disease correlate with reduced frontal CB 1 R levels. J. Nucl. Med. 2019, 60, 115–121. [Google Scholar] [CrossRef] [Green Version]
- You, T.; Disanzo, B.L.; Wang, X.; Yang, R.; Gong, D. Adipose tissue endocannabinoid system gene expression: Depot differences and effects of diet and exercise. Lipids Health Dis. 2011, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes-Correa, L.M.; Nobre, T.S.; Groehs, R.V.; Alves, M.J.N.N.; Fernandes, T.; Couto, G.K.; Rondon, M.U.P.B.; Oliveira, P.; Lima, M.; Mathias, W.; et al. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1655–H1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Luis, D.A.; Mulero, I.; Primo, D.; Izaola, O.; Aller, R. Effects of polymorphism rs3123554 in the cannabinoid receptor gene type 2 (CB2R) on metabolic and adiposity parameters after weight loss with two hypocaloric diets. Diabetes Res. Clin. Pract. 2018, 139, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Mancusi, S.; Bellini, G.; Roberti, D.; Punzo, F.; Vetrella, S.; Matarese, S.M.R.; Nobili, B.; Maione, S.; Perrotta, S. CNR2 functional variant (Q63R) influences childhood immune thrombocytopenic purpura. Haematologica 2011, 96, 1883–1885. [Google Scholar] [CrossRef] [Green Version]
- Sánchez López, A.J.; Román-Vega, L.; Ramil Tojeiro, E.; Giuffrida, A.; García-Merino, A. Regulation of cannabinoid receptor gene expression and endocannabinoid levels in lymphocyte subsets by interferon-β: A longitudinal study in multiple sclerosis patients. Clin. Exp. Immunol. 2015, 179, 119–127. [Google Scholar] [CrossRef] [Green Version]
- López-Sendón Moreno, J.L.; García Caldentey, J.; Trigo Cubillo, P.; Ruiz Romero, C.; García Ribas, G.; Alonso Arias, M.A.A.; García de Yébenes, M.J.; Tolón, R.M.; Galve-Roperh, I.; Sagredo, O.; et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J. Neurol. 2016, 263, 1390–1400. [Google Scholar] [CrossRef]
- Flores-Contreras, L.; Sandoval-Rodríguez, A.S.; Mena-Enriquez, M.G.; Lucano-Landeros, S.; Arellano-Olivera, I.; Álvarez-Álvarez, A.; Sanchez-Parada, M.G.; Armendáriz-Borunda, J. Treatment with pirfenidone for two years decreases fibrosis, cytokine levels and enhances CB2 gene expression in patients with chronic hepatitis C. Bmc Gastroenterol. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engeli, S.; Lehmann, A.C.; Kaminski, J.; Haas, V.; Janke, J.; Zoerner, A.A.; Luft, F.C.; Tsikas, D.; Jordan, J. Influence of dietary fat intake on the endocannabinoid system in lean and obese subjects. Obesity 2014, 22. [Google Scholar] [CrossRef] [Green Version]
- Sathyapalan, T.; Javed, Z.; Kilpatrick, E.S.; Coady, A.M.; Atkin, S.L. Endocannabinoid receptor blockade increases vascular endothelial growth factor and inflammatory markers in obese women with polycystic ovary syndrome. Clin. Endocrinol. Oxf. 2017, 86, 384–387. [Google Scholar] [CrossRef]
- Andries, A.; Frystyk, J.; Flyvbjerg, A.; Støving, R.K. Changes in IGF-I, urinary free cortisol and adipokines during dronabinol therapy in anorexia nervosa: Results from a randomised, controlled trial. Growth Horm. Igf Res. 2015, 25, 247–252. [Google Scholar] [CrossRef]
- Rabinak, C.A.; Angstadt, M.; Sripada, C.S.; Abelson, J.L.; Liberzon, I.; Milad, M.R.; Phan, K.L. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology 2013, 64, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, C.W.; Carlson, O.D.; Liu, D.D.; González-Mariscal, I.; Santa-Cruz Calvo, S.; Egan, J.M. Incretin secretion in humans is under the influence of cannabinoid receptors. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E359–E366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronne, L.J.; Finer, N.; Hollander, P.A.; England, R.D.; Klioze, S.S.; Chew, R.D.; Fountaine, R.J.; Powell, C.M.; Obourn, J.D. Efficacy and safety of CP-945,598, a selective cannabinoid CB1 receptor antagonist, on weight loss and maintenance. Obesity 2011, 19, 1404–1414. [Google Scholar] [CrossRef]
- Riggs, P.K.; Vaida, F.; Rossi, S.S.; Sorkin, L.S.; Gouaux, B.; Grant, I.; Ellis, R.J. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012, 1431, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J.; Bousser, M.-G.; Fox, K.A.; Creager, M.A.; Despres, J.-P.; Easton, J.D.; Hamm, C.W.; Montalescot, G.; Steg, P.G.; Pearson, T.A.; et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): A randomised, multicentre, placebo-controlled trial. Lancet 2010, 376, 517–523. [Google Scholar] [CrossRef]
- Wadden, T.A.; Fujioka, K.; Toubro, S.; Gantz, I.; Erondu, N.E.; Chen, M.; Suryawanshi, S.; Carofano, W.; Johnson-Levonas, A.O.; Shapiro, D.R.; et al. A randomized trial of lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity 2010, 18, 2301–2310. [Google Scholar] [CrossRef] [Green Version]
- Proietto, J.; Rissanen, A.; Harp, J.B.; Erondu, N.; Yu, Q.; Suryawanshi, S.; Jones, M.E.; Johnson-Levonas, A.O.; Heymsfield, S.B.; Kaufman, K.D.; et al. A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: Low-dose study. Int. J. Obes. 2010, 34, 1243–1254. [Google Scholar] [CrossRef] [Green Version]
- Rzepa, E.; Tudge, L.; McCabe, C. The CB1 Neutral Antagonist Tetrahydrocannabivarin Reduces Default Mode Network and Increases Executive Control Network Resting State Functional Connectivity in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2015, 19, pyv092. [Google Scholar] [CrossRef] [Green Version]
- Tudge, L.; Williams, C.; Cowen, P.J.; McCabe, C. Neural Effects of Cannabinoid CB1 Neutral Antagonist Tetrahydrocannabivarin on Food Reward and Aversion in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef] [Green Version]
- Firsching, R.; Piek, J.; Skalej, M.; Rohde, V.; Schmidt, U.; Striggow, F. Early survival of comatose patients after severe traumatic brain injury with the dual cannabinoid CB1/CB2 receptor agonist KN38-7271: A randomized, double-blind, placebo-controlled phase II trial. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2012, 73, 204–216. [Google Scholar] [CrossRef]
- Kalliomäki, J.; Annas, P.; Huizar, K.; Clarke, C.; Zettergren, A.; Karlsten, R.; Segerdahl, M. Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin. Exp. Pharmacol. Physiol. 2013, 40, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.; Ball, S.; Wright, D.; Vickery, J.; Nunn, A.; Miller, D.; Cano, M.G.; McManus, D.; Mallik, S.; Hobart, J. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): A randomised, placebo-controlled trial. Lancet Neurol. 2013, 12, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Malik, Z.; Bayman, L.; Valestin, J.; Rizvi-Toner, A.; Hashmi, S.; Schey, R. Dronabinol increases pain threshold in patients with functional chest pain: A pilot double-blind placebo-controlled trial. Dis. Esophagus 2017, 30. [Google Scholar] [CrossRef] [PubMed]
- Bergholm, R.; Sevastianova, K.; Santos, A.; Kotronen, A.; Urjansson, M.; Hakkarainen, A.; Lundbom, J.; Tiikkainen, M.; Rissanen, A.; Lundbom, N.; et al. CB 1 blockade-induced weight loss over 48 weeks decreases liver fat in proportion to weight loss in humans. Int. J. Obes. 2013, 37, 699–703. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, D.H.; Reuwer, A.Q.; Nissen, S.E.; Després, J.P.; Deanfield, J.E.; Brown, M.W.; Zhou, R.; Zabbatino, S.M.; Job, B.; Kastelein, J.J.P.; et al. Effect of rimonabant on carotid intimaemedia thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: The AUDITOR Trial. Heart 2011, 97, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Pataky, Z.; Gasteyger, C.; Ziegler, O.; Rissanen, A.; Hanotin, C.; Golay, A. Efficacy of rimonabant in obese patients with binge eating disorder. Exp. Clin. Endocrinol. Diabetes 2013, 121, 20–26. [Google Scholar] [CrossRef]
- Boggs, D.L.; Kelly, D.L.; McMahon, R.P.; Gold, J.M.; Gorelick, D.A.; Linthicum, J.; Conley, R.R.; Liu, F.; Waltz, J.; Huestis, M.A.; et al. Rimonabant for neurocognition in schizophrenia: A 16-week double blind randomized placebo controlled trial. Schizophr. Res. 2012, 134, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Backhouse, K.; Sarac, I.; Shojaee-Moradie, F.; Stolinski, M.; Robertson, M.D.; Frost, G.S.; Bell, J.D.; Thomas, E.L.; Wright, J.; Russell-Jones, D.; et al. Fatty acid flux and oxidation are increased by rimonabant in obese women. Metabolism 2012, 61, 1220–1223. [Google Scholar] [CrossRef] [Green Version]
- Triay, J.; Mundi, M.; Klein, S.; Toledo, F.G.; Smith, S.R.; Abu-Lebdeh, H.; Jensen, M. Does rimonabant independently affect free fatty acid and glucose metabolism? J. Clin. Endocrinol. Metab. 2012, 97, 819–827. [Google Scholar] [CrossRef]
- Van Den Elsen, G.A.H.; Ahmed, A.I.A.; Verkes, R.J.; Kramers, C.; Feuth, T.; Rosenberg, P.B.; Van Der Marck, M.A.; Olde Rikkert, M.G.M. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: A randomized controlled trial. Neurology 2015, 84, 2338–2346. [Google Scholar] [CrossRef] [Green Version]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elphick, M.R.; Egertová, M. The phylogenetic distribution and evolutionary origins of endocannabinoid signalling. Handb. Exp. Pharmacol. 2005, 168, 283–297. [Google Scholar] [CrossRef]
- Paria, B.C.; Das, S.K.; Dey, S.K. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc. Natl. Acad. Sci. USA 1995, 92, 9460–9464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.M.; Paria, B.C.; Dey, S.K. Activation of brain-type cannabinoid receptors interferes with preimplantation mouse embryo development. Biol. Reprod. 1996, 55, 756–761. [Google Scholar] [CrossRef]
- Paria, B.C.; Ma, W.; Andrenyak, D.M.; Schmid, P.C.; Schmid, H.H.; Moody, D.E.; Deng, H.; Makriyannis, A.; Dey, S.K. Effects of cannabinoids on preimplantation mouse embryo development and implantation are mediated by brain-type cannabinoid receptors. Biol. Reprod. 1998, 58, 1490–1495. [Google Scholar] [CrossRef] [Green Version]
- Buckley, N.E.; Hansson, S.; Harta, G.; Mezey, É. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 1997, 82, 1131–1149. [Google Scholar] [CrossRef]
- Habayeb, O.M.H.; Taylor, A.H.; Bell, S.C.; Taylor, D.J.; Konje, J.C. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology 2008, 149, 5052–5060. [Google Scholar] [CrossRef] [Green Version]
- Sufian, M.S.; Amin, M.R.; Kanyo, R.; Ted Allison, W.; Ali, D.W. CB1 and CB2 receptors play differential roles in early zebrafish locomotor development. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Fu, Y.; Williams, J.; Wood, J.; Pandarinathan, L.; Avraham, S.; Makriyannis, A.; Avraham, S.; Avraham, H.K. Expression and function of cannabinoid receptors CB1 and CB2 and their cognate cannabinoid ligands in murine embryonic stem cells. PLoS ONE 2007, 2, e641. [Google Scholar] [CrossRef]
- Bari, M.; Tedesco, M.; Battista, N.; Pasquariello, N.; Pucci, M.; Gasperi, V.; Scaldaferri, M.L.; Farini, D.; De Felici, M.; Maccarrone, M. Characterization of the endocannabinoid system in mouse embryonic stem cells. Stem Cells Dev. 2011, 20, 139–147. [Google Scholar] [CrossRef]
- Nones, J.; Spohr, T.C.; Furtado, D.R.; Sartore, R.C.; Paulsen, B.S.; Guimarães, M.Z.; Rehen, S.K. Cannabinoids modulate cell survival in embryoid bodies. Cell Biol. Int. 2010, 34, 399–408. [Google Scholar] [CrossRef]
- Berrendero, F.; García-Gil, L.; Hernández, M.L.; Romero, J.; Cebeira, M.; De Miguel, R.; Ramos, J.A.; Fernández-Ruiz, J.J. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development 1998, 125, 3179–3188. [Google Scholar] [PubMed]
- Rueda, D.; Navarro, B.; Martinez-Serrano, A.; Guzman, M.; Galve-Roperh, I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J. Biol. Chem. 2002, 277, 46645–46650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K.; Xie, L.; Kim, S.H.; Parmentier-Batteur, S.; Sun, Y.; Mao, X.O.; Childs, J.; Greenberg, D.A. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 2004, 66, 204–208. [Google Scholar] [CrossRef] [Green Version]
- Aguado, T.; Monory, K.; Palazuelos, J.; Stella, N.; Cravatt, B.; Lutz, B.; Marsicano, G.; Kokaia, Z.; Guzmán, M.; Galve-Roperh, I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005, 19, 1704–1706. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Xiao, L.; Van Cleemput, J.; Ji, S.-P.; Bai, G.; Zhang, X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Investig. 2005, 115, 3104–3116. [Google Scholar] [CrossRef] [Green Version]
- Berghuis, P.; Rajnicek, A.M.; Morozov, Y.M.; Ross, R.A.; Mulder, J.; Urbán, G.M.; Monory, K.; Marsicano, G.; Matteoli, M.; Canty, A.; et al. Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science 2007, 316, 1212–1216. [Google Scholar] [CrossRef] [Green Version]
- Mulder, J.; Aguado, T.; Keimpema, E.; Barabás, K.; Ballester Rosado, C.J.; Nguyen, L.; Monory, K.; Marsicano, G.; Di Marzo, V.; Hurd, Y.L.; et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl. Acad. Sci. USA 2008, 105, 8760–8765. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-S.; Zhu, J.; Wager-Miller, J.; Wang, S.; O’Leary, D.; Monory, K.; Lutz, B.; Mackie, K.; Lu, H.-C. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur. J. Neurosci. 2010, 32, 693–706. [Google Scholar] [CrossRef] [Green Version]
- Aguado, T.; Palazuelos, J.; Monory, K.; Stella, N.; Cravatt, B.; Lutz, B.; Marsicano, G.; Kokaia, Z.; Guzmán, M.; Galve-Roperh, I. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J. Neurosci. 2006, 26, 1551–1561. [Google Scholar] [CrossRef] [Green Version]
