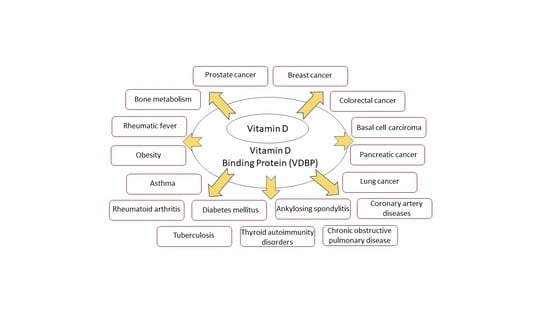
Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms—The Risk of Malignant Tumors and Other Diseases
Abstract
:1. Introduction
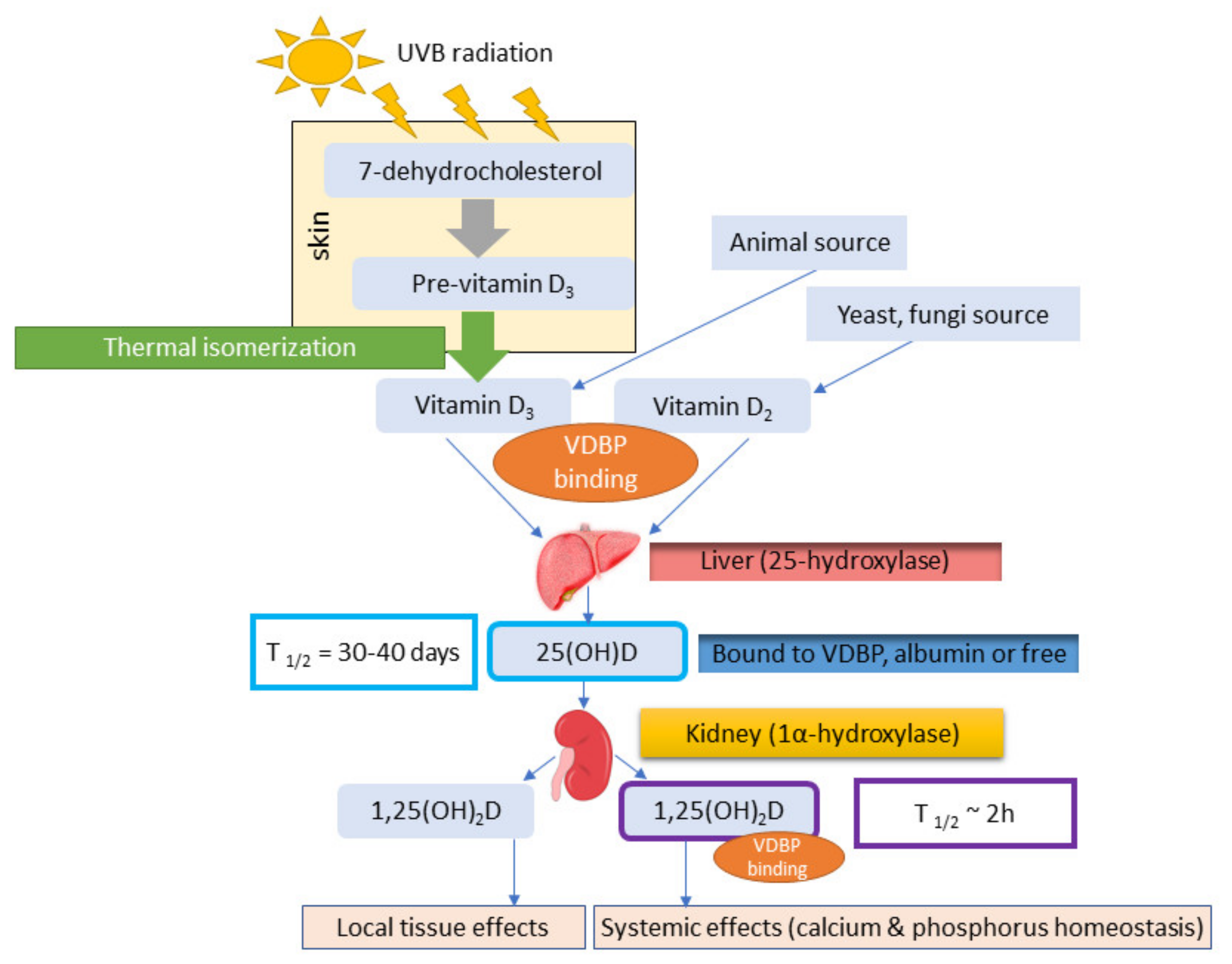
2. Vitamin D and Gene Regulation
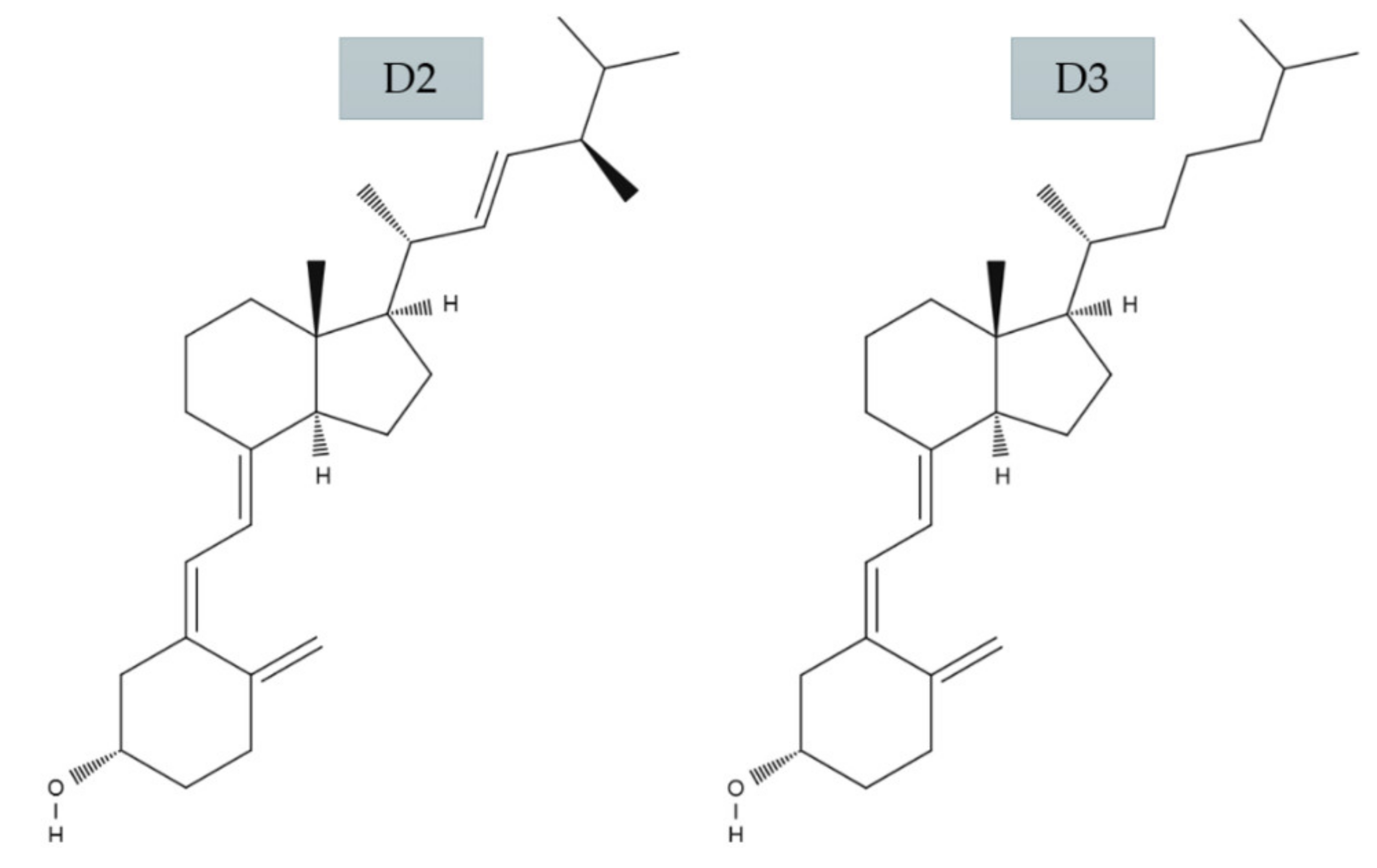
2.1. Vitamin D3 and D2
2.2. Role of VDBP
2.3. VDBP Gene Family Polymorphism
3. Association of VDBP with Human Diseases
3.1. Cancers
3.1.1. Breast Cancer
3.1.2. Prostate Cancer
3.1.3. Pancreatic Cancer
3.1.4. Lung and Colorectal Cancer
3.1.5. Basal Cell Carcinoma
3.1.6. Cutaneous Melanoma
3.2. Other Important Diseases
3.2.1. Diabetes Mellitus
3.2.2. Thyroid Autoimmunity Disorders
3.2.3. Obesity
3.2.4. Bone Metabolism
3.2.5. Rheumatoid Arthritis
3.2.6. Ankylosing Spondylitis
3.2.7. Asthma
3.2.8. Chronic Obstructive Pulmonary Disease (COPD)
3.2.9. Tuberculosis
3.2.10. Coronary Artery Diseases (CAD)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fleet, J.C. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell. Endocrinol. 2017, 453, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almesri, N.; Das, N.S.; Ali, M.E.; Gumaa, K.; Giha, H.A. Independent associations of polymorphisms in vitamin D binding protein (GC) and vitamin D receptor (VD) genes with obesity and plasma 25OHD3 levels demonstrate sex dimorphism. Appl. Physiol. Nutr. Metab. 2016, 41, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, D.R.; Holmstrom, S.R.; Fon Tacer, K.; Bookout, A.L.; Kliewer, S.A.; Mangelsdorf, D.J. Regulation of Bile Acid Synthesis by Fat-soluble Vitamins A and D. J. Biol. Chem. 2010, 285, 14486–14494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef]
- Aktürk, T.; Turan, Y.; Tanik, N.; Karadağ, M.E.; Sacmaci, H.; Inan, L.E. Vitamin D, vitamin D binding protein, vitamin D receptor levels and cardiac dysautonomia in patients with multiple sclerosis: A cross-sectional study. Arq. Neuropsiquiatr. 2019, 77, 848–854. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Whitfield, G.K.; Hsieh, J.-C.; Thompson, P.D.; Barthel, T.K.; Bartik, L.; Egan, J.B.; Wu, Y.; Kubicek, J.L.; et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J. Steroid Biochem. Mol. Biol. 2010, 121, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and Cardiovascular Disease. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D Deficiency in Adults: When to Test and How to Treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Pike, J.W.; Meyer, M.B. The unsettled science of nonrenal calcitriol production and its clinical relevance. J. Clin. Investig. 2020, 130, 4519–4521. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The Role of Vitamin D in Cancer Prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef]