- Ilyasov, A.A.; Milligan, C.E.; Pharr, E.P.; Howlett, A.C. The Endocannabinoid System and Oligodendrocytes in Health and Disease. Front. Neurosci. 2018, 12, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazuelos, J.; Ortega, Z.; Díaz-Alonso, J.; Guzmán, M.; Galve-Roperh, I. CB 2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling. J. Biol. Chem. 2012, 287, 1198–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mereu, G.; Fà, M.; Ferraro, L.; Cagiano, R.; Antonelli, T.; Tattoli, M.; Ghiglieri, V.; Tanganelli, S.; Gessa, G.L.; Cuomo, V. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc. Natl. Acad. Sci. USA 2003, 100, 4915–4920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelli, T.; Tomasini, M.C.; Tattoli, M.; Cassano, T.; Tanganelli, S.; Finetti, S.; Mazzoni, E.; Trabace, L.; Steardo, L.; Cuomo, V.; et al. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 2005, 15, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Milh, M.; Morozov, Y.M.; Ben-Ari, Y.; Freund, T.F.; Gozlan, H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc. Natl. Acad. Sci. USA 2005, 102, 9388–9393. [Google Scholar] [CrossRef] [Green Version]
- MacCarrone, M.; Rossi, S.; Bari, M.; De Chiara, V.; Rapino, C.; Musella, A.; Bernardi, G.; Bagni, C.; Centonze, D. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology 2010, 35, 1500–1509. [Google Scholar] [CrossRef]
- Zhang, L.; Alger, B.E. Enhanced Endocannabinoid Signaling Elevates Neuronal Excitability in Fragile X Syndrome. J. Neurosci. 2010, 30, 5724–5729. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Gomis-González, M.; Guegan, T.; Agustín-Pavón, C.; Pastor, A.; Mato, S.; Pérez-Samartín, A.; Matute, C.; de la Torre, R.; Dierssen, M.; et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat. Med. 2013, 19, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Purcell, A.E.; Jeon, O.H.; Zimmerman, A.W.; Blue, M.E.; Pevsner, J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 2001, 57, 1618–1628. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, B.; Baron-Cohen, S. Variation in the human cannabinoid receptor CNR1 gene modulates gaze duration for happy faces. Mol. Autism 2011, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, B.; Persico, A.; Battista, N.; Maccarrone, M. Endocannabinoid Signaling in Autism. Neurotherapeutics 2015, 12, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, A.T.; Ogdie, M.N.; Järvelin, M.R.; Moilanen, I.K.; Loo, S.K.; McCracken, J.T.; McGough, J.J.; Yang, M.H.; Peltonen, L.; Nelson, S.F.; et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147, 1488–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltramo, M.; Rodríguez De Fonseca, F.; Navarro, M.; Calignano, A.; Gorriti, M.A.; Grammatikopoulos, G.; Sadile, A.G.; Giuffrida, A.; Piomelli, D. Reversal of dopamine D2 receptor responses by an anandamide transport inhibitor. J. Neurosci. 2000, 20, 3401–3407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriani, W.; Caprioli, A.; Granstrem, O.; Carli, M.; Laviola, G. The spontaneously hypertensive-rat as an animal model of ADHD: Evidence for impulsive and non-impulsive subpopulations. Neurosci. Biobehav. Rev. 2003, 27, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Strohbeck-Kuehner, P.; Skopp, G.; Mattern, R. Cannabis improves symptoms of ADHD. Cannabinoids 2008, 3, 1–3. [Google Scholar]
- Hupli, A.M.M. Medical Cannabis for Adult Attention Deficit Hyperactivity Disorder: Sociological Patient Case Report of Cannabinoid Therapeutics in Finland. Med. Cannabis Cannabinoids 2018, 1, 112–118. [Google Scholar] [CrossRef]
- Saito, A.; Ballinger, M.D.L.; Pletnikov, M.V.; Wong, D.F.; Kamiya, A. Endocannabinoid system: Potential novel targets for treatment of schizophrenia. Neurobiol. Dis. 2013, 53, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Marconi, A.; Di Forti, M.; Lewis, C.M.; Murray, R.M.; Vassos, E. Meta-Analysis of the association between the level of cannabis use and risk of psychosis. Schizophr. Bull. 2016, 42, 1262–1269. [Google Scholar] [CrossRef]
- Renard, J.; Krebs, M.O.; Le Pen, G.; Jay, T.M. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Rubino, T.; Parolaro, D. The impact of exposure to cannabinoids in adolescence: Insights from animal models. Biol. Psychiatry 2016, 79, 578–585. [Google Scholar] [CrossRef]
- Dean, B.; Sundram, S.; Bradbury, R.; Scarr, E.; Copolov, D.D. Studies on [3H]CP-55940 binding in the human central nervous system: Regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 2001, 103, 9–15. [Google Scholar] [CrossRef]
- Newell, K.A.; Deng, C.; Huang, X.F. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp. Brain Res. 2006, 172, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Eggan, S.M.; Hashimoto, T.; Lewis, D.A. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch. Gen. Psychiatry 2008, 65, 772–784. [Google Scholar] [CrossRef]
- Eggan, S.M.; Stoyak, S.R.; Verrico, C.D.; Lewis, D.A. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology 2010, 35, 2060–2071. [Google Scholar] [CrossRef]
- Wong, D.F.; Kuwabara, H.; Horti, A.G.; Raymont, V.; Brasic, J.; Guevara, M.; Ye, W.; Dannals, R.F.; Ravert, H.T.; Nandi, A.; et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage 2010, 52, 1505–1513. [Google Scholar] [CrossRef]
- Gomes, F.V.; Edelson, J.R.; Volk, D.W.; Grace, A.A. Altered brain cannabinoid 1 receptor mRNA expression across postnatal development in the MAM model of schizophrenia. Schizophr. Res. 2018, 201, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Black, M.D.; Stevens, R.J.; Rogacki, N.; Featherstone, R.E.; Senyah, Y.; Giardino, O.; Borowsky, B.; Stemmelin, J.; Cohen, C.; Pichat, P.; et al. AVE1625, a cannabinoid CB1 receptor antagonist, as a co-treatment with antipsychotics for schizophrenia: Improvement in cognitive function and reduction of antipsychotic-side effects in rodents. Psychopharmacol. Berl. 2011, 215, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Kruk-Slomka, M.; Budzynska, B.; Slomka, T.; Banaszkiewicz, I.; Biala, G. The Influence of the CB1 Receptor Ligands on the Schizophrenia-Like Effects in Mice Induced by MK-801. Neurotox. Res. 2016, 30, 658–676. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.I.; Nicoll, R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001, 410, 588–592. [Google Scholar] [CrossRef]
- Ohno-Shosaku, T.; Maejima, T.; Kano, M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 2001, 29, 729–738. [Google Scholar] [CrossRef]
- Alger, B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286. [Google Scholar] [CrossRef]
- Pitler, T.A.; Alger, B.E. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron 1994, 13, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Freund, T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008, 14, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, A.C.; Regehr, W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 2001, 29, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Maejima, T.; Hashimoto, K.; Yoshida, T.; Aiba, A.; Kano, M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 2001, 31, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Galante, M.; Diana, M.A. Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J. Neurosci. 2004, 24, 4865–4874. [Google Scholar] [CrossRef] [Green Version]
- Robbe, D.; Kopf, M.; Remaury, A.; Bockaert, J.; Manzoni, O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2002, 99, 8384–8388. [Google Scholar] [CrossRef] [Green Version]
- Safo, P.K.; Regehr, W.G. Endocannabinoids control the induction of cerebellar LTD. Neuron 2005, 48, 647–659. [Google Scholar] [CrossRef] [Green Version]
- Auclair, N.; Otani, S.; Soubrie, P.; Crepel, F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J. Neurophysiol. 2000, 83, 3287–3293. [Google Scholar] [CrossRef]
- Carlson, G.; Wang, Y.; Alger, B.E. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat. Neurosci. 2002, 5, 723–724. [Google Scholar] [CrossRef]
- Bohme, G.A.; Laville, M.; Ledent, C.; Parmentier, M.; Imperato, A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience 1999, 95, 5–7. [Google Scholar] [CrossRef]
- Slanina, K.A.; Roberto, M.; Schweitzer, P. Endocannabinoids restrict hippocampal long-term potentiation via CB1. Neuropharmacology 2005, 49, 660–668. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Nagre, N.N.; Xie, S.; Subbanna, S. Elevation of endogenous anandamide impairs LTP, learning, and memory through CB1 receptor signaling in mice. Hippocampus 2014, 24, 808–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Cruz, A.; Carlström, M.; Ribeiro, J.A.; Sebastião, A.M. Dual influence of endocannabinoids on long-term potentiation of synaptic transmission. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puighermanal, E.; Marsicano, G.; Busquets-Garcia, A.; Lutz, B.; Maldonado, R.; Ozaita, A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat. Neurosci. 2009, 12, 1152–1158. [Google Scholar] [CrossRef]
- Wilson, R.I.; Kunos, G.; Nicoll, R.A. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 2001, 31, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Chevaleyre, V.; Heifets, B.D.; Kaeser, P.S.; Südhof, T.C.; Castillo, P.E. Endocannabinoid-Mediated Long-Term Plasticity Requires cAMP/PKA Signaling and RIM1α. Neuron 2007, 55, 169. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.H.; Davis, M.I.; Ronesi, J.A.; Lovinger, D.M. The role of protein synthesis in striatal long-term depression. J. Neurosci. 2006, 26, 11811–11820. [Google Scholar] [CrossRef] [Green Version]
- Heifets, B.D.; Castillo, P.E. Endocannabinoid Signaling and Long-Term Synaptic Plasticity. Annu. Rev. Physiol. 2009, 71, 283–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellin, T. Communication between neurons and astrocytes: Relevance to the modulation of synaptic and network activity. J. Neurochem. 2009, 108, 533–544. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Navarrete, M.; Diez, A.; Araque, A. Astrocytes in endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130599. [Google Scholar] [CrossRef]
- Navarrete, M.; Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 2010, 68, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Gonzalo, M.; Navarrete, M.; Perea, G.; Covelo, A.; Martín-Fernández, M.; Shigemoto, R.; Luján, R.; Araque, A. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb. Cortex 2015, 25, 3699–3712. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Kesner, P.; Metna-Laurent, M.; Duan, T.; Xu, L.; Georges, F.; Koehl, M.; Abrous, D.N.; Mendizabal-Zubiaga, J.; Grandes, P.; et al. Acute Cannabinoids Impair Working Memory through Astroglial CB1 Receptor Modulation of Hippocampal LTD. Cell 2012, 148, 1039–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira da Cruz, J.F.; Robin, L.M.; Drago, F.; Marsicano, G.; Metna-Laurent, M. Astroglial type-1 cannabinoid receptor (CB1): A new player in the tripartite synapse. Neuroscience 2016, 323, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Gomes, S.; Sousa, N.; Pinto, L.; Oliveira, J.F. Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front. Cell. Neurosci. 2018, 11, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef]
- Onaivi, E.S. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology 2007, 54, 231–246. [Google Scholar] [CrossRef]
- Zhang, J.; Hoffert, C.; Vu, H.K.; Groblewski, T.; Ahmad, S.; O’Donnell, D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003, 17, 2750–2754. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C. The use of knockout mice to test the specificity of antibodies for cannabinoid receptors. Hippocampus 2012, 22, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Cécyre, B.; Thomas, S.; Ptito, M.; Casanova, C.; Bouchard, J.F. Evaluation of the specificity of antibodies raised against cannabinoid receptor type 2 in the mouse retina. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014, 387, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience 2015, 311, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Y.; Gao, M.; Liu, Q.R.; Bi, G.H.; Li, X.; Yang, H.J.; Gardner, E.L.; Wu, J.; Xi, Z.X. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E5007–E5015. [Google Scholar] [CrossRef] [Green Version]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: From mice to human subjects. PLoS ONE 2008, 3, e1640. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Gao, F.; Larsen, B.; Gao, M.; Luo, Z.; Chen, D.; Ma, X.; Qiu, S.; Zhou, Y.; Xie, J.; et al. Mechanisms of cannabinoid CB 2 receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area. EBioMedicine 2019, 42, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Marchalant, Y.; Cerbai, F.; Brothers, H.M.; Wenk, G.L. Cannabinoid receptor stimulation is anti-inflammatory and improves memory in old rats. Neurobiol. Aging 2008, 29, 1894–1901. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Blázquez, P.; Rodríguez-Muñoz, M.; Vicente-Sánchez, A.; Garzón, J. Cannabinoid receptors couple to NMDA receptors to reduce the production of NO and the mobilization of zinc induced by glutamate. Antioxid. Redox Signal. 2013, 19, 1766–1782. [Google Scholar] [CrossRef]
- Jia, J.; Ma, L.; Wu, M.; Zhang, L.; Zhang, X.; Zhai, Q.; Jiang, T.; Wang, Q.; Xiong, L. Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.N.; Baban, B.; et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain. Behav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Mudgal, J.; Arora, D.; Kinra, M.; Mallik, S.B.; Rao, C.M.; Pai, K.S.R.; Nampoothiri, M. Cannabinoid receptor 2 activation mitigates lipopolysaccharide-induced neuroinflammation and sickness behavior in mice. Psychopharmacol. Berl. 2019, 236, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S.T. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflammation 2004, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank-Cannon, T.C.; Alto, L.T.; McAlpine, F.E.; Tansey, M.G. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol. Neurodegener. 2009, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Bélanger, M.; Magistretti, P.J. The role of astroglia in neuroprotection. Dialogues Clin. Neurosci. 2009, 11, 281–295. [Google Scholar]
- Molina-Holgado, F.; Pinteaux, E.; Moore, J.D.; Molina-Holgado, E.; Guaza, C.; Gibson, R.M.; Rothwell, N.J. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J. Neurosci. 2003, 23, 6470–6474. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.; Min, X.; Cabral, G.A.; Lokensgard, J.R.; Peterson, P.K. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia 2005, 49, 211–219. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Gómez Del Pulgar, T.; De Ceballos, M.L.; Guzmán, M.; Velasco, G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2002, 277, 36527–36533. [Google Scholar] [CrossRef] [Green Version]
- Carracedo, A.; Geelen, M.J.H.; Diez, M.; Hanada, K.; Guzmán, M.; Velasco, G. Ceramide sensitizes astrocytes to oxidative stress: Protective role of cannabinoids. Biochem. J. 2004, 380, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Luo, X.; Liu, S.; Shen, Y. Neuroprotective effect of cannabinoid receptor 1 antagonist in the MNU-induced retinal degeneration model. Exp. Eye Res. 2018, 167, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Dong, X.; Zhu, R.; Jiang, J.; Ye, Y.; Jiang, Y. Cannabinoid 1 Receptor Antagonists Play a Neuroprotective Role in Chronic Alcoholic Hippocampal Injury Related to Pyroptosis Pathway. Alcohol. Clin. Exp. Res. 2020, 14391. [Google Scholar] [CrossRef]
- Molina-Holgado, F.; Molina-Holgado, E.; Guaza, C.; Rothwell, N.J. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J. Neurosci. Res. 2002, 67, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waksman, Y.; Olson, J.M.; Carlisle, S.J.; Cabral, G.A. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J. Pharmacol. Exp. Ther. 1999, 288, 1357–1366. [Google Scholar]
- Correa, F.; Hernangómez, M.; Mestre, L.; Loría, F.; Spagnolo, A.; Docagne, F.; Di Marzo, V.; Guaza, C. Anandamide enhances IL-10 production in activated microglia by targeting CB2 receptors: Roles of ERK1/2, JNK, and NF-κB. Glia 2010, 58, 135–147. [Google Scholar] [CrossRef]
- Merighi, S.; Gessi, S.; Varani, K.; Simioni, C.; Fazzi, D.; Mirandola, P.; Borea, P.A. Cannabinoid CB2 receptors modulate ERK-1/2 kinase signalling and NO release in microglial cells stimulated with bacterial lipopolysaccharide. Br. J. Pharmacol. 2012, 165, 1773–1788. [Google Scholar] [CrossRef] [Green Version]
- Malek, N.; Popiolek-Barczyk, K.; Mika, J.; Przewlocka, B.; Starowicz, K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015, 2015, 130639. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Jia, J.; Liu, X.; Bai, F.; Wang, Q.; Xiong, L. Activation of murine microglial N9 cells is attenuated through cannabinoid receptor CB2 signaling. Biochem. Biophys. Res. Commun. 2015, 458, 92–97. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Haskó, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J. Neurosci. 2012, 32, 4004–4016. [Google Scholar] [CrossRef]
- Elliott, M.B.; Tuma, R.F.; Amenta, P.S.; Barbe, M.F.; Jallo, J.I. Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J. Neurotrauma 2011, 28, 973–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Craig Hooper, D.; Elliott, M.B. Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J. Neuroinflammation 2014, 11, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, G.A.; Griffin-Thomas, L.T. Emerging role of the cannabinoid receptor CB 2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, J.A.; Wake, A.; Sato, M.; Corcoran, M.E. Antiepileptic and prophylactic effects of tetrahydrocannabinols in amygdaloid kindled cats. Epilepsia 1975, 16, 503–510. [Google Scholar] [CrossRef]
- Karler, R.; Turkanis, S.A. Subacute cannabinoid treatment: Anticonvulsant activity and withdrawal excitability in mice. Br. J. Pharmacol. 1980, 68, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Wallace, M.J.; Wiley, J.L.; Martin, B.R.; DeLorenzo, R.J. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur. J. Pharmacol. 2001, 428, 51–57. [Google Scholar] [CrossRef]
- Wallace, M.J.; Blair, R.E.; Falenski, K.W.; Martin, B.R.; DeLorenzo, R.J. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 2003, 307, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Ortega-Gutiérrez, S.; Van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Monory, K.; Massa, F.; Egertová, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The Endocannabinoid System Controls Key Epileptogenic Circuits in the Hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Karanian, D.A.; Karim, S.L.; Wood, J.T.; Williams, J.S.; Lin, S.; Makriyannis, A.; Bahr, B.A. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J. Pharmacol. Exp. Ther. 2007, 322, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Guggenhuber, S.; Monory, K.; Lutz, B.; Klugmann, M. AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Bhaskaran, M.D.; Smith, B.N. Cannabinoid-mediated inhibition of recurrent excitatoryCircuitry in the dentate gyrus in a mouse model of temporal lobe epilepsy. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Neu, A.; Howard, A.L.; Földy, C.; Echegoyen, J.; Hilgenberg, L.; Smith, M.; Mackie, K.; Soltesz, I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J. Neurosci. 2007, 27, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Ratzliff, A.; Hilgenberg, L.; Gulyás, A.; Freund, T.F.; Smith, M.; Dinh, T.P.; Piomelli, D.; Mackie, K.; Soltesz, I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron 2003, 39, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Ludányi, A.; Eross, L.; Czirják, S.; Vajda, J.; Halász, P.; Watanabe, M.; Palkovits, M.; Maglóczky, Z.; Freund, T.F.; Katona, I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 2008, 28, 2976–2990. [Google Scholar] [CrossRef] [Green Version]
- Goffin, K.; Van Paesschen, W.; Van Laere, K. In Vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain 2011, 134, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Falenski, K.W.; Carter, D.S.; Harrison, A.J.; Martin, B.R.; Blair, R.E.; DeLorenzo, R.J. Temporal characterization of changes in hippocampal cannabinoid CB(1) receptor expression following pilocarpine-induced status epilepticus. Brain Res. 2009, 1262, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Karlócai, M.R.; Tóth, K.; Watanabe, M.; Ledent, C.; Juhász, G.; Freund, T.F.; Maglóczky, Z. Redistribution of CB1 cannabinoid receptors in the acute and chronic phases of pilocarpine-induced epilepsy. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Sales-Carbonell, C.; Rueda-Orozco, P.E.; Soria-Gómez, E.; Buzsáki, G.; Marsicano, G.; Robbe, D. Striatal GABAergic and cortical glutamatergic neurons mediate contrasting effects of cannabinoids on cortical network synchrony. Proc. Natl. Acad. Sci. USA 2013, 110, 719–724. [Google Scholar] [CrossRef] [Green Version]
- Vilela, L.R.; Medeiros, D.C.; Rezende, G.H.S.; de Oliveira, A.C.P.; Moraes, M.F.D.; Moreira, F.A. Effects of cannabinoids and endocannabinoid hydrolysis inhibition on pentylenetetrazole-induced seizure and electroencephalographic activity in rats. Epilepsy Res. 2013, 104, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Huizenga, M.N.; Wicker, E.; Beck, V.C.; Forcelli, P.A. Anticonvulsant effect of cannabinoid receptor agonists in models of seizures in developing rats. Epilepsia 2017, 58, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Aziz Ahari, F.; Shafaghi, B.; Najarkolaei, A.H.; Motamedi, F. Evaluation of interactions between cannabinoid compounds and diazepam in electroshock-induced seizure model in mice. J. Neural Transm. 2008, 115, 1501–1511. [Google Scholar] [CrossRef]
- Turkanis, S.A.; Karler, R. Central excitatory properties of delta 9-tetrahydrocannabinol and its metabolites in iron-induced epileptic rats. Neuropharmacology 1982, 21, 7–13. [Google Scholar] [CrossRef]
- Gordon, E.; Devinsky, O. Alcohol and marijuana: Effects on epilepsy and use by patients with epilepsy. Epilepsia 2001, 42, 1266–1272. [Google Scholar] [CrossRef]
- Clement, A.B.; Hawkins, E.G.; Lichtman, A.H.; Cravatt, B.F. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J. Neurosci. 2003, 23, 3916–3923. [Google Scholar] [CrossRef]
- Deshpande, L.S.; Sombati, S.; Blair, R.E.; Carter, D.S.; Martin, B.R.; DeLorenzo, R.J. Cannabinoid CB1 receptor antagonists cause status epilepticus-like activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci. Lett. 2007, 411, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braakman, H.M.H.; Van Oostenbrugge, R.J.; Van Kranen-Mastenbroek, V.H.J.M.; De Krom, M.C.T.F.M. Rimonabant induces partial seizures in a patient with a history of generalized epilepsy: Letters/commentary. Epilepsia 2009, 50, 2171–2172. [Google Scholar] [CrossRef]
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef]
- Rizzo, V.; Ferraro, G.; Carletti, F.; Lonobile, G.; Cannizzaro, C.; Sardo, P. Evidences of cannabinoids-induced modulation of paroxysmal events in an experimental model of partial epilepsy in the rat. Neurosci. Lett. 2009, 462, 135–139. [Google Scholar] [CrossRef]
- Hill, T.D.M.; Cascio, M.G.; Romano, B.; Duncan, M.; Pertwee, R.G.; Williams, C.M.; Whalley, B.J.; Hill, A.J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 2013, 170, 679–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, V.; Carletti, F.; Gambino, G.; Schiera, G.; Cannizzaro, C.; Ferraro, G.; Sardo, P. Role of CB2 receptors and cGMP pathway on the cannabinoid-dependent antiepileptic effects in an in vivo model of partial epilepsy. Epilepsy Res. 2014, 108, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Liu, X.; Yang, F.; Wang, H. CB2R induces a protective response for epileptic seizure via the PI3K 110α-AKT signaling pathway. Exp. Ther. Med. 2018, 16, 4784–4790. [Google Scholar] [CrossRef]
- Shapiro, L.; Wong, J.C.; Escayg, A. Reduced cannabinoid 2 receptor activity increases susceptibility to induced seizures in mice. Epilepsia 2019, 60, 2359–2369. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H. The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Res. 2018, 148, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Berrendero, F.; Pérez-Rosado, A.; Manzanares, J.; Rojo, A.; Fernández-Ruiz, J.J.; de Yebenes, J.G.; Ramos, J.A. Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci. 2000, 66, 485–494. [Google Scholar] [CrossRef]
- Di Marzo, V.; Hill, M.P.; Bisogno, T.; Crossman, A.R.; Brotchie, J.M. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000, 14, 1432–1438. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Cebeira, M.; De Ceballos, M.L.; Zeng, B.Y.; Jenner, P.; Ramos, J.A.; Fernández-Ruiz, J.J. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur. J. Neurosci. 2001, 14, 1827–1832. [Google Scholar] [CrossRef]
- Pisani, A.; Fezza, F.; Galati, S.; Battista, N.; Napolitano, S.; Finazzi-Agrò, A.; Bernardi, G.; Brusa, L.; Pierantozzi, M.; Stanzione, P.; et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005, 57, 777–779. [Google Scholar] [CrossRef]
- Pisani, V.; Moschella, V.; Bari, M.; Fezza, F.; Galati, S.; Bernardi, G.; Stanzione, P.; Pisani, A.; Maccarrone, M. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov. Disord. 2010, 25, 920–924. [Google Scholar] [CrossRef]
- Mesnage, V.; Houeto, J.L.; Bonnet, A.M.; Clavier, I.; Arnulf, I.; Cattelin, F.; Le Fur, G.; Damier, P.; Welter, M.L.; Agid, Y. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin. Neuropharmacol. 2004, 27, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Sieradzan, K.A.; Fox, S.H.; Hill, M.; Dick, J.P.; Crossman, A.R.; Brotchie, J.M. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: A pilot study. Neurology 2001, 57, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.B.; Bain, P.O.; Teare, L.; Liu, X.; Joint, C.; Wroath, C.; Parkin, S.G.; Fox, P.; Wright, D.; Hobart, J.; et al. Cannabis for dyskinesia in Parkinson disease: A randomized double-blind crossover study. Neurology 2004, 63, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Kirsten, G.P.; Mazucanti, C.H.Y.; Real, C.C.; Souza, B.M.; Britto, L.R.G.; Torrão, A.S. Temporal changes of CB1 cannabinoid receptor in the basal ganglia as a possible structure-specific plasticity process in 6-OHDA lesioned rats. PLoS ONE 2013, 8, e76874. [Google Scholar] [CrossRef] [Green Version]
- Lotan, I.; Treves, T.A.; Roditi, Y.; Djaldetti, R. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: An open-label observational study. Clin. Neuropharmacol. 2014, 37, 41–44. [Google Scholar] [CrossRef]
- García-Arencibia, M.; González, S.; de Lago, E.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: Importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007, 1134, 162–170. [Google Scholar] [CrossRef]