- Gorham, E.D.; Garland, C.F.; Garland, F.C.; Grant, W.B.; Mohr, S.B.; Lipkin, M.; Newmark, H.L.; Giovannucci, E.; Wei, M.; Holick, M.F. Vitamin D and prevention of colorectal cancer. J. Steroid Biochem. Mol. Biol. 2005, 97, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The Vitamin D Epidemic and its Health Consequences. J. Nutr. 2005, 135, 2739S–2748S. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 16, 266–281. [Google Scholar] [CrossRef]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.T. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Manousaki, D.; Richards, J.B. Low vitamin D levels as a risk factor for cancer. BMJ 2017, 359. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou, V.I.; Tsilidis, K.K.; Haycock, P.C.; Dimou, N.L.; Al-Dabhani, K.; Martin, R.M.; Lewis, S.J.; Gunter, M.J.; Mondul, A.; Shui, I.M.; et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaaby, T.; Thuesen, B.H.; Linneberg, A. Vitamin D, Cardiovascular Disease and Risk Factors. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 221–230. ISBN 978-3-319-56017-5. [Google Scholar]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin. Hypertens. 2018, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Lucato, P.; Solmi, M.; Maggi, S.; Bertocco, A.; Bano, G.; Trevisan, C.; Manzato, E.; Sergi, G.; Schofield, P.; Kouidrat, Y.; et al. Low vitamin D levels increase the risk of type 2 diabetes in older adults: A systematic review and meta-analysis. Maturitas 2017, 100, 8–15. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Watad, A.; Neumann, S.G.; Simon, M.; Brown, S.B.; Abu Much, A.; Harari, A.; Tiosano, S.; Amital, H.; Shoenfeld, Y. Vitamin D and rheumatoid arthritis: An ongoing mystery. Curr. Opin. Rheumatol. 2017, 29, 378–388. [Google Scholar] [CrossRef]
- Hajjaj-Hassouni, N.; Mawani, N.; Allali, F.; Rkain, H.; Hassouni, K.; Hmamouchi, I.; Dougados, M. Evaluation of Vitamin D Status in Rheumatoid Arthritis and Its Association with Disease Activity across 15 Countries: “The COMORA Study”. Available online: https://www.hindawi.com/journals/ijr/2017/5491676/ (accessed on 26 September 2020).