- Chung, Y.C.; Shin, W.H.; Baek, J.Y.; Cho, E.J.; Baik, H.H.; Kim, S.R.; Won, S.Y.; Jin, B.K. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2016, 48, e205. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br. J. Pharmacol. 2009, 156, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Glass, M.; Faull, R.L.; Dragunow, M. Loss of cannabinoid receptors in the substantia nigra in Huntington’s disease. Neuroscience 1993, 56, 523–527. [Google Scholar] [CrossRef]
- Richfield, E.K.; Herkenham, M. Selective vulnerability in Huntington’s disease: Preferential loss of cannabinoid receptors in lateral globus pallidus. Ann. Neurol. 1994, 36, 577–584. [Google Scholar] [CrossRef]
- Glass, M.; Dragunow, M.; Faull, R.L. The pattern of neurodegeneration in Huntington’s disease: A comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 2000, 97, 505–519. [Google Scholar] [CrossRef]
- Denovan-Wright, E.M.; Robertson, H.A. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington’s disease mice. Neuroscience 2000, 98, 705–713. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Fezza, F.; Cebeira, M.; Bisogno, T.; Ramos, J.A.; Milone, A.; Fernández-Ruiz, J.; Di Marzo, V. Changes in endocannabinoid transmission in the basal ganglia in a rat model of Huntington’s disease. Neuroreport 2001, 12, 2125–2129. [Google Scholar] [CrossRef]
- Van Laere, K.; Casteels, C.; Dhollander, I.; Goffin, K.; Grachev, I.; Bormans, G.; Vandenberghe, W. Widespread decrease of type 1 cannabinoid receptor availability in Huntington disease In Vivo. J. Nucl. Med. 2010, 51, 1413–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, A.; Mitchell, I.; Patel, S.; Ives, N.; Rickards, H. A pilot study using nabilone for symptomatic treatment in Huntington’s disease. Mov. Disord. 2009, 24, 2254–2259. [Google Scholar] [CrossRef]
- Sagredo, O.; González, S.; Aroyo, I.; Pazos, M.R.; Benito, C.; Lastres-Becker, I.; Romero, J.P.; Tolón, R.M.; Mechoulam, R.; Brouillet, E.; et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: Relevance for Huntington’s disease. Glia 2009, 57, 1154–1167. [Google Scholar] [CrossRef] [Green Version]
- Westlake, T.M.; Howlett, A.C.; Bonner, T.I.; Matsuda, L.A.; Herkenham, M. Cannabinoid receptor binding and messenger RNA expression in human brain: An in vitro receptor autoradiography and In Situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience 1994, 63, 637–652. [Google Scholar] [CrossRef]
- Ramírez, B.G.; Blázquez, C.; Gómez Del Pulgar, T.; Guzmán, M.; De Ceballos, M.L. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005, 25, 1904–1913. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Agacinski, G.; Williams, J.H.; Wilcock, G.K.; Esiri, M.M.; Francis, P.T.; Wong, P.T.H.; Chen, C.P.; Lai, M.K.P. Intact cannabinoid CB1 receptors in the Alzheimer’s disease cortex. Neurochem. Int. 2010, 57, 985–989. [Google Scholar] [CrossRef]
- Mulder, J.; Zilberter, M.; Pasquaré, S.J.; Alpár, A.; Schulte, G.; Ferreira, S.G.; Köfalvi, A.; Martín-Moreno, A.M.; Keimpema, E.; Tanila, H.; et al. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain 2011, 134, 1041–1060. [Google Scholar] [CrossRef]
- Bedse, G.; Romano, A.; Cianci, S.; Lavecchia, A.M.; Lorenzo, P.; Elphick, M.R.; Laferla, F.M.; Vendemiale, G.; Grillo, C.; Altieri, F.; et al. Altered expression of the CB1 cannabinoid receptor in the triple transgenic mouse model of alzheimer’s disease. J. Alzheimer’s Dis. 2014, 40, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Goffin, K.; Van den Stock, J.; De Winter, F.L.; Cleeren, E.; Bormans, G.; Tournoy, J.; Persoons, P.; Van Laere, K.; Vandenbulcke, M. In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur. Neuropsychopharmacol. 2014, 24, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Eikelenboom, P.; Veerhuis, R.; Scheper, W.; Rozemuller, A.J.M.; Van Gool, W.A.; Hoozemans, J.J.M. The significance of neuroinflammation in understanding Alzheimer’s disease. J. Neural Transm. 2006, 113, 1685–1695. [Google Scholar] [CrossRef]
- Tolón, R.M.; Núñez, E.; Pazos, M.R.; Benito, C.; Castillo, A.I.; Martínez-Orgado, J.A.; Romero, J. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Res. 2009, 1283, 148–154. [Google Scholar] [CrossRef]
- Wu, J.; Bie, B.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol. Aging 2013, 34, 791–804. [Google Scholar] [CrossRef]
- Naguib, M.; Giordano, T. DT-02-05: A novel therapeutic (NTRX-07) targeting neuroinflammation in alzheimer’s disease is undergoing phase I trials. Alzheimer’s Dement. 2019, 15, 1490–1491. [Google Scholar] [CrossRef]
- Martin, M.; Ledent, C.; Parmentier, M.; Maldonado, R.; Valverde, O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacol. Berl. 2002, 159, 379–387. [Google Scholar] [CrossRef]
- Viveros, M.P.; Marco, E.M.; File, S.E. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 2005, 81, 331–342. [Google Scholar] [CrossRef]
- Moreira, F.A.; Wotjak, C.T. Cannabinoids and anxiety. Curr. Top. Behav. Neurosci. 2010, 2, 429–450. [Google Scholar] [CrossRef]
- Rubino, T.; Guidali, C.; Vigano, D.; Realini, N.; Valenti, M.; Massi, P.; Parolaro, D. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology 2008, 54, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.A.; Purrio, M.; Viveros, M.P.; Lutz, B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of gabaergic and glutamatergic neurotransmission. Neuropsychopharmacology 2012, 37, 2624–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, J.; Bakos, N.; Szirmay, M.; Ledent, C.; Freund, T.F. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 2002, 16, 1395–1398. [Google Scholar] [CrossRef]
- Moreira, F.A.; Aguiar, D.C.; Terzian, A.L.B.; Guimarães, F.S.; Wotjak, C.T. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience 2012, 204, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; De Chiara, V.; Musella, A.; Kusayanagi, H.; Mataluni, G.; Bernardi, G.; Usiello, A.; Centonze, D. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J. Neurosci. 2008, 28, 7284–7292. [Google Scholar] [CrossRef] [PubMed]
- Häring, M.; Enk, V.; Rey, A.A.; Loch, S.; de Azua, I.R.; Weber, T.; Bartsch, D.; Monory, K.; Lutz, B. Cannabinoid type-1 receptor signaling in central serotonergic neurons regulates anxiety-like behavior and sociability. Front. Behav. Neurosci. 2015, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Su, F.; Yi, H.; Xu, L.; Zhang, Z. Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia In Vitro. Neuroscience 2015, 294, 60–68. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Gilio, L.; Maffei, P.; Dolcetti, E.; Bruno, A.; Buttari, F.; Centonze, D.; Iezzi, E. Exploiting the multifaceted effects of cannabinoids on mood to boost their therapeutic use against anxiety and depression. Front. Mol. Neurosci. 2018, 11, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef] [Green Version]
- García-Gutiérrez, M.S.; Manzanares, J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J. Psychopharmacol. 2011, 25, 111–120. [Google Scholar] [CrossRef] [Green Version]
- García-Gutiérrez, M.S.; García-Bueno, B.; Zoppi, S.; Leza, J.C.; Manzanares, J. Chronic blockade of cannabinoid CB 2 receptors induces anxiolytic-like actions associated with alterations in GABA A receptors. Br. J. Pharmacol. 2012, 165, 951–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.R.; Canseco-Alba, A.; Zhang, H.Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Escosteguy-Neto, J.C.; Ishiguro, H.; et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, T.; Sinor, A.D.; Simon, R.P.; Chen, J.; Graham, S.H.; Jin, K.; Greenberg, D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999, 19, 2987–2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.J.; Reiner, D.; Shen, H.; Wu, K.J.; Liu, Q.R.; Wang, Y. Time-dependent protection of CB2 receptor agonist in stroke. PLoS ONE 2015, 10, e0132487. [Google Scholar] [CrossRef]
- Caltana, L.; Saez, T.M.; Aronne, M.P.; Brusco, A. Cannabinoid receptor type 1 agonist ACEA improves motor recovery and protects neurons in ischemic stroke in mice. J. Neurochem. 2015, 135, 616–629. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Han, J.; Higashimori, H.; Wang, J.; Liu, J.; Tong, L.; Yang, Y.; Dong, H.; Zhang, X.; Xiong, L. Long-term depression induced by endogenous cannabinoids produces neuroprotection via astroglial CB1R after stroke in rodents. J. Cereb. Blood Flow Metab. 2019, 39, 1122–1137. [Google Scholar] [CrossRef]
- Kolb, B.; Saber, H.; Fadel, H.; Rajah, G. The endocannabinoid system and stroke: A focused review. Brain Circ. 2019, 5, 1. [Google Scholar] [CrossRef]
- Reichenbach, Z.W.; Li, H.; Ward, S.J.; Tuma, R.F. The CB1 antagonist, SR141716A, is protective in permanent photothrombotic cerebral ischemia. Neurosci. Lett. 2016, 630, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, M.D.; de la Tremblaye, P.B.; Azogu, I.; Plamondon, H. Endocannabinoid CB1 receptor activation upon global ischemia adversely impact recovery of reward and stress signaling molecules, neuronal survival and behavioral impulsivity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 66, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Ferrer, I.; Cuartero, M.I.; Zarruk, J.G.; Pradillo, J.M.; Hurtado, O.; Romera, V.G.; Díaz-Alonso, J.; García-Segura, J.M.; Guzmán, M.; Lizasoain, I.; et al. Cannabinoid Type-2 Receptor Drives Neurogenesis and Improves Functional Outcome After Stroke. Stroke 2017, 48, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Miao, H.; Jiang, B.; Chen, Q.; Tan, L.; Tao, Y.; Zhang, J.; Gao, F.; Feng, H.; Zhu, G.; et al. A selective CB2R agonist (JWH133) restores neuronal circuit after Germinal Matrix Hemorrhage in the preterm via CX3CR1+ microglia. Neuropharmacology 2017, 119, 157–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Martin, B.R.; Adler, M.W.; Razdan, R.J.; Kong, W.; Ganea, D.; Tuma, R.F. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J. Neuroimmune Pharmacol. 2009, 4, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mahadevan, A.; Amere, M.; Li, H.; Ganea, D.; Tuma, R.F. Unique Effects of Compounds Active at Both Cannabinoid and Serotonin Receptors During Stroke. Transl. Stroke Res. 2012, 3, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Martin, B.R. Spinal and supraspinal components of cannabinoid-induced antinociception. J. Pharmacol. Exp. Ther. 1991, 258, 517–523. [Google Scholar] [PubMed]
- Mao, J.; Price, D.D.; Lu, J.; Keniston, L.; Mayer, D.J. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci. Lett. 2000, 280, 13–16. [Google Scholar] [CrossRef]
- Edsall, S.A.; Knapp, R.J.; Vanderah, T.W.; Roeske, W.R.; Consroe, P.; Yamamura, H.I. Antisense oligodeoxynucleotide treatment to the brain cannabinoid receptor inhibits antinociception. Neuroreport 1996, 7, 593–596. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Cook, S.A.; Martin, B.R. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: Evidence supporting periaqueductal gray involvement. J. Pharmacol. Exp. Ther. 1996, 276, 585–593. [Google Scholar]
- Lichtman, A.H.; Martin, B.R. The selective cannabinoid antagonist SR 141716A blocks cannabinoid- induced antinociception in rats. Pharmacol. Biochem. Behav. 1997, 57, 7–12. [Google Scholar] [CrossRef]
- Bridges, D.; Ahmad, K.; Rice, A.S.C. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br. J. Pharmacol. 2001, 133, 586–594. [Google Scholar] [CrossRef]
- Fox, A.; Kesingland, A.; Gentry, C.; McNair, K.; Patel, S.; Urban, L.; James, I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 2001, 92, 91–100. [Google Scholar] [CrossRef]
- Lim, G.; Sung, B.; Ji, R.-R.; Mao, J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain 2003, 105, 275–283. [Google Scholar] [CrossRef]
- Hama, A.T.; Urban, M.O. Antihyperalgesic effect of the cannabinoid agonist WIN55,212-2 is mediated through an interaction with spinal metabotropic glutamate-5 receptors in rats. Neurosci. Lett. 2004, 358, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Shafaghi, B.; Khodayar, M.J.; Zarindast, M.R. Interaction between gamma-aminobutyric acid GABAB and cannabinoid CB1 receptors in spinal pain pathways in rat. Eur. J. Pharmacol. 2005, 514, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Nackley, A.G.; Makriyannis, A.; Hohmann, A.G. Selective activation of cannabinoid CB2 receptors suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience 2003, 119, 747–757. [Google Scholar] [CrossRef]
- Elmes, S.J.R.; Winyard, L.A.; Medhurst, S.J.; Clayton, N.M.; Wilson, A.W.; Kendall, D.A.; Chapman, V. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain 2005, 118, 327–335. [Google Scholar] [CrossRef]
- Racz, I.; Nadal, X.; Alferink, J.; Baños, J.E.; Rehnelt, J.; Martín, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Bellora, N.; et al. Interferon-γ is a critical modulator of CB2 cannabinoid receptor signaling during neuropathic pain. J. Neurosci. 2008, 28, 12136–12145. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.M.; Deng, H.; Zvonok, A.; Cockayne, D.A.; Kwan, J.; Mata, H.P.; Vanderah, T.W.; Lai, J.; Porreca, F.; Makriyannis, A.; et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: Pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. USA 2003, 100, 10529–10533. [Google Scholar] [CrossRef] [Green Version]
- Beltramo, M.; Bernardini, N.; Bertorelli, R.; Campanella, M.; Nicolussi, E.; Fredduzzi, S.; Reggiani, A. CB2 receptor-mediated antihyperalgesia: Possible direct involvement of neural mechanisms. Eur. J. Neurosci. 2006, 23, 1530–1538. [Google Scholar] [CrossRef]
- Jhaveri, M.D.; Elmes, S.J.R.; Richardson, D.; Barrett, D.A.; Kendall, D.A.; Mason, R.; Chapman, V. Evidence for a novel functional role of cannabinoid CB2 receptors in the thalamus of neuropathic rats. Eur. J. Neurosci. 2008, 27, 1722–1730. [Google Scholar] [CrossRef] [Green Version]
- Rahn, E.J.; Zvonok, A.M.; Thakur, G.A.; Khanolkar, A.D.; Makriyannis, A.; Hohmann, A.G. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J. Pharmacol. Exp. Ther. 2008, 327, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Wilkerson, J.L.; Gentry, K.R.; Dengler, E.C.; Wallace, J.A.; Kerwin, A.A.; Armijo, L.M.; Kuhn, M.N.; Thakur, G.A.; Makriyannis, A.; Milligan, E.D. Intrathecal cannabilactone CB 2R agonist, AM1710, controls pathological pain and restores basal cytokine levels. Pain 2012, 153, 1091–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quartilho, A.; Mata, H.P.; Ibrahim, M.M.; Vanderah, T.W.; Porreca, F.; Makriyannis, A.; Malan, T.P. Inhibition of inflammatory hyperalgesia by activation of peripheral CB 2 cannabinoid receptors. Anesthesiology 2003, 99, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, S.G.; Mahadevan, A.; Zhao, B.; Sun, H.; Naidu, P.S.; Razdan, R.K.; Selley, D.E.; Imad Damaj, M.; Lichtman, A.H. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology 2011, 60, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.S.C.; Chan, W.S.; Cheung, C.W. Analgesic Effects of Cannabinoids for Chronic Non-cancer Pain: A Systematic Review and Meta-Analysis with Meta-Regression. J. Neuroimmune Pharmacol. 2020, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Agabio, R.; Diaz, G.; Lobina, C.; Reali, R.; Gessa, G.L. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998, 63, PL113–PL117. [Google Scholar] [CrossRef]
- Di Marzo, V.; Goparaju, S.K.; Wang, L.; Liu, J.; Bátkai, S.; Járai, Z.; Fezza, F.; Miura, G.I.; Palmiter, R.D.; Sugiura, T.; et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001, 410, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Ligresti, A.; Cristino, L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int. J. Obes. 2009, 33, S18–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucci, S.A.; Rogers, E.K.; Korbonits, M.; Kirkham, T.C. The cannabinoid CB 1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br. J. Pharmacol. 2004, 143, 520–523. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.H.; Chen, Y.J.J.; Chua, S.C.; Talmage, D.A.; Role, L.W. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron 2005, 48, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Kola, B.; Farkas, I.; Christ-Crain, M.; Wittmann, G.; Lolli, F.; Amin, F.; Harvey-White, J.; Liposits, Z.; Kunos, G.; Grossman, A.B.; et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE 2008, 3. [Google Scholar] [CrossRef]
- Miller, C.C.; Murray, T.F.; Freeman, K.G.; Edwards, G.L. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol. Behav. 2004, 80, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Melis, T.; Succu, S.; Sanna, F.; Boi, A.; Argiolas, A.; Melis, M.R. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci. Lett. 2007, 419, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Koch, M. Cannabinoid receptor signaling in central regulation of feeding behavior: A mini-review. Front. Neurosci. 2017, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Ravinet Trillou, C.; Delgorge, C.; Menet, C.; Arnone, M.; Soubrié, P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. 2004, 28, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Doyle, M.E.; Liu, Z.; Lao, Q.; Shin, Y.K.; Carlson, O.D.; Kim, H.S.; Thomas, S.; Napora, J.K.; Lee, E.K.; et al. Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes 2011, 60, 1198–1209. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Han, J.H.; Yoon, J.; Sim, H.J.; Park, T.J.; Yang, S.; Lee, E.K.; Kulkarni, R.N.; Egan, J.M.; Kim, W. Blockade of cannabinoid 1 receptor improves glucose responsiveness in pancreatic beta cells. J. Cell. Mol. Med. 2018, 22, 2337–2345. [Google Scholar] [CrossRef] [Green Version]
- Rohrbach, K.; Thomas, M.A.; Glick, S.; Fung, E.N.; Wang, V.; Watson, L.; Gregory, P.; Antel, J.; Pelleymounter, M.A. Ibipinabant attenuates β-cell loss in male Zucker diabetic fatty rats independently of its effects on body weight. Diabetesobes. Metab. 2012, 14, 555–564. [Google Scholar] [CrossRef]
- González-Mariscal, I.; Montoro, R.A.; Doyle, M.E.; Liu, Q.R.; Rouse, M.; O’Connell, J.F.; Santa-Cruz Calvo, S.; Krzysik-Walker, S.M.; Ghosh, S.; Carlson, O.D.; et al. Absence of cannabinoid 1 receptor in beta cells protects against high-fat/high-sugar diet-induced beta cell dysfunction and inflammation in murine islets. Diabetologia 2018, 61, 1470–1483. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, T.; Godlewski, G.; Cinar, R.; Bertola, A.; Szanda, G.; Liu, J.; Tam, J.; Han, T.; Mukhopadhyay, B.; Skarulis, M.C.; et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013, 19, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, T.; Godlewski, G.; Kunos, G. Endocannabinoid regulation of β -cell functions: Implications for glycaemic control and diabetes. Diabetesobes. Metab. 2016, 18, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Ruby, M.A.; Nomura, D.K.; Hudak, C.S.S.; Mangravite, L.M.; Chiu, S.; Casida, J.E.; Krauss, R.M. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proc. Natl. Acad. Sci. USA 2008, 105, 14561–14566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, I.; Gonthier, M.-P.; Orlando, P.; Martiadis, V.; De Petrocellis, L.; Cervino, C.; Petrosino, S.; Hoareau, L.; Festy, F.; Pasquali, R.; et al. Regulation, Function, and Dysregulation of Endocannabinoids in Models of Adipose and β-Pancreatic Cells and in Obesity and Hyperglycemia. J. Clin. Endocrinol. Metab. 2006, 91, 3171–3180. [Google Scholar] [CrossRef]
- Furuya, D.T.; Poletto, A.C.; Freitas, H.S.; Machado, U.F. Inhibition of cannabinoid CB1 receptor upregulates Slc2a4 expression via nuclear factor-κB and sterol regulatory element-binding protein-1 in adipocytes. J. Mol. Endocrinol. 2012, 49, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Shin, H.; Rho, J.G.; Kim, J.-E.; Son, D.H.; Yoon, J.; Lee, Y.J.; Park, J.-H.; Song, B.J.; Choi, C.-S.; et al. Peripheral cannabinoid 1 receptor blockade mitigates adipose tissue inflammation via NLRP3 inflammasome in mouse models of obesity. Diabetesobes. Metab. 2018, 20, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, L.; Valerio, A.; Cervino, C.; Cardile, A.; Pagano, C.; Vettor, R.; Pasquali, R.; Carruba, M.O.; Marsicano, G.; Lutz, B.; et al. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes 2008, 57, 2028–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedesco, L.; Valerio, A.; Dossena, M.; Cardile, A.; Ragni, M.; Pagano, C.; Pagotto, U.; Carruba, M.O.; Vettor, R.; Nisoli, E. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: The role of eNOS, p38 MAPK, and AMPK pathways. Diabetes 2010, 59, 2826–2836. [Google Scholar] [CrossRef] [Green Version]
- Arrabal, S.; Lucena, M.A.; Canduela, M.J.; Ramos-Uriarte, A.; Rivera, P.; Serrano, A.; Pavón, F.J.; Decara, J.; Vargas, A.; Baixeras, E.; et al. Pharmacological blockade of cannabinoid CB1 receptors in diet-induced obesity regulates mitochondrial dihydrolipoamide dehydrogenase in muscle. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yu, Q.; Yue, H.; Zeng, S.; Cui, F. Effect of Intermittent Hypoxia and Rimonabant on Glucose Metabolism in Rats: Involvement of Expression of GLUT4 in Skeletal Muscle. Med. Sci. Monit. 2015, 21, 3252–3260. [Google Scholar] [CrossRef] [Green Version]
- Eckardt, K.; Sell, H.; Taube, A.; Koenen, M.; Platzbecker, B.; Cramer, A.; Horrighs, A.; Lehtonen, M.; Tennagels, N.; Eckel, J. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia 2009, 52, 664–674. [Google Scholar] [CrossRef] [Green Version]
- Lipina, C.; Stretton, C.; Hastings, S.; Hundal, J.S.; Mackie, K.; Irving, A.J.; Hundal, H.S. Regulation of MAP kinase-directed mitogenic and protein kinase B-mediated signaling by cannabinoid receptor type 1 in skeletal muscle cells. Diabetes 2010, 59, 375–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavuoto, P.; McAinch, A.J.; Hatzinikolas, G.; Cameron-Smith, D.; Wittert, G.A. Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol. Cell. Endocrinol. 2007, 267, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, F.X.; Aronne, L.J.; Heshmati, H.M.; Devin, J.; Rosenstock, J. RIO-North America Study Group, for the Effect of Rimonabant, a Cannabinoid-1 Receptor Blocker, on Weight and Cardiometabolic Risk Factors in Overweight or Obese Patients. JAMA 2006, 295, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendizábal, V.E.; Adler-Graschinsky, E. Cannabinoids as therapeutic agents in cardiovascular disease: A tale of passions and illusions. Br. J. Pharmacol. 2007, 151, 427–440. [Google Scholar] [CrossRef]
- Moreira, F.A.; Crippa, J.A.S. The psychiatric side-effects of rimonabant. Rev. Bras. Psiquiatr. 2009, 31, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Sam, A.H.; Salem, V.; Ghatei, M.A. Rimonabant: From RIO to Ban. J. Obes. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Peters, R.J. Rimonabant: Obituary for a wonder drug. Lancet 2010, 376, 489–490. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Fridberg, M.; De Kam, M.L.; Little, P.B.; Jensen, N.O.; Kleinloog, H.D.; Elling, C.E.; Van Gerven, J.M.A. Peripheral selectivity of the novel cannabinoid receptor antagonist TM38837 in healthy subjects. Br. J. Clin. Pharmacol. 2013, 76, 846–857. [Google Scholar] [CrossRef] [Green Version]
- Cluny, N.L.; Vemuri, V.K.; Chambers, A.P.; Limebeer, C.L.; Bedard, H.; Wood, J.T.; Lutz, B.; Zimmer, A.; Parker, L.A.; Makriyannis, A.; et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br. J. Pharmacol. 2010, 161, 629–642. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.; Cinar, R.; Liu, J.; Godlewski, G.; Wesley, D.; Jourdan, T.; Szanda, G.; Mukhopadhyay, B.; Chedester, L.; Liow, J.S.; et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012, 16, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.; Serrano, A.; Vida, M.; Crespillo, A.; Hernandez-Folgado, L.; Jagerovic, N.; Goya, P.; Reyes-Cabello, C.; Perez-Valero, V.; Decara, J.; et al. Anti-obesity efficacy of LH-21, a cannabinoid CB 1 receptor antagonist with poor brain penetration, in diet-induced obese rats. Br. J. Pharmacol. 2012, 165, 2274–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Shui, F.; Liu, C.; Zhou, X.; Li, W.; Zheng, Z.; Fu, W.; Wang, L. Novel peripherally restricted cannabinoid 1 receptor selective antagonist TXX-522 with prominent weight-loss efficacy in diet induced obese mice. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zhang, G.; Mou, C.; Fu, X.; Chen, Y. Peripheral CB1 receptor neutral antagonist, AM6545, ameliorates hypometabolic obesity and improves adipokine secretion in monosodium glutamate induced obese mice. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueiras, R.; Veyrat-Durebex, C.; Suchanek, P.M.; Klein, M.; Tschöp, J.; Caldwell, C.; Woods, S.C.; Wittmann, G.; Watanabe, M.; Liposits, Z.; et al. Peripheral, but Not Central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 2008, 57, 2977–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowles, N.P.; Karatsoreos, I.N.; Li, X.; Vemuri, V.K.; Wood, J.A.; Li, Z.; Tamashiro, K.L.K.; Schwartz, G.J.; Makriyannis, A.M.; Kunos, G.; et al. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.; Vemuri, V.K.; Liu, J.; Bátkai, S.; Mukhopadhyay, B.; Godlewski, G.; Osei-Hyiaman, D.; Ohnuma, S.; Ambudkar, S.V.; Pickel, J.; et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Investig. 2010, 120, 2953–2966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barutta, F.; Grimaldi, S.; Gambino, R.; Vemuri, K.; Makriyannis, A.; Annaratone, L.; di Marzo, V.; Bruno, G.; Gruden, G. Dual therapy targeting the endocannabinoid system prevents experimental diabetic nephropathy. Nephrol. Dial. Transplant. 2017, 32, 1655–1665. [Google Scholar] [CrossRef]
- Vidot, D.C.; Prado, G.; Hlaing, W.M.; Florez, H.J.; Arheart, K.L.; Messiah, S.E. Metabolic Syndrome Among Marijuana Users in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am. J. Med. 2016, 129, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Anker, S.D.; Chua, T.P.; Ponikowski, P.; Harrington, D.; Swan, J.W.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 1997, 96, 526–534. [Google Scholar] [CrossRef]
- Burckart, K.; Beca, S.; Urban, R.J.; Sheffield-Moore, M. Pathogenesis of muscle wasting in cancer cachexia: Targeted anabolic and anticatabolic therapies. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Marco, E.M.; Romero-Zerbo, S.Y.; Viveros, M.-P.; Bermudez-Silva, F.J.; Bermú Dez-Silva, F.J. The role of the endocannabinoid system in eating disorders: Pharmacological implications A brief update on the endocannabinoid system. Behav. Pharmacol. 2012, 23, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Osei-Hyiaman, D. Endocannabinoid system in cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Strasser, F.; Luftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of orally administered cannabis extract and delta-9- tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, C.; Corsi, O.; Pérez-Cruz, P. ¿Son los cannabinoides una opción para el síndrome anorexia-caquexia en pacientes con cáncer avanzado? Medwave 2017, 17, e7130. [Google Scholar] [CrossRef]