- Huang, S.-J.; Wang, X.-H.; Liu, Z.-D.; Cao, W.-L.; Han, Y.; Ma, A.-G.; Xu, S.-F. Vitamin D deficiency and the risk of tuberculosis: A meta-analysis. Drug Des. Devel. Ther. 2016, 11, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef]
- Barsony, J.; Renyi, I.; McKoy, W. Subcellular Distribution of Normal and Mutant Vitamin D Receptors in Living Cells: STUDIES WITH A NOVEL FLUORESCENT LIGAND. J. Biol. Chem. 1997, 272, 5774–5782. [Google Scholar] [CrossRef] [Green Version]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2015, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Yu, R.T.; Subramaniam, N.; Sherman, M.H.; Wilson, C.; Rao, R.; Leblanc, M.; Coulter, S.; He, M.; Scott, C.; et al. A Vitamin D Receptor/SMAD Genomic Circuit Gates Hepatic Fibrotic Response. Cell 2013, 153, 601–613. [Google Scholar] [CrossRef] [Green Version]
- Pike, J.W.; Meyer, M.B.; Benkusky, N.A.; Lee, S.M.; St. John, H.; Carlson, A.; Onal, M.; Shamsuzzaman, S. Genomic Determinants of Vitamin D-Regulated Gene Expression. Vitam. Horm. 2016, 100, 21–44. [Google Scholar] [CrossRef] [Green Version]
- Keegan, R.-J.H.; Lu, Z.; Bogusz, J.M.; Williams, J.E.; Holick, M.F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Dermato-Endocrinology 2013, 5, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. The Influence of Vitamin D on Bone Health across the Life Cycle. J. Nutr. 2005, 135, 2726S–2727S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D 2 Is Much Less Effective than Vitamin D 3 in Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef] [Green Version]
- Glendenning, P.; Chew, G.T.; Seymour, H.M.; Gillett, M.J.; Goldswain, P.R.; Inderjeeth, C.A.; Vasikaran, S.D.; Taranto, M.; Musk, A.A.; Fraser, W.D. Serum 25-hydroxyvitamin D levels in vitamin D-insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone 2009, 45, 870–875. [Google Scholar] [CrossRef]
- Romagnoli, E.; Mascia, M.L.; Cipriani, C.; Fassino, V.; Mazzei, F.; D’Erasmo, E.; Carnevale, V.; Scillitani, A.; Minisola, S. Short and Long-Term Variations in Serum Calciotropic Hormones after a Single Very Large Dose of Ergocalciferol (Vitamin D2) or Cholecalciferol (Vitamin D3) in the Elderly. J. Clin. Endocrinol. Metab. 2008, 93, 3015–3020. [Google Scholar] [CrossRef]
- Trang, H.M.; Cole, D.E.; Rubin, L.A.; Pierratos, A.; Siu, S.; Vieth, R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am. J. Clin. Nutr. 1998, 68, 854–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschfeld, J. Immune-electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta Pathol. Microbiol. Scand. 2009, 47, 160–168. [Google Scholar] [CrossRef]
- Cooke, N.E.; Haddad, J.G. Vitamin D Binding Protein (Gc-Globulin)*. Endocr. Rev. 1989, 10, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-H.; Naumova, A.K.; Liebhaber, S.A.; Cooke, N.E. Physical and Meiotic Mapping of the Region of Human Chromosome 4q11–q13 Encompassing the Vitamin D Binding Protein DBP/Gc-Globulin and Albumin Multigene Cluster. Genome Res. 1999, 9, 581–587. [Google Scholar] [PubMed]
- Speeckaert, M.; Huang, G.; Delanghe, J.R.; Taes, Y.E.C. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta 2006, 372, 33–42. [Google Scholar] [CrossRef]
- Jung, K.-H.; Kim, T.-H.; Sheen, D.-H.; Lim, M.-K.; Lee, S.-K.; Kim, J.-Y.; Park, H.; Chae, S.-C.; Shim, S.-C. Associations of Vitamin D Binding Protein Gene Polymorphisms with the Development of Peripheral Arthritis and Uveitis in Ankylosing Spondylitis. J. Rheumatol. 2011, 38, 2224–2229. [Google Scholar] [CrossRef]
- Metcalf, J.P.; Thompson, A.B.; Gossman, G.L.; Nelson, K.J.; Koyama, S.; Rennard, S.I.; Robbins, R.A. GcGlobulin Functions as a Cochemotaxin in the Lower Respiratory Tract: A Potential Mechanism for Lung Neutrophil Recruitment in Cigarette Smokers. Am. Rev. Respir. Dis. 1991, 143, 844–849. [Google Scholar] [CrossRef]
- Haldar, D.; Agrawal, N.; Patel, S.; Kambale, P.R.; Arora, K.; Sharma, A.; Tripathi, M.; Batra, A.; Kabi, B.C. Association of VDBP and CYP2R1 gene polymorphisms with vitamin D status in women with polycystic ovarian syndrome: A north Indian study. Eur. J. Nutr. 2018, 57, 703–711. [Google Scholar] [CrossRef]
- Abbas, S.; Linseisen, J.; Slanger, T.; Kropp, S.; Mutschelknauss, E.J.; Flesch-Janys, D.; Chang-Claude, J. The Gc2 Allele of the Vitamin D Binding Protein Is Associated with a Decreased Postmenopausal Breast Cancer Risk, Independent of the Vitamin D Status. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1339–1343. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, J.; Constans, J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum. Genet. 1993, 92. [Google Scholar] [CrossRef]
- Braun, A.; Bichlmaier, R.; Cleve, H. Molecular analysis of the gene for the human vitamin-D-binding protein (group-specific component): Allelic differences of the common genetic GC types. Hum. Genet. 1992, 89. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Homma, S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 8539–8543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Eliassen, A.H.; Spiegelman, D.; Willett, W.C.; Hankinson, S.E. Plasma free 25-hydroxyvitamin D, vitamin D binding protein, and risk of breast cancer in the Nurses’ Health Study II. Cancer Causes Control 2014, 25, 819–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamboh, M.I.; Ferrell, R.E. Ethnic variation in vitamin D-binding protein (GC): A review of isoelectric focusing studies in human populations. Hum. Genet. 1986, 72, 281–293. [Google Scholar] [CrossRef]
- Perna, L.; Felix, J.F.; Breitling, L.P.; Haug, U.; Raum, E.; Burwinkel, B.; Schöttker, B.; Brenner, H. Genetic Variations in the Vitamin D Binding Protein and Season-Specific Levels of Vitamin D Among Older Adults. Epidemiology 2013, 24, 104–109. [Google Scholar] [CrossRef]
- Nagasawa, H.; Uto, Y.; Sasaki, H.; Okamura, N.; Murakami, A.; Kubo, S.; Kirk, K.L.; Hori, H. Gc protein (vitamin D-binding protein): Gc genotyping and GcMAF precursor activity. Anticancer Res. 2005, 25, 3689–3695. [Google Scholar]
- Saburi, E.; Saburi, A.; Ghanei, M. Promising role for Gc-MAF in cancer immunotherapy: From bench to bedside. Casp. J. Intern. Med. 2017, 8. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Bouariu, A.; Ion, D.; Andronic, O.; Dumitrașcu, M.C.; Bolocan, A. Considerations Regarding GCMAF Treatement in Breast Cancer. Romanian Biotechnol. Lett. 2019, 24, 851–855. [Google Scholar] [CrossRef]
- Francis, I.; AlAbdali, N.; Kapila, K.; John, B.; Al-Temaimi, R.A. Vitamin D pathway related polymorphisms and vitamin D receptor expression in breast cancer. Int. J. Vitam. Nutr. Res. Int. Z. Vitam. Ernahrungsforschung J. Int. Vitaminol. Nutr. 2019, 1–9. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs <20 ng/ml (150 vs 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-Hydroxyvitamin D Concentrations ≥40 ng/ml Are Associated with >65% Lower Cancer Risk: Pooled Analysis of Randomized Trial and Prospective Cohort Study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, S.J.; Stolzenberg-Solomon, R.Z.; Kopp, W.; Rager, H.; Virtamo, J.; Albanes, D. Impact of Circulating Vitamin D Binding Protein Levels on the Association between 25-Hydroxyvitamin D and Pancreatic Cancer Risk: A Nested Case-Control Study. Cancer Res. 2012, 72, 1190–1198. [Google Scholar] [CrossRef] [Green Version]
- Mondul, A.M.; Shui, I.M.; Yu, K.; Travis, R.C.; Stevens, V.L.; Campa, D.; Schumacher, F.R.; Ziegler, R.G.; Bueno-de-Mesquita, H.B.; Berndt, S.; et al. Genetic Variation in the Vitamin D Pathway in Relation to Risk of Prostate Cancer—Results from Breast and Prostate Cancer Cohort Consortium (BPC3). Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2013, 22, 688–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Wei, W.; Wang, G.; Zhou, H.; Fu, Y.; Liu, N. Circulating vitamin D concentration and risk of prostate cancer: A dose-response meta-analysis of prospective studies. Ther. Clin. Risk Manag. 2018, 14, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, X.; Sun, X.; Lu, S.; Liu, S. Vitamin intake and pancreatic cancer risk reduction: A meta-analysis of observational studies. Medicine (Baltimore) 2018, 97, e0114. [Google Scholar] [CrossRef]
- Wilson, K.M.; Shui, I.M.; Mucci, L.A.; Giovannucci, E. Calcium and phosphorus intake and prostate cancer risk: A 24-y follow-up study. Am. J. Clin. Nutr. 2015, 101, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batai, K.; Murphy, A.B.; Ruden, M.; Newsome, J.; Shah, E.; Dixon, M.A.; Jacobs, E.T.; Hollowell, C.M.P.; Ahaghotu, C.; Kittles, R.A. Race and BMI modify associations of calcium and vitamin D intake with prostate cancer. BMC Cancer 2017, 17, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, M.R.; Freedman, D.M.; Robien, K.; Kopp, W.; Rager, H.; Horst, R.L.; Stolzenberg-Solomon, R.Z. Vitamin D–binding protein and pancreatic cancer: A nested case-control study. Am. J. Clin. Nutr. 2015, 101, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Tagliabue, E.; Raimondi, S.; Gandini, S. Meta-analysis of Vitamin D-Binding Protein and Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1758–1765. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.M.; McGowan, L.; Millen, A.; Rajesh, P.; Webster, C.; Langman, G.; Rock, G.; Tachibana, I.; Tomlinson, M.G.; Berditchevski, F.; et al. Circulating DBP level and prognosis in operated lung cancer: An exploration of pathophysiology. Eur. Respir. J. 2013, 41, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Mick, P.J.; Peng, S.A.; Loftus, J.P. Serum Vitamin D Metabolites and CXCL10 Concentrations Associate with Survival in Dogs with Immune Mediated Disease. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maneechay, W.; Boonpipattanapong, T.; Kanngurn, S.; Puttawibul, P.; Geater, S.L.; Sangkhathat, S. Single Nucleotide Polymorphisms in the Gc Gene for Vitamin D Binding Protein in Common Cancers in Thailand. Asian Pac. J. Cancer Prev. 2015, 16, 3339–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Cheng, J.; Yang, K. Vitamin D-Related Gene Polymorphisms, Plasma 25-Hydroxy-Vitamin D, Cigarette Smoke and Non-Small Cell Lung Cancer (NSCLC) Risk. Int. J. Mol. Sci. 2016, 17, 1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, T.I.; Vogel, U.; Andersen, V. Associations between common polymorphisms in CYP2R1 and GC, Vitamin D intake and risk of colorectal cancer in a prospective case-cohort study in Danes. PLoS ONE 2020, 15, e0228635. [Google Scholar] [CrossRef]
- Flohil, S.C.; Vries, E.D.; Meurs, J.B.J.V.; Fang, Y.; Stricker, B.H.C.; Uitterlinden, A.G.; Nijsten, T. Vitamin D-binding protein polymorphisms are not associated with development of (multiple) basal cell carcinomas. Exp. Dermatol. 2010, 19, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Peña-Chilet, M.; Ibarrola-Villava, M.; Martin-González, M.; Feito, M.; Gomez-Fernandez, C.; Planelles, D.; Carretero, G.; Lluch, A.; Nagore, E.; Ribas, G. rs12512631 on the Group Specific Complement (Vitamin D-Binding Protein GC) Implicated in Melanoma Susceptibility. PLoS ONE 2013, 8, e59607. [Google Scholar] [CrossRef]
- Yin, J.; Liu, H.; Yi, X.; Wu, W.; Amos, C.I.; Fang, S.; Lee, J.E.; Han, J.; Wei, Q. Genetic Variants in the Vitamin D Pathway Genes VDBP and RXRA Modulate Cutaneous Melanoma Disease-Specific Survival. Pigment Cell Melanoma Res. 2016, 29, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.; Emmert, S.; Kruppa, J.; Schubert, S.; Tzvetkov, M.; Mössner, R.; Reich, K.; Berking, C.; Volkenandt, M.; Pföhler, C.; et al. No association of vitamin D metabolism-related polymorphisms and melanoma risk as well as melanoma prognosis: A case–control study. Arch. Dermatol. Res. 2012, 304, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Hirai, M.; Suzuki, S.; Hinokio, Y.; Chiba, M.; Kasuga, S.; Hirai, A.; Toyota, T. Group specific component protein genotype is associated with NIDDM in Japan. Diabetologia 1998, 41, 742–743. [Google Scholar] [CrossRef] [Green Version]
- Hirai, M.; Suzuki, S.; Hinokio, Y.; Hirai, A.; Chiba, M.; Akai, H.; Suzuki, C.; Toyota, T. Variations in Vitamin D-Binding Protein (Group-Specific Component Protein) Are Associated with Fasting Plasma Insulin Levels in Japanese with Normal Glucose Tolerance. J. Clin. Endocrinol. Metab. 2000, 85, 1951–1953. [Google Scholar] [CrossRef]