- Malinowska, B.; Baranowska-Kuczko, M.; Schlicker, E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br. J. Pharmacol. 2012, 165, 2073–2088. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, S.W.; Lapa, A.J.; Ribeiro Do Valle, J. The triple effect ineuced by Δ9-tetrahydrocannabinol on the rat blood pressure. Eur. J. Pharmacol. 1979, 58, 351–357. [Google Scholar] [CrossRef]
- Varga, K.; Lake, K.; Martin, B.R.; Kunos, G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur. J. Pharmacol. 1995, 278, 279–283. [Google Scholar] [CrossRef]
- Malinowska, B.; Kwolek, G.; Göthert, M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2001, 364, 562–569. [Google Scholar] [CrossRef]
- Niederhoffer, N.; Schmid, K.; Szabo, B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn. Schmiedebergs. Arch. Pharmacol. 2003, 367, 434–443. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J. Physiol. 2004, 558, 647–657. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology 2005, 48, 1130–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkow, B. Physiological aspects of primary hypertension. Physiol. Rev. 1982, 62, 347–504. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. 2009, 148, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, J.P.; Paton, J.F.R. The sympathetic nervous system and blood pressure in humans: Implications for hypertension. J. Human Hypertens. 2012, 26, 463–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haspula, D.; Clark, M.A. Neuroinflammation and sympathetic overactivity: Mechanisms and implications in hypertension. Auton. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Seagard, J.L.; Dean, C.; Patel, S.; Rademacher, D.J.; Hopp, F.A.; Schmeling, W.T.; Hillard, C.J. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H992–H1000. [Google Scholar] [CrossRef] [Green Version]
- Seagard, J.L.; Hopp, F.A.; Hillard, C.J.; Dean, C. Effects of endocannabinoids on discharge of baroreceptive NTS neurons. Neurosci. Lett. 2005, 381, 334–339. [Google Scholar] [CrossRef]
- Brozoski, D.T.; Dean, C.; Hopp, F.A.; Seagard, J.L. Uptake blockade of endocannabinoids in the NTS modulates baroreflex-evoked sympathoinhibition. Brain Res. 2005, 1059, 197–202. [Google Scholar] [CrossRef]
- Durakoglugil, M.S.; Orer, H.S. Cannabinoid receptor activation in the nucleus tractus solitaries produces baroreflex-like responses in the rat. Int. J. Biomed. Sci. 2008, 4, 229–237. [Google Scholar]
- Chen, C.Y.; Bonham, A.C.; Dean, C.; Hopp, F.A.; Hillard, C.J.; Seagard, J.L. Retrograde release of endocannabinoids inhibits presynaptic GABA release to second-order baroreceptive neurons in NTS. Auton. Neurosci. Basic Clin. 2010, 158, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Padley, J.R.; Li, Q.; Pilowsky, P.M.; Goodchild, A.K. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br. J. Pharmacol. 2003, 140, 384–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, C. Endocannabinoid modulation of sympathetic and cardiovascular responses to acute stress in the periaqueductal gray of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, B.M.; Abdel-Rahman, A.A. Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor responses in conscious rats. Brain Res. 2011, 1414, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, B.M.; Abdel-Rahman, A.A. Differential modulation of brainstem phosphatidylinositol 3-kinase/Akt and extracellular signal-regulated kinase 1/2 signaling underlies WIN55,212-2 centrally mediated pressor response in conscious rats. J. Pharmacol. Exp. Ther. 2012, 340, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, B.M.; Abdel-Rahman, A.A. Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-Nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressor response in conscious rats. J. Pharmacol. Exp. Ther. 2012, 341, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, B.M.; Abdel-Rahman, A.A. A pivotal role for enhanced brainstem Orexin receptor 1 signaling in the central cannabinoid receptor 1-mediated pressor response in conscious rats. Brain Res. 2015, 1622, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Smiley, K.A.; Karler, R.; Turkanis, S.A. Effects of cannabinoids on the perfused rat heart. Res. Commun. Chem. Pathol. Pharmacol. 1976, 14, 659–675. [Google Scholar]
- Krylatov, A.V.; Maslov, L.N.; Ermakov, S.Y.; Lasukova, O.V.; Barzakh, E.I.; Crawford, D.; Pertwee, R.G. Significance of cardiac cannabinoid receptors in regulation of cardiac rhythm, myocardial contractility, and electrophysiologic processes in heart. Biol. Bull. 2007, 34, 28–35. [Google Scholar] [CrossRef]
- Sterin-Borda, L.; Del Zar, C.F.; Borda, E. Differential CB1 and CB2 cannabinoid receptor-inotropic response of rat isolated atria: Endogenous signal transduction pathways. Biochem. Pharmacol. 2005, 69, 1705–1713. [Google Scholar] [CrossRef]
- Liao, Y.; Bin, J.; Luo, T.; Zhao, H.; Ledent, C.; Asakura, M.; Xu, D.; Takashima, S.; Kitakaze, M. CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice. Int. J. Cardiol. 2013, 167, 1936–1944. [Google Scholar] [CrossRef]
- Pacher, P.; Mukhopadhyay, P.; Mohanraj, R.; Godlewski, G.; Bátkai, S.; Kunos, G. Modulation of the endocannabinoid system in cardiovascular disease: Therapeutic potential and limitations. Hypertension 2008, 52, 601–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledent, C.; Valverde, O.; Cossu, G.; Petitet, F.; Aubert, J.F.; Beslot, F.; Böhme, G.A.; Imperato, A.; Pedrazzini, T.; Roques, B.P.; et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999, 283, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Bin, J.; Asakura, M.; Xuan, W.; Chen, B.; Huang, Q.; Xu, D.; Ledent, C.; Takashima, S.; Kitakaze, M. Deficiency of type 1 cannabinoid receptors worsens acute heart failure induced by pressure overload in mice. Eur. Heart J. 2012, 33, 3124–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Kaminski, N.E.; Wang, D.H. Anandamide-Induced Depressor Effect in Spontaneously Hypertensive Rats: Role of the Vanilloid Receptor. Hypertension 2003, 41, 757–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, R.; Vanessa Ho, W.S.V.; Bottrill, F.E.; Ford, W.R.; Hiley, C.R. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br. J. Pharmacol. 2001, 134, 921–929. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Kendall, D.A.; Randall, M.D. The effects of Δ9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br. J. Pharmacol. 2005, 145, 514–526. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, S.E.; Kendall, D.A.; Randall, M.D. Vascular effects of Δ9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. Eur. J. Pharmacol. 2005, 507, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Abesser, M.; Karcher, J.; Laser, M.; Kunos, G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. J. Cardiovasc. Pharmacol. 2005, 46, 348–355. [Google Scholar] [CrossRef]
- Dannert, M.T.; Alsasua, A.; Herradon, E.; Martín, M.I.; López-Miranda, V. Vasorelaxant effect of Win 55,212-2 in rat aorta: New mechanisms involved. Vascul. Pharmacol. 2007, 46, 16–23. [Google Scholar] [CrossRef]
- Szekeres, M.; Nádasy, G.L.; Soltész-Katona, E.; Hunyady, L. Control of myogenic tone and agonist induced contraction of intramural coronary resistance arterioles by cannabinoid type 1 receptors and endocannabinoids. Prostaglandins Other Lipid Mediat. 2018, 134, 77–83. [Google Scholar] [CrossRef]
- Waldeck-Welermair, M.; Zoratti, C.; Osibow, K.; Balenga, N.; Goessnitzer, E.; Waldhoer, M.; Malli, R.; Graier, W.F. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J. Cell Sci. 2008, 121, 1704–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, A.M.; Llanos, M.; Tamayo, D.; Hirsch, S. Anandamide Inhibits Endothelin-1 Production by Human Cultured Endothelial Cells: A New Vascular Action of This Endocannabinoid. Pharmacology 2007, 79, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, A.I. Endothelial atypical cannabinoid receptor: Do we have enough evidence? Br. J. Pharmacol. 2014, 171, 5573–5588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenkrantz, H.; Braude, M. Acute, subacute and 23-day chronic marihuana inhalation toxicities in the rat. Toxicol. Appl. Pharmacol. 1974, 28, 428–441. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Jones, R.T. Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin. Pharmacol. Ther. 1975, 18, 287–297. [Google Scholar] [CrossRef]
- Crawford, W.J.; Merritt, J.C. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 191–196. [Google Scholar] [PubMed]
- Vollmer, R.R.; Cavero, I.; Ertel, R.J.; Solomon, T.A.; Buckley, J.P. Role of the central autonomic nervous system in the hypotension and bradycardia induced by (-)-delta 9-trans-tetrahydrocannabinol. J. Pharm. Pharmacol. 1974, 26, 186–192. [Google Scholar] [CrossRef]
- Brozoski, D.T.; Dean, C.; Hopp, F.A.; Hillard, C.J.; Seagard, J.L. Differential endocannabinoid regulation of baroreflex-evoked sympathoinhibition in normotensive versus hypertensive rats. Auton. Neurosci. 2009, 150, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Haspula, D.; Clark, M.A. Heterologous regulation of the cannabinoid type 1 receptor by angiotensin II in astrocytes of spontaneously hypertensive rats. J. Neurochem. 2016, 139, 523–536. [Google Scholar] [CrossRef]
- Anderson, E.A.; Sinkey, C.A.; Lawton, W.J.; Mark, A.L. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertens. Dallas Tex. 1979 1989, 14, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Niederhoffer, N.; Szabo, B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000, 294, 707–713. [Google Scholar] [PubMed]
- Schaich, C.L.; Shaltout, H.A.; Brosnihan, K.B.; Howlett, A.C.; Diz, D.I. Acute and chronic systemic CB1 cannabinoid receptor blockade improves blood pressure regulation and metabolic profile in hypertensive (mRen2)27 rats. Physiol. Rep. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Schaich, C.L.; Grabenauer, M.; Thomas, B.F.; Shaltout, H.A.; Gallagher, P.E.; Howlett, A.C.; Diz, D.I. Medullary Endocannabinoids Contribute to the Differential Resting Baroreflex Sensitivity in Rats with Altered Brain Renin-Angiotensin System Expression. Front. Physiol. 2016, 7, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavic, S.; Lauer, D.; Sommerfeld, M.; Kemnitz, U.R.; Grzesiak, A.; Trappiel, M.; Thöne-Reineke, C.; Baulmann, J.; Paulis, L.; Kappert, K.; et al. Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J. Mol. Med. 2013, 91, 811–823. [Google Scholar] [CrossRef]
- Rajesh, M.; Bátkai, S.; Kechrid, M.; Mukhopadhyay, P.; Lee, W.S.; Horváth, B.; Holovac, E.; Cinar, R.; Liaudet, L.; Mackie, K.; et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 2012, 61, 716–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiyerili, V.; Zimmer, S.; Jung, S.; Wassmann, K.; Naehle, C.P.; Lütjohann, D.; Zimmer, A.; Nickenig, G.; Wassmann, S. CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic Res. Cardiol. 2010, 105, 465–477. [Google Scholar] [CrossRef]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Benyó, Z.; Offermanns, S.; Ruisanchez, É.; Szabó, E.; Takáts, Z.; Bátkai, S.; et al. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol. Cell. Endocrinol. 2015, 403, 46–56. [Google Scholar] [CrossRef]
- Mihrimah Ozturk, H.; Yetkin, E.; Ozturk, S. Synthetic Cannabinoids and Cardiac Arrhythmia Risk: Review of the Literature. Cardiovasc. Toxicol. 2012, 19, 191–197. [Google Scholar] [CrossRef]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef] [Green Version]
- McKallip, R.J.; Lombard, C.; Martin, B.R.; Nagarkatti, M.; Nagarkatti, P.S. Δ9-Tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J. Pharmacol. Exp. Ther. 2002, 302, 451–465. [Google Scholar] [CrossRef]
- Lombard, C.; Nagarkatti, M.; Nagarkatti, P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: Potential role for CB2-selective ligands as immunosuppressive agents. Clin. Immunol. 2007, 122, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cencioni, M.T.; Chiurchiù, V.; Catanzaro, G.; Borsellino, G.; Bernardi, G.; Battistini, L.; Maccarrone, M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Taylor, L.; Feuerborn, A.; Valaris, S.; Hussain, M.T.; Rainger, G.E.; Greaves, D.R.; Iqbal, A.J. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB J. 2019, 33, 6154–6167. [Google Scholar] [CrossRef] [Green Version]
- Ziring, D.; Wei, B.; Velazquez, P.; Schrage, M.; Buckley, N.E.; Braun, J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics 2006, 58, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Muppidi, J.R.; Arnon, T.I.; Bronevetsky, Y.; Veerapen, N.; Tanaka, M.; Besra, G.S.; Cyster, J.G. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J. Exp. Med. 2011, 208, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Derocq, J.M.; Ségui, M.; Marchand, J.; Le Fur, G.; Casellas, P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 1995, 369, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.M.; Stella, N. CB 2 receptor-mediated migration of immune cells: It can go either way. Br. J. Pharmacol. 2008, 153, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BOUABOULA, M.; RINALDI, M.; CARAYON, P.; CARILLON, C.; DELPECH, B.; SHIRE, D.; LE FUR, G.; CASELLAS, P. Cannabinoid-receptor expression in human leukocytes. Eur. J. Biochem. 1993, 214, 173–180. [Google Scholar] [CrossRef]