- Sandler, S.; Buschard, K.; Bendtzen, K. Effects of 1,25-dihydroxyvitamin D3 and the analogues MC903 and KH1060 on interleukin-1 beta-induced inhibition of rat pancreatic islet beta-cell function in vitro. Immunol. Lett. 1994, 41, 73–77. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hosen, M.B.; Faruk, M.O.; Hasan, M.M.; Kabir, Y.; Howlader, M.Z.H. Association of vitamin D and vitamin D binding protein (DBP) gene polymorphism with susceptibility of type 2 diabetes mellitus in Bangladesh. Gene 2017, 636, 42–47. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Pani, M.A.; Regulla, K.; Segni, M.; Hofmann, S.; Fner, M.H.; Pasquino, A.M.; Usadel, K.-H.; Badenhoop, K. A Polymorphism within the Vitamin D-Binding Protein Gene Is Associated with Graves’ Disease but Not with Hashimoto’s Thyroiditis. J. Clin. Endocrinol. Metab. 2002, 87, 2564–2567. [Google Scholar] [CrossRef] [Green Version]
- Kurylowicz, A.; Ramos-Lopez, E.; Bednarczuk, T.; Badenhoop, K. Vitamin D-Binding Protein (DBP) Gene Polymorphism is Associated with Graves’ Disease and the Vitamin D Status in a Polish Population Study. Exp. Clin. Endocrinol. Diabetes 2006, 114, 329–335. [Google Scholar] [CrossRef]
- Jiang, H.; Xiong, D.-H.; Guo, Y.-F.; Shen, H.; Xiao, P.; Yang, F.; Chen, Y.; Zhang, F.; Recker, R.R.; Deng, H.-W. Association analysis of vitamin D-binding protein gene polymorphisms with variations of obesity-related traits in Caucasian nuclear families. Int. J. Obes. 2007, 31, 1319–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Ricciardi, C.; Berg, A.H.; Erdenesanaa, D.; Collerone, G.; Ankers, E.; Wenger, J.; Karumanchi, S.A.; Thadhani, R.; Bhan, I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J. Bone Miner. Res. 2011, 26, 1609–1616. [Google Scholar] [CrossRef]
- Aloia, J.; Mikhail, M.; Dhaliwal, R.; Shieh, A.; Usera, G.; Stolberg, A.; Ragolia, L.; Islam, S. Free 25(OH)D and the Vitamin D Paradox in African Americans. J. Clin. Endocrinol. Metab. 2015, 100, 3356–3363. [Google Scholar] [CrossRef]
- Martínez-Aguilar, M.M.; Aparicio-Bautista, D.I.; Ramírez-Salazar, E.G.; Reyes-Grajeda, J.P.; De la Cruz-Montoya, A.H.; Antuna-Puente, B.; Hidalgo-Bravo, A.; Rivera-Paredez, B.; Ramírez-Palacios, P.; Quiterio, M.; et al. Serum Proteomic Analysis Reveals Vitamin D-Binding Protein (VDBP) as a Potential Biomarker for Low Bone Mineral Density in Mexican Postmenopausal Women. Nutrients 2019, 11, 2853. [Google Scholar] [CrossRef] [Green Version]
- Ezura, Y.; Nakajima, T.; Kajita, M.; Ishida, R.; Inoue, S.; Yoshida, H.; Suzuki, T.; Shiraki, M.; Hosoi, T.; Orimo, H.; et al. Association of Molecular Variants, Haplotypes, and Linkage Disequilibrium Within the Human Vitamin D-Binding Protein (DBP) Gene With Postmenopausal Bone Mineral Density. J. Bone Miner. Res. 2003, 18, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Sahmoud, S.; Ibrahim, M.S.; Toraih, E.A.; Kamel, N.; Fawzy, M.S.; Elfiky, S. Association of VDBP rs4701 Variant, but not VDR/RXR-α Over-Expression with BoneMineral Density in Pediatric Well-Chelated β-Thalassemia Patients. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020037. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhao, Y.; Pan, J.; Fang, K.; Wang, Y.; Li, Z.; Chang, X. Vitamin D-binding protein (group-specific component) has decreased expression in rheumatoid arthritis. Clin. Exp. Rheumatol. 2012, 30, 525–533. [Google Scholar]
- Brink, M.; Johansson, L.; Nygren, E.; Ärlestig, L.; Hultdin, J.; Rantapää-Dahlqvist, S. Vitamin D in individuals before onset of rheumatoid arthritis—Relation to vitamin D binding protein and its associated genetic variants. BMC Rheumatol. 2018, 2, 26. [Google Scholar] [CrossRef]
- Haneul, K.; Seungye, B.; Seung-Min, H.; Jaeseon, L.; Min, J.S.; Jennifer, L.; Mi-La, C.; Seung-Ki, K.; Sung-Hwan, P. 1,25-dihydroxy Vitamin D3 and Interleukin-6 Blockade Synergistically Regulate Rheumatoid Arthritis by Suppressing Interleukin-17 Production and Osteoclastogenesis. J. Korean Med. Sci. 2020, 35. [Google Scholar] [CrossRef]
- Zarei, A.; Morovat, A.; Javaid, K.; Brown, C.P. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Orhan, C.; Seyhan, B.; Baykara, O.; Yildiz, M.; Kasapcopur, O.; Buyru, N. Vitamin D binding protein genotype frequency in familial Mediterranean fever patients. Scand. J. Rheumatol. 2020, 1–5. [Google Scholar] [CrossRef]
- Gu, J.; Tong, X.; Chen, Y.; Zhang, C.; Ma, T.; Li, S.; Min, W.; Yuan, Y.; Liu, X.; Bian, J.; et al. Vitamin D Inhibition of TRPV5 Expression During Osteoclast Differentiation. Int. J. Endocrinol. Metab. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Laufs, J.; Andrason, H.; Sigvaldason, A.; Halapi, E.; Thorsteinsson, L.; Jónassen, K.; Söebech, E.; Gislason, T.; Gulcher, J.R.; Stefansson, K.; et al. Association of Vitamin D Binding Protein Variants with Chronic Mucus Hypersecretion in Iceland. Am. J. PharmacoGenomics 2004, 4, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiang, L.; Willis-Owen, S.A.; Zhang, Y.; Gao, J. Vitamin D binding protein variants associate with asthma susceptibility in the Chinese han population. BMC Med. Genet. 2011, 12, 103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Z.; Ma, T. Associations of Genetic Polymorphisms Relevant to Metabolic Pathway of Vitamin D3 with Development and Prognosis of Childhood Bronchial Asthma. DNA Cell Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.S.; Elgazzaz, M.G.; Ibrahim, A.; Hussein, M.H.; Khashana, M.S.; Toraih, E.A. Association of group-specific component exon 11 polymorphisms with bronchial asthma in children and adolescents. Scand. J. Immunol. 2019, 89, e12740. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Kalmarzi, R.; Abdi, M.; Hosseini, J.; Tavana, S.; Mokarizadeh, A.; Rahbari, R. Association of vitamin D genetic pathway with asthma susceptibility in the Kurdish population. J. Clin. Lab. Anal. 2020, 34, e23039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, A.M.; Bassford, C.; Webster, D.; Newby, P.; Rajesh, P.; Stockley, R.A.; Thickett, D.R. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax 2011, 66, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martineau, A.R.; Leandro, A.C.C.S.; Anderson, S.T.; Newton, S.M.; Wilkinson, K.A.; Nicol, M.P.; Pienaar, S.M.; Skolimowska, K.H.; Rocha, M.A.; Rolla, V.C.; et al. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur. Respir. J. 2010, 35, 1106–1112. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Kim, Y.S.; Kang, Y.A.; Shin, J.H.; Oh, Y.M.; Seo, J.B.; Jung, J.Y.; Lee, S.D. Relationship between Vitamin D-Binding Protein Polymorphisms and Blood Vitamin D Level in Korean Patients with COPD. Available online: https://www.dovepress.com/relationship-between-vitamin-d-binding-protein-polymorphisms-and-blood-peer-reviewed-article-COPD (accessed on 24 September 2020).
- Gao, J.; Törölä, T.; Li, C.-X.; Ohlmeier, S.; Toljamo, T.; Nieminen, P.; Hattori, N.; Pulkkinen, V.; Iwamoto, H.; Mazur, W. Sputum Vitamin D Binding Protein (VDBP) GC1S/1S Genotype Predicts Airway Obstruction: A Prospective Study in Smokers with COPD. Available online: https://www.dovepress.com/sputum-vitamin-d-binding-protein-vdbp-gc1s1s-genotype-predicts-airway--peer-reviewed-article-COPD (accessed on 24 September 2020).
- Brighenti, S.; Bergman, P.; Martineau, A.R. Vitamin D and tuberculosis: Where next? J. Intern. Med. 2018, 284, 145–162. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-W.; Chuang, T.-Y.; Huang, H.-H.; Liu, C.-W.; Kao, Y.-H.; Wu, L.S.-H. VDR and VDBP genes polymorphisms associated with susceptibility to tuberculosis in a Han Taiwanese population. J. Microbiol. Immunol. Infect. 2016, 49, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Junaid, K.; Rehman, A.; Jolliffe, D.A.; Saeed, T.; Wood, K.; Martineau, A.R. Vitamin D deficiency associates with susceptibility to tuberculosis in Pakistan, but polymorphisms in VDR, DBP and CYP2R1 do not. BMC Pulm. Med. 2016, 16, 73. [Google Scholar] [CrossRef] [Green Version]
- Kiani, A.; Mohamadi-Nori, E.; Vaisi-Raygani, A.; Tanhapour, M.; Elahi-Rad, S.; Bahrehmand, F.; Rahimi, Z.; Pourmotabbed, T. Vitamin D-binding protein and vitamin D receptor genotypes and 25-hydroxyvitamin D levels are associated with development of aortic and mitral valve calcification and coronary artery diseases. Mol. Biol. Rep. 2019, 46, 5225–5236. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Z.; van Duijnhoven, F.J.B.; Dong, M.; Qian, Y.; Yu, H.; Yang, J.; Cui, L.; Han, R.; Su, J.; et al. Genetic Variants in Group-Specific Component (GC) Gene Are Associated with Breast Cancer Risk among Chinese Women. Available online: https://www.hindawi.com/journals/bmri/2019/3295781/ (accessed on 23 September 2020).
- Maalmi, H.; Walter, V.; Jansen, L.; Chang-Claude, J.; Owen, R.W.; Ulrich, A.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Relationship of very low serum 25-hydroxyvitamin D3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur. J. Epidemiol. 2017, 32, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Mondul, A.M.; Kopp, W.; Rager, H.; Virtamo, J.; Albanes, D. Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of prostate cancer. Int. J. Cancer 2013, 132, 2940–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speeckaert, M.M.; Taes, Y.E.; De Buyzere, M.L.; Christophe, A.B.; Kaufman, J.-M.; Delanghe, J.R. Investigation of the potential association of vitamin D binding protein with lipoproteins. Ann. Clin. Biochem. 2010, 47, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Bolland, M.J.; Grey, A.B.; Ames, R.W.; Horne, A.M.; Mason, B.H.; Wattie, D.J.; Gamble, G.D.; Bouillon, R.; Reid, I.R. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin D are not mediated by vitamin D binding protein. Clin. Endocrinol. 2007, 67, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Winters, S.J.; Chennubhatla, R.; Wang, C.; Miller, J.J. Influence of obesity on vitamin D–binding protein and 25-hydroxy vitamin D levels in African American and white women. Metabolism 2009, 58, 438–442. [Google Scholar] [CrossRef]
- Fang, Y.; van Meurs, J.B.J.; d’Alesio, A.; Jhamai, M.; Zhao, H.; Rivadeneira, F.; Hofman, A.; van Leeuwen, J.P.T.; Jehan, F.; Pols, H.A.P.; et al. Promoter and 3′-Untranslated-Region Haplotypes in the Vitamin D Receptor Gene Predispose to Osteoporotic Fracture: The Rotterdam Study. Am. J. Hum. Genet. 2005, 77, 807–823. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; van Meurs, J.B.J.; Rivadeneira, F.; van Schoor, N.M.; van Leeuwen, J.P.T.; Lips, P.; Pols, H.A.P.; Uitterlinden, A.G. Vitamin D Receptor Gene Haplotype Is Associated with Body Height and Bone Size. J. Clin. Endocrinol. Metab. 2007, 92, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Blanton, D.; Han, Z.; Bierschenk, L.; Linga-Reddy, M.V.P.; Wang, H.; Clare-Salzler, M.; Haller, M.; Schatz, D.; Myhr, C.; She, J.-X.; et al. Reduced Serum Vitamin D-Binding Protein Levels Are Associated with Type 1 Diabetes. Diabetes 2011, 60, 2566–2570. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Qureshi, A.M.; Murad, S. Vitamin D binding protein gene variants rs4588 and rs7041 and low serum concentration of 25-hydroxy (OH) vitamin D3 in type-2 diabetes patients: A pilot study. Sci. Lett. 2015, 3, 39–41. [Google Scholar]
- Ongagna, J.C.; Kaltenbacher, M.C.; Sapin, R.; Pinget, M.; Belcourt, A. The HLA-DQB alleles and amino acid variants of the vitamin D-binding protein in diabetic patients in Alsace. Clin. Biochem. 2001, 34, 59–63. [Google Scholar] [CrossRef]
- Ongagna, J.C.; Pinget, M.; Belcourt, A. Vitamin D-binding protein gene polymorphism association with IA-2 autoantibodies in type 1 diabetes. Clin. Biochem. 2005, 38, 415–419. [Google Scholar] [CrossRef]
- Xu, X.-H.; Xiong, D.-H.; Liu, X.-G.; Guo, Y.; Chen, Y.; Zhao, J.; Recker, R.R.; Deng, H.-W. Association analyses of vitamin D-binding protein gene with compression strength index variation in Caucasian nuclear families. Osteoporos. Int. 2010, 21, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, A.; Bichlmaier, R.; Muller, B.; Cleve, H. Molecular evaluation of an Alu repeat including a polymorphic variable poly(dA) (AluVpA) in the vitamin D binding protein (DBP) gene. Hum. Genet. 1993, 90. [Google Scholar] [CrossRef] [PubMed]