- Lee, S.F.; Newton, C.; Widen, R.; Friedman, H.; Klein, T.W. Differential expression of cannabinoid CB2 receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur. J. Pharmacol. 2001, 423, 235–241. [Google Scholar] [CrossRef]
- Correa, F.; Mestre, L.; Docagne, F.; Guaza, C. Activation of cannabinoid CB 2 receptor negatively regulates IL-12p40 production in murine macrophages: Role of IL-10 and ERK1/2 kinase signaling. Br. J. Pharmacol. 2005, 145, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Mbvundula, E.C.; Bunning, R.A.D.; Rainsford, K.D. Arthritis and cannabinoids: HU-210 and Win-55,212-2 prevent IL-1 α-induced matrix degradation in bovine articular chondrocytes in-vitro. J. Pharm. Pharmacol. 2006, 58, 351–358. [Google Scholar] [CrossRef]
- Gui, H.; Liu, X.; Wang, Z.-W.; He, D.-Y.; Su, D.-F.; Dai, S.-M. Expression of cannabinoid receptor 2 and its inhibitory effects on synovial fibroblasts in rheumatoid arthritis. Rheumatol. Oxf. 2014, 53, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Kohsaka, H.; Takayasu, A.; Yokoyama, W.; Miyabe, C.; Miyabe, Y.; Harigai, M.; Miyasaka, N.; Nanki, T. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. Bmc Musculoskelet. Disord. 2014, 15, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Ge, G.; Wang, Y.; Zhang, W.; Wang, Q.; Wang, W.; Guo, X.; Yu, B.; Xu, Y.; Yang, H.; et al. A selective CB2 agonist protects against the inflammatory response and joint destruction in collagen-induced arthritis mice. Biomed. Pharmacother. 2019, 116, 109025. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, S.; Singh, A.K.; Srivastava, I.; Szlenk, C.T.; Muench, T.R.; Natesan, S.; Ahmed, S. Cannabinoid Receptor 2 Agonist JWH-015 Inhibits Interleukin-1β-Induced Inflammation in Rheumatoid Arthritis Synovial Fibroblasts and in Adjuvant Induced Arthritis Rat via Glucocorticoid Receptor. Front. Immunol. 2019, 10, 1027. [Google Scholar] [CrossRef]
- Ashwood, P.; Wills, S.; Van de Water, J. The immune response in autism: A new frontier for autism research. J. Leukoc. Biol. 2006, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Careaga, M.; Van de Water, J.; Ashwood, P. Immune dysfunction in autism: A pathway to treatment. Neurotherapeutics 2010, 7, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siniscalco, D.; Sapone, A.; Giordano, C.; Cirillo, A.; De Magistris, L.; Rossi, F.; Fasano, A.; Bradstreet, J.J.; Maione, S.; Antonucci, N. Cannabinoid receptor type 2, but not type 1, is up-regulated in peripheral blood mononuclear cells of children affected by autistic disorders. J. Autism Dev. Disord. 2013, 43, 2686–2695. [Google Scholar] [CrossRef]
- Siniscalco, D.; Bradstreet, J.J.; Cirillo, A.; Antonucci, N. The in vitro GcMAF effects on endocannabinoid system transcriptionomics, receptor formation, and cell activity of autism-derived macrophages. J. Neuroinflammation 2014, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean-Gilles, L.; Feng, S.; Tench, C.R.; Chapman, V.; Kendall, D.A.; Barrett, D.A.; Constantinescu, C.S. Plasma endocannabinoid levels in multiple sclerosis. J. Neurol. Sci. 2009, 287, 212–215. [Google Scholar] [CrossRef]
- Docagne, F.; Muñetón, V.; Clemente, D.; Ali, C.; Loría, F.; Correa, F.; Hernangómez, M.; Mestre, L.; Vivien, D.; Guaza, C. Excitotoxicity in a chronic model of multiple sclerosis: Neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol. Cell. Neurosci. 2007, 34, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, C.; Romero, J.P.; Tolón, R.M.; Clemente, D.; Docagne, F.; Hillard, C.J.; Guaza, C.; Romero, J. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J. Neurosci. 2007, 27, 2396–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docagne, F.; Mestre, L.; Loría, F.; Hernangómez, M.; Correa, F.; Guaza, C. Therapeutic potential of CB2 targeting in multiple sclerosis. Expert Opin. Ther. Targets 2008, 12, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, X.; Geller, E.B.; Eppihimer, M.J.; Eisenstein, T.K.; Adler, M.W.; Tuma, R.F. Win 55212-2, a cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune encephalomyelitis model. Mult. Scler. 2004, 10, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, A.M.; Laezza, C.; D’Alessandro, A.; Procaccini, C.; Saccomanni, G.; Tuccinardi, T.; Manera, C.; Macchia, M.; Matarese, G.; Gazzerro, P.; et al. Effects on Immune Cells of a New 1,8-Naphthyridin-2-One Derivative and Its Analogues as Selective CB2 Agonists: Implications in Multiple Sclerosis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, P.; Cioni, C.; Mugnaini, C.; Corelli, F. Potent immunomodulatory activity of a highly selective cannabinoid CB2 agonist on immune cells from healthy subjects and patients with multiple sclerosis. J. Neuroimmunol. 2017, 303, 66–74. [Google Scholar] [CrossRef]
- Sánchez, A.J.; González-Pérez, P.; Galve-Roperh, I.; García-Merino, A. R-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)-pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphtalenylmethanone (WIN-2) ameliorates experimental autoimmune encephalomyelitis and induces encephalitogenic T cell apoptosis: Partial involvement of the CB2 receptor. Biochem. Pharmacol. 2006, 72, 1697–1706. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Ludovic Croxford, J.; Brown, P.; Pertwee, R.G.; Huffman, J.W.; Layward, L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 2000, 404, 84–87. [Google Scholar] [CrossRef]
- Pryce, G.; Baker, D. Control of spasticity in a multiple sclerosis model is mediated by CB 1, not CB 2, cannabinoid receptors. Br. J. Pharmacol. 2007, 150, 519–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K.; et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Veillard, N.R.; Arnaud, C.; Pelli, G.; Burger, F.; Staub, C.; Zimmer, A.; Frossard, J.L.; Mach, F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005, 434, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Zhang, W.; Xue, J.; Wu, Y.Z.; Xu, W.; Liang, X.; Chen, T.; Kishimoto, C.; Yuan, Z. WIN55212-2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur. J. Pharmacol. 2010, 649, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Netherland, C.D.; Pickle, T.G.; Bales, A.; Thewke, D.P. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic Ldlr-null mice. Atherosclerosis 2010, 213, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montecucco, F.; Burger, F.; Mach, F.; Steffens, S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1145–H1155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Ulmann, L.; Hirbec, H.; Rassendren, F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010, 29, 2290–2300. [Google Scholar] [CrossRef] [Green Version]
- Hickernell, T.R.; Lakra, A.; Berg, A.; Cooper, H.J.; Geller, J.A.; Shah, R.P. Should Cannabinoids Be Added to Multimodal Pain Regimens After Total Hip and Knee Arthroplasty? J. Arthroplast. 2018, 33, 3637–3641. [Google Scholar] [CrossRef]
- Gazendam, A.; Nucci, N.; Gouveia, K.; Abdel Khalik, H.; Rubinger, L.; Johal, H. Cannabinoids in the Management of Acute Pain: A Systematic Review and Meta-analysis. Cannabis Cannabinoid Res. 2020. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.D.; Kilo, S.; Hargreaves, K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain 1998, 75, 111–119. [Google Scholar] [CrossRef]
- Agarwal, N.; Pacher, P.; Tegeder, I.; Amaya, F.; Constantin, C.E.; Brenner, G.J.; Rubino, T.; Michalski, C.W.; Marsicano, G.; Monory, K.; et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007, 10, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.H.; Cao, C.Q.; Martino, G.; Puma, C.; Morinville, A.; St-Onge, S.; Lessard, É.; Perkins, M.N.; Laird, J.M.A. A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain. Pain 2010, 151, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Shimosato, G.; Kawasaki, Y.; Hashimoto, S.; Tanaka, Y.; Ji, R.R.; Tanaka, M. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 2006, 124, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.; Levin, E.; Varanelli, C. Prostaglandins and cannabis-II inhibition of biosynthesis by the naturally occurring cannabinoids. Biochem. Pharmacol. 1973, 22, 2905–2910. [Google Scholar] [CrossRef]
- Fimiani, C.; Liberty, T.; Aquirre, A.J.; Amin, I.; Ali, N.; Stefano, G.B. Opiate, cannabinoid, and eicosanoid signaling converges on common intracellular pathways nitric oxide coupling. Prostaglandins Other Lipid Mediat. 1999, 57, 23–34. [Google Scholar] [CrossRef]
- Kunos, G.; Osei-Hyiaman, D.; Bátkai, S.; Sharkey, K.A.; Makriyannis, A. Should peripheral CB1 cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol. Sci. 2009, 30, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009, 60, 77–84. [Google Scholar] [CrossRef]
- McPartland, J.M.; Guy, G.W.; Di Marzo, V. Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System. PLoS ONE 2014, 9, e89566. [Google Scholar] [CrossRef]
- Bushlin, I.; Rozenfeld, R.; Devi, L.A. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr. Opin. Pharmacol. 2010, 10, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojo, M.; Sudo, Y.; Ando, Y.; Minami, K.; Takada, M.; Matsubara, T.; Kanaide, M.; Taniyama, K.; Sumikawa, K.; Uezono, Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: Electrophysiological and FRET assay analysis. J. Pharmacol. Sci. 2008, 108, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Somvanshi, R.K.; Kumar, U. Somatostatin receptor 5 is a prominent regulator of signaling pathways in cells with coexpression of Cannabinoid receptors 1. Neuroscience 2017, 340, 218–231. [Google Scholar] [CrossRef]
- Tóth, A.D.; Turu, G.; Hunyady, L.; Balla, A. Novel mechanisms of G-protein-coupled receptors functions: AT1 angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbdAlla, S.; Lother, H.; El Massiery, A.; Quitterer, U. Increased AT1 receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat. Med. 2001, 7, 1003–1009. [Google Scholar] [CrossRef]
- Rozenfeld, R.; Devi, L.A. Receptor heteromerization and drug discovery. Trends Pharmacol. Sci. 2010, 31, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The endocannabinoid system as a target in cancer diseases: Are we there yet? Front. Pharmacol. 2019, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Perrey, D.A.; Gilmour, B.P.; Thomas, B.F.; Zhang, Y. Toward the development of bivalent ligand probes of cannabinoid CB1 and Orexin OX1 receptor heterodimers. Acs Med. Chem. Lett. 2014, 5, 634–638. [Google Scholar] [CrossRef]
- Grant, P.S.; Kahlcke, N.; Govindpani, K.; Hunter, M.; MacDonald, C.; Brimble, M.A.; Glass, M.; Furkert, D.P. Divalent cannabinoid-1 receptor ligands: A linker attachment point survey of SR141716A for development of high-affinity CB1R molecular probes. Bioorganic Med. Chem. Lett. 2019, 29, 126644. [Google Scholar] [CrossRef]
- Cinar, R.; Iyer, M.R.; Kunos, G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol. Ther. 2020, 208, 107477. [Google Scholar] [CrossRef]
- Morales, P.; Goya, P.; Jagerovic, N.; Hernandez-Folgado, L. Allosteric Modulators of the CB1 Cannabinoid Receptor: A Structural Update Review. Cannabis Cannabinoid Res. 2016, 1, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.R.; Garai, S.; Janero, D.R.; Thakur, G.A. Design and Synthesis of Cannabinoid 1 Receptor (CB1R) Allosteric Modulators: Drug Discovery Applications. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 593, pp. 281–315. [Google Scholar] [CrossRef]
- Horswill, J.G.; Bali, U.; Shaaban, S.; Keily, J.F.; Jeevaratnam, P.; Babbs, A.J.; Reynet, C.; Wong Kai In, P. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB 1 receptors with hypophagic effects in rats. Br. J. Pharmacol. 2007, 152, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, C.J.; Ali, H.I.; Ahn, K.H.; Kolluru, S.; Kendall, D.A.; Lu, D. Synthesis and biological evaluation of indole-2-carboxamides bearing photoactivatable functionalities as novel allosteric modulators for the cannabinoid CB1 receptor. Eur. J. Med. Chem. 2016, 121, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Wu, J. Cannabis, cannabinoid receptors, and endocannabinoid system: Yesterday, today, and tomorrow. Acta Pharmacol. Sin. 2019, 40, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Dhopeshwarkar, A.; Mackie, K. CB 2 Cannabinoid Receptors as a Therapeutic Target-What Does the Future Hold? Mol. Pharmacol. Mol Pharm. 2014, 86, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Tai, S.; Vasiljevik, T.; Sherwood, A.M.; Eddington, S.; Wilson, C.D.; Prisinzano, T.E.; Fantegrossi, W.E. Assessment of rimonabant-like adverse effects of purported CB1R neutral antagonist/CB2R agonist aminoalkylindole derivatives in mice. Drug Alcohol Depend. 2018, 192, 285–293. [Google Scholar] [CrossRef]
- Ostenfeld, T.; Price, J.; Albanese, M.; Bullman, J.; Guillard, F.; Meyer, I.; Leeson, R.; Costantin, C.; Ziviani, L.; Nocini, P.F.; et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin. J. Pain 2011, 27, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N. Cannabinoid receptor with an “identity crisis” gets a second look. Nat. Med. 2015, 21, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Clapper, J.R.; Henry, C.L.; Niphakis, M.J.; Knize, A.M.; Coppola, A.R.; Simon, G.M.; Ngo, N.; Herbst, R.A.; Herbst, D.M.; Reed, A.W.; et al. Monoacylglycerol lipase inhibition in human and rodent systems supports clinical evaluation of endocannabinoid modulatorss. J. Pharmacol. Exp. Ther. 2018, 367, 494–508. [Google Scholar] [CrossRef] [Green Version]