- Chupeerach, C.; Tungtrongchitr, A.; Phonrat, B.; Schweigert, F.J.; Tungtrongchitr, R.; Preutthipan, S. Association of Thr420Lys polymorphism in DBP gene with fat-soluble vitamins and low radial bone mineral density in postmenopausal Thai women. Biomark. Med. 2012, 6, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Papiha, S.S.; Allcroft, L.C.; Kanan, R.M.; Francis, R.M.; Datta, H.K. Vitamin D Binding Protein Gene in Male Osteoporosis: Association of Plasma DBP and Bone Mineral Density with (TAAA)n-Alu Polymorphism in DBP. Calcif. Tissue Int. 1999, 65, 262–266. [Google Scholar] [CrossRef]
- Eichner, J.E.; Cauley, J.A.; Ferrell, R.E.; Cummings, S.R.; Kuller, L.H.; Vogler, G.P. Genetic variation in two bone-related proteins: Is there an association with bone mineral density or skeletal size in postmenopausal women?: Variation in Bone-Related Proteins in Women. Genet. Epidemiol. 1992, 9, 177–184. [Google Scholar] [CrossRef]
- Rapado, A.; Hawkins, F.; Sobrinho, L.; Díaz-Curiel, M.; Galvao-Telles, A.; Arver, S.; Melo Gomes, J.; Mazer, N.; Garcia e Costa, J.; Horcajada, C.; et al. Bone Mineral Density and Androgen Levels in Elderly Males. Calcif. Tissue Int. 1999, 65, 417–421. [Google Scholar] [CrossRef]
- Ritter, C.S.; Brown, A.J. Suppression of PTH by the vitamin D analog eldecalcitol is modulated by its high affinity for the serum vitamin D-binding protein and resistance to metabolism. J. Cell. Biochem. 2011, 112, 1348–1352. [Google Scholar] [CrossRef]
- Martineau, A.R.; Wilkinson, R.J.; Wilkinson, K.A.; Newton, S.M.; Kampmann, B.; Hall, B.M.; Packe, G.E.; Davidson, R.N.; Eldridge, S.M.; Maunsell, Z.J.; et al. A Single Dose of Vitamin D Enhances Immunity to Mycobacteria. Am. J. Respir. Crit. Care Med. 2007, 176, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Daffara, V.; Verdoia, M.; Rolla, R.; Nardin, M.; Marino, P.; Bellomo, G.; Carriero, A.; Luca, G.D. Impact of polymorphism rs7041 and rs4588 of Vitamin D Binding Protein on the extent of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 775–783. [Google Scholar] [CrossRef]
| SNP Locus | GC Name | Codon Variant | Amino Acid Variant |
|---|---|---|---|
| Rs4588 | GC1 | ACG (Thr->Lys) | Thr-436 |
| Rs4588 | GC2 | AAG (Thr->Lys) | Lys-436 |
| Rs7041 | GC1F | GAT (Asp->Glu) | Asp-432 (416/mature VDBP) |
| Rs7041 | GC1S | GAG (Asp->Glu) | Glu-432 (420/mature VDBP) |
| Disease | VDBP Influence | Mechanism | Reference |
|---|---|---|---|
| Cancers | |||
| Breast cancer | Gc2-2 genotype associated with decreased risk of postmenopausal breast cancer (n = 1402, control: 2608) SNPs: rs17467825, rs2298850 and rs3755967 are associated to the breast cancer risk (n = 818, controls = 935); another study does not support an important role of either calculated circulating free 25(OH)D or circulating VDBP levels in breast cancer risk among predominantly premenopausal women; (controls = 584) | The carcinogenic mechanism is based on the potential to convert Gc to GcMAF, which is a macrophage activator. GcMAF may enhance proapoptotic enzymes activity and induce cell apoptosis via JNK1/2 and p387 pathway—that may inhibit cancer development | [51,54,114,115,116] |
| Prostate cancer | Decreased risk in of prostate cancer associated with higher serum VDBP levels in men with lower than median 25(OH)D status, where elevated risk in men with higher than median 25(OH)D concentration (n = 950, control = 964); SNP: Rs2282679 in Gc has no significant correlation with non-aggressive and aggressive prostate cancer (n = 10,572, controls = 4975) | Extracellular concentrations of VDBP and 25(OH)D result in an upregulation of megalin-mediated internalization of SHBG-bound testosterone | [65,117] |
| Pancreatic cancer | Higher serum 25(OH)D and serum VDBP are associated with higher pancreatic cancer risk (n = 234, control = 234) among Finnish men population; VDBP or 25(OH)D were not associated with pancreatic cancer (n = 295, two controls n = 590); rs2282679, rs7041 and rs4588 found no significant correlation with pancreas cancer | Reducing free 25(OH)D by VDBP decreases bioavailability; high concentration of VDBP and 25(OH)D could potentially displace 1,25(OH)D with its antitumorigenic properties | [64,70,71] |
| Lung cancer | VDBP low serum concentration might be a predictor of subsequent death from non-small cell lung cancer (n = 148 lung cancer patients, 68 patients with other intrathoracic tumors and 33 noncancer controls); GC2-1f combination (TT-CA) has significant and protective association with lung cancer (n = 113, control = 113); Rs7041 in GC gene reduces the risk of Non-Small Cell Lung Cancer risk (n = 446, controls = 425) | Conversion of VDBP to GcMAF may be reduced in malignancy due to the action of α-N-acetylogalactosaminidasa and as a result it might lower macrophage activation | [72,75,90] |
| Colorectal cancer | Rs7041 (TG/GG) significant association with colorectal cancers among age 60 years old and older (n = 282, control = 113); Rs4588 (CA/AA) significant association with cancer in males aged 60 years old or less (n = 282, control = 113); Both: Gc/Rs7041 and CYP2R1/rs10741657 polymorphisms decreases the risk of colorectal cancer about 9–12% (n = 920, controls = 1743) | [74,76] | |
| Basal cell carcinoma | SNP may affect skin carcinogenesis. Among patients with rs7041 and rs4588 233 of them developed BCC and 52.4% among those patients developed multiple BCCs (n = 7983). GC1s homozygotes had lower BCC risk. Rs7041 was associated with BCC development among the youngest group. | SNPs may be associated with BCC development among younger patients | [77] |
| Cutaneous Melanoma | Association between VDBP rs12512631 and risk of cutaneous melanoma among Spanish population (n = 530, controls = 314); No association between VDBP rs1155563 and rs7041 and melanoma risk or prognosis (n = 305, controls = 370) | VDBP variants may influence on vitamin synthesis and distribution | [78,79,80] |
| Other important diseases | |||
| Thyroid autoimmunity disorders | Intron 8 (TAAA)n-Alu repeat polymorphism correlates with Graves’ disease (n = 561) but no association with Hashimoto’s thyroiditis; VDBP polymorphisms may contribute to development of autoimmune diseases (n = 332, control = 185). | Ability of VDBP to binding; linkage with nearby gene, affecting on immune system by VDBP’s macrophage activating role | [86,87] |
| Obesity | Possible role of VDBP in the relation between body fat mass and vitamin D metabolism rs17467825 and its corresponding haplotype GAA—strongest association in females; VDBP has an influence on PFM (percentage of fat mass), more significant associations are more female-specific | A lipid-bound VDBP fraction | [47,88,89,118,119,120,121,122] |
| Diabetes mellitus | People with Gc1S-2 and 1S-1S had higher fasting plasma insulin concentration than 1F-1F; rs7041 (Glu/Glu-416) and rs4588 (Lys/Lys-420) variants of VDBP were higher in type 2 diabetic comparing to control group (n = 104, controls = 107) | Polymorphisms of VDBP might be associated with insulin resistance in Japanese population with normal glucose tolerance. It might contribute to type 2 diabetes development. VDBP affects glucose metabolism by modulating the action of metabolites of vitamin D; Vitamin D stimulates synthesis of insulin, effects on β-cells and protects them against destruction by inflammatory cytokines | [81,82,83,84,123,124,125,126] |
| Bone metabolism | An inverse correlation between serum VDBP levels and BMD; A highly significant difference in premenopausal bone fracture risk among women with different VDBP phenotypes (VDBP1-1>VDBP2-1>VDBP2-2); SNPs in the VDBP gene might be associated with BMD; VDBP rs4701 is associated with lower BMD-L4 and higher risk of osteoporosis; multiple of the VDBP SNPs might increase the risk of osteoporosis in postmenopausal women | Phenotype of VDBP is mediated by VDBP-MAF and activation osteoclasts | [90,93,127,128,129,130,131,132,133] |
| Rheumatoid arthritis | correlation between RA and rs2282679 SNP | 1,25(OH)2D3 may have inhibitory effect on osteoclasts formation that is induced by IL-22 | [95,97] |
| Ankylosing spondylitis | Patients with G alleles at rs222016 and rs222020 and an allele at rs3733359 show decreased risk of peripheral arthritis; rs4752 polymorphisms are associated with development of uveitis. Haplotype analysis showed that AGGA haplotype protects against peripheral arthritis development in ankylosing spondylitis patients (n = 223, control = 239). | [48] | |
| Asthma | Upregulation of DBP expression in patients with diphenyl-methane disocyanate occupational asthma DBP SNPs rs4588 and rs7041 are associated with the risk of asthma and the DBP1 allele might confer a protective effect; patients with GC2 (compared to GC1) haplotype are more susceptible for the development of asthma; Gc1, Gc2 was significantly associated with the risk of asthma (n = 467, control = 288); Rs7041 and rs4588 associated with increased risk of bronchial asthma (n = 143, controls = 143); Rs4588 CA and AA genotypes had protective effect, while rs7041 GG genotype had significantly higher frequency among patients diagnosed with asthma (n = 96, controls = 96) | VDBP and enhancing the chemotactic activity of monocytes and neutrophils; VDBP modules Th2-mediated inflammation and influences the susceptibility to asthma | [101,102,103,104,105] |
| Chronic obstructive pulmonary disease | GC1f and GC2 alleles may be linked to sputum hypersecretion in COPD patients; Gc2 protects against COPD (n = 140, control = 480) 1F-1S genotype was protective factor against deficiency of vitamin D among Korean patients (n = 175); High frequencies of the haplotypes in rs7041 and rs4588—GC1S/1S among COPD patients (n = 233) | VDBP has the potential to influence the respiratory function by determining vitamin D bioavailability and via direct effects on innate cell function | [101,106,108,109] |
| Tuberculosis | VDBP2-2 phenotype strongly associated to susceptibility to TB among Gujarati Asians (n = 534, control = 400); Among Taiwan patients the GC1F carriers were associated with tuberculosis (n = 198, controls = 170) | Reduced ability of VDBP2 to conversion VDBP to VDBP-MAF | [107,134] |
| Coronary artery diseases | A strong interaction between A allele VDR rs1544410 and G allele of VDBP rs7041 genes in a protective role; strong association between vitamin D deficiency, lipid profile and the VDR rs1544410G>A and rs7T41>G VDBP genes polymorphisms (n = 157, control = 182) No correlation between rs7041, rs4588 and CAD (n = 1080). | [113,135] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms—The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217822
Rozmus D, Ciesielska A, Płomiński J, Grzybowski R, Fiedorowicz E, Kordulewska N, Savelkoul H, Kostyra E, Cieślińska A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms—The Risk of Malignant Tumors and Other Diseases. International Journal of Molecular Sciences. 2020; 21(21):7822. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217822
Chicago/Turabian StyleRozmus, Dominika, Alicja Ciesielska, Janusz Płomiński, Roman Grzybowski, Ewa Fiedorowicz, Natalia Kordulewska, Huub Savelkoul, Elżbieta Kostyra, and Anna Cieślińska. 2020. "Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms—The Risk of Malignant Tumors and Other Diseases" International Journal of Molecular