- Huggins, J.P.; Smart, T.S.; Langman, S.; Taylor, L.; Young, T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012, 153, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Cortes-Briones, J.; Creatura, G.; Bluez, G.; Thurnauer, H.; Deaso, E.; Bielen, K.; Surti, T.; Radhakrishnan, R.; Gupta, A.; et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: A double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 2019, 6, 35–45. [Google Scholar] [CrossRef]
- Kerbrat, A.; Ferré, J.-C.; Fillatre, P.; Ronzière, T.; Vannier, S.; Carsin-Nicol, B.; Lavoué, S.; Vérin, M.; Gauvrit, J.-Y.; Le Tulzo, Y.; et al. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N. Engl. J. Med. 2016, 375, 1717–1725. [Google Scholar] [CrossRef] [Green Version]
- Treister-Goltzman, Y.; Freud, T.; Press, Y.; Peleg, R. Trends in Publications on Medical Cannabis from the Year 2000. Popul. Health Manag. 2019, 22, 362–368. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Arkells, N.; Milella, L.; Stankiewicz, A.M.; Huminiecki, Ł.; Horbanczuk, O.K.; Atanasov, A.G. Molecular neuroscience at its “high”: Bibliometric analysis of the most cited papers on endocannabinoid system, cannabis and cannabinoids. J. Cannabis Res. 2019, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Ligresti, A.; Dimarzo, V. The endocannabinoid signalling system: Biochemical aspects. Pharmacol. Biochem. Behav. 2005, 81, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Mallipeddi, S.; Janero, D.R.; Zvonok, N.; Makriyannis, A. Functional selectivity at G-protein coupled receptors: Advancing cannabinoid receptors as drug targets. Biochem. Pharmacol. 2017, 128, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB 1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity Signaling of CB 1 Receptors in the Brain: Intrinsic or Emerging Features? Neuropsychopharmacol. Rev. 2018, 43, 4–20. [Google Scholar] [CrossRef]
- Hryhorowicz, S.; Kaczmarek-Ryś, M.; Andrzejewska, A.; Staszak, K.; Hryhorowicz, M.; Korcz, A.; Słomski, R. Allosteric modulation of cannabinoid receptor 1—Current challenges and future opportunities. Int. J. Mol. Sci. 2019, 20, 5874. [Google Scholar] [CrossRef] [Green Version]
- Urits, I.; Borchart, M.; Hasegawa, M.; Kochanski, J.; Orhurhu, V.; Viswanath, O. An Update of Current Cannabis-Based Pharmaceuticals in Pain Medicine. Pain Ther. 2019, 8, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Onaivi, E.S. Endocannabinoid system, pharmacogenomics and response to therapy. Pharmacogenomics 2010, 11, 907–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, V.; Grogan, D.; Ahluwalia, M.; Salles, É.L.; Ahluwalia, P.; Khodadadi, H.; Alverson, K.; Nguyen, A.; Raju, S.P.; Gaur, P.; et al. Targeting the endocannabinoid system: A predictive, preventive, and personalized medicine-directed approach to the management of brain pathologies. EPMA J. 2020, 11, 217–250. [Google Scholar] [CrossRef] [PubMed]

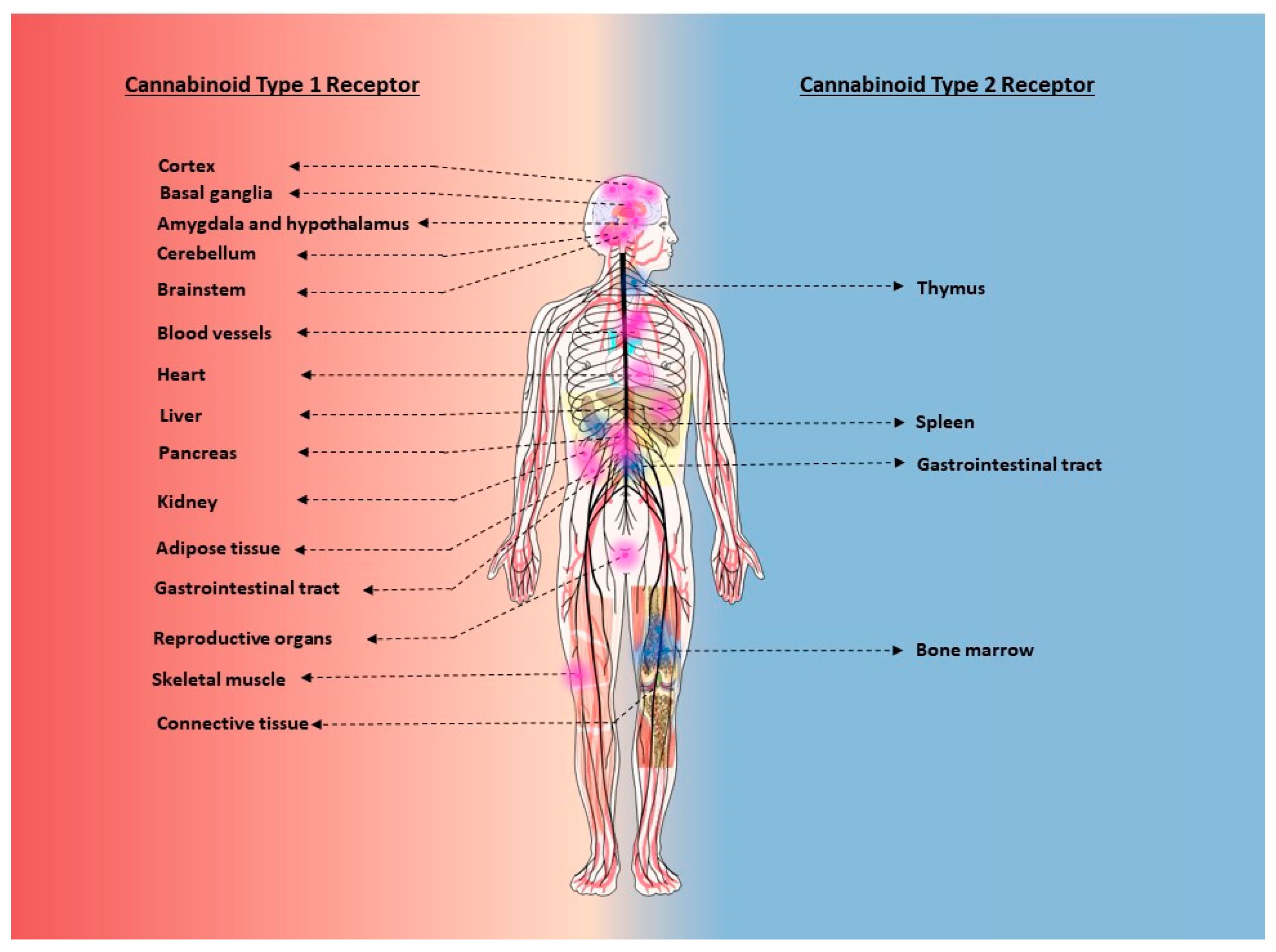

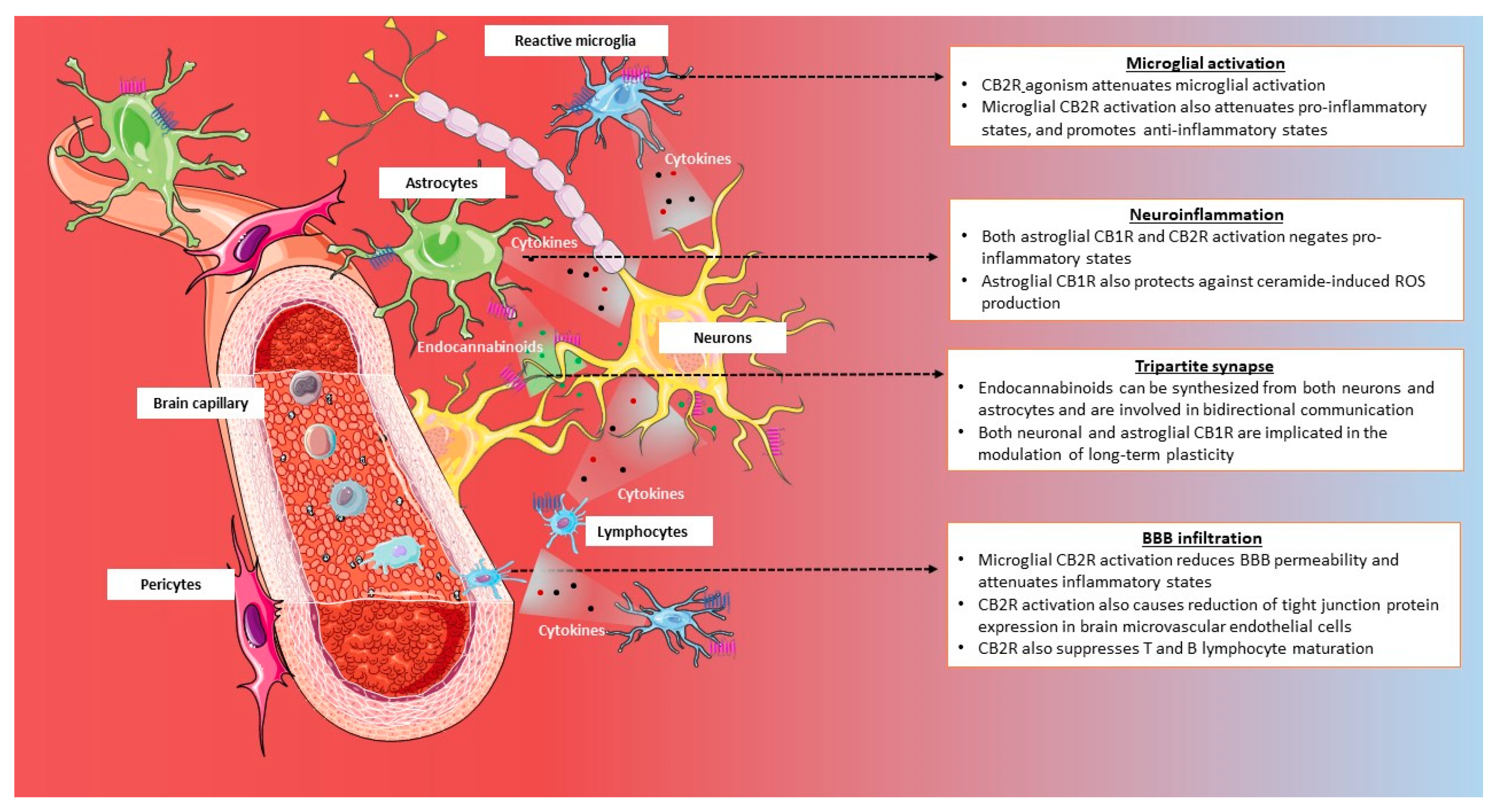

| Subjects and Measurement Indices | Study Design | CB receptors as Biomarkers | Outcome of Pharmacological Intervention | Reference |
|---|---|---|---|---|
| Measurement of CB1R density in brown adipose tissue in lean and obese healthy males | Non-randomized, crossover clinical trial | CB1R upregulation in obese individuals | CB1R blockade increased lipolysis | [186] |
| Determination of the effect of dronabinol in gut transit in irritable bowel syndrome with diarrhea. | double-blind, randomized, placebo-controlled, parallel-group study | CNR1 rs806378 CT/TT is associated with a delay in colonic transit compared with CC | Dronabinol delays transit in individuals with CNR1 rs806378 CT/TT | [187] |
| Impact of Sativex on CNR1 and CNR2 expression in peripheral blood mononuclear cells (PBMCs) in patients with multiple sclerosis (MS) secondary progressive (MSS-SP). | Controlled clinical trial | - | Significant decrease in CNR2 expression | [188] |
| Measurement of CB1R density in the brains of schizophrenic individuals with or without antipsychotic medication | prospective study | Increased CB1R binding in mesocorticolimbic regions of individuals with schizophrenia | - | [189] |
| Measurement of CB1R density in the brains of pre- Huntington disease mutation carriers | prospective study | Decrease in CB1R density in prefrontal cortex compared to controls | - | [190] |
| Determination of whether hypocaloric diet and/or aerobic exercise alters subcutaneous adipose tissue CB1R and FAAH expression in obese women | Randomized clinical trial | Caloric restriction alone lowered gluteal CB1R and FAAH, while both caloric restriction plus aerobic exercise reduced abdominal adipose tissue FAAH gene expression | - | [191] |
| Determination of whether exercise training resulted in changes in muscle CB1R and TRPV1 expression in heart failure patients | Randomized controlled trial | Exercise training significantly increased gene expression of the TRPV1 receptor and the CB1R | - | [192] |
| Impact of single nucleotide polymorphism rs3123554 in CNR2 on metabolic and adiposity parameters in obese induvial on two hypocaloric diets | Randomized controlled trial | Individuals that are carriers for CNR2 genetic variant loose less body weight. | - | [193] |
| Determination of whether CNR2 gene variation rs35761398 (Q63R) is significantly associated with chronic idiopathic thrombocytopenic purpura (ITP) in children | Case-control association study | CNR2 gene variation is significantly associated with childhood chronic ITP | - | [194] |
| Impact of interferon therapy on CB1R and CB2R gene expression in immune cells from patients on interferon therapy | Controlled trial | Reduction in both CB1R and CB2R after interferon therapy | - | [195] |
| Impact of Sativex on the clinical improvement of motor, cognitive and psychiatric measures in patients with Huntington’s Disease. | double-blind, randomized, cross-over, placebo-controlled, pilot trial | - | No improvement in motor, cognitive, and behavioral functional scores when compared to the placebo | [196] |
| Impact of pirfenidone on CB1 and CB2 gene expression in liver biopsies, in individuals with chronic hepatitis C | Open-label, non-controlled, and non-randomized clinical trial | - | Significant upregulation of CNR2, while no statistical difference in CNR1. | [197] |
| Determination of the effects of isocaloric low and high-fat diets on endocannabinoid system in obese individuals | randomized cross-over study | Reductions in skeletal muscle CB1R in high fat diet group | - | [198] |
| Measurement of VEGF and cytokines in sera of obese PCOS women in response to rimonabant. | Randomized open-labelled parallel study | - | CB1R blockade raises VEGF and the pro-inflammatory cytokine IL-8 in obese women with PCOS | [199] |
| Measurement of insulin-like growth factor I levels in women with anorexia nervosa (AN) in response to dronabinol | Prospective, double-blind randomized crossover study | - | Dronabinol affected neither the concentration nor the activity of the circulating IGF-system in women with severe and chronic AN. | [200] |
| Measurement of skin conductance response to determine whether dronabinol facilitates fear extinction learning in healthy individuals | Randomized, double-blind, placebo-controlled trial | - | Dronabinol facilitates extinction of conditioned fear in humans | [201] |
| Measurement of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) responses during oral glucose tolerance test (OGTT) in the plasma of lean and obese participants | Randomized, double-blind, crossover, placebo-controlled trial | - | CBR agonist increased circulating GIP levels, but not GLP levels, in the fasting (nonstimulated) state. | [202] |
| Measurement of body weight and HbA1c in obese and overweight individuals with and without diabetes in response to CB1R antagonist, CP-945,598. | double-blind, placebo-controlled trial | - | CP-945,598 resulted in a reduction in body weight and better glycemic control. | [203] |
| Impact of smoked medicinal cannabis on appetite hormones ghrelin, leptin and PYY in individuals with HIV-associated neuropathic pain | double-blind cross-over, placebo-controlled trial | - | Medical cannabis resulted in significant increases in plasma levels of ghrelin and leptin, and decreases in PYY | [204] |
| Impact of rimonabant on improving the risk of cardiovascular death, myocardial infarction, or stroke in individuals with cardiovascular risk factors or prior cardiovascular events. | Randomized, double-blind, placebo-controlled trial | - | Termination of trial due to neuropsychiatric effects | [205] |
| Impact of CB1R inverse agonist, taranabant, for maintenance of prior weight loss achieved on a low-calorie diet | Randomized, double-blind, placebo-controlled trial | - | Improvement in weight loss with taranabant, compared to maintenance therapy alone | [206] |
| Impact of CB1R inverse agonist, taranabant, on weight loss in obese and overweight patients | Randomized, double-blind, placebo-controlled trial | - | Taranabant treatment resulted in statistically significant weight loss | [207] |
| Impact of CB1 neutral antagonist tetrahydrocannabivarin on resting state functional connectivity in key brain regions relevant to development of obesity, in healthy individuals | Randomized, within-subject, double-blind, placebo-controlled trial | - | Alteration in resting state functional connectivity without significant effects on mood. | [208] |
| Impact of CB1 neutral antagonist tetrahydrocannabivarin on activation of brain regions involved in food aversion in healthy individuals | Randomized, double-blind, placebo-controlled trial | - | Treatment increased neural responses to aversive stimuli | [209] |
| Impact of CB1/CB2 receptor agonist KN38-7271 on survival rates following head injury | Randomized, double-blind, placebo-controlled phase II trial | - | Improved survival rates in the early phase of the comatose patient after a head injury | [210] |
| Impact of peripherally acting CB1/CB2 receptor agonist, AZD 1940, on capsaicin-evoked pain and hyperalgesia | Randomized, double-blind, placebo-controlled | - | No evidence of analgesic efficacy in the human capsaicin pain model. | [211] |
| Impact of oral dronabinol on the progression of primary and secondary progressive multiple sclerosis in patients with prior diagnosis | Randomized, double-blind, placebo-controlled study | - | No significant effect on the progression of multiple sclerosis in the progressive phase | [212] |
| Impact of oral dronabinol on altering pain threshold in individuals diagnosed with functional chest pain (FCP) | Randomized, double-blind, placebo-controlled study | - | Pain threshold was increased, and the frequency and intensity of pain was reduced, in FCP | [213] |
| Impact of rimonabant on changes in liver fat in individuals with metabolic syndrome | Randomized, double-blind, placebo-controlled trial | - | Reduction in liver fat is proportional to weight loss | [214] |
| Impact of rimonabant on carotid atherosclerosis in obese individuals | Randomized, double-blind, placebo-controlled trial | - | No significant effect on the progression of atherosclerosis | [215] |
| Impact of rimonabant on body weight in obese patients with binge eating disorders | randomized, double-blind, placebo-controlled study | - | Reduction in body weight when compared to placebo group | [216] |
| Impact of rimonabant on neurocognitive impairments in individuals with schizophrenia | randomized, double-blind, placebo-controlled study | - | Improvement on a probabilistic learning task, with no improvement in global cognitive functioning | [217] |
| Impact of rimonabant on fatty acid and triglyceride metabolism and insulin sensitivity after controlling for metabolic effects of weight loss in obese women | Randomized controlled trial | - | Increased lipolysis and fatty acid oxidation without any effect on insulin sensitivity | [218] |
| Impact of rimonabant on insulin regulation of free fatty acid and glucose metabolism, after controlling for weight loss, in obese, metabolic syndrome individuals | randomized, double-blind, placebo-controlled study | - | Improvement in insulin regulation of free fatty acids and glucose metabolism was due to weight loss | [219] |
| Treatment of dementia-related neuropsychiatric symptoms (NPS) in response to low-dose oral THC | Prospective study | - | low-dose THC does not significantly reduce NPS | [220] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207693
Haspula D, Clark MA. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. International Journal of Molecular Sciences. 2020; 21(20):7693. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207693
Chicago/Turabian StyleHaspula, Dhanush, and Michelle A. Clark. 2020. "Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases" International Journal of Molecular Sciences 21, no. 20: 7693. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207693





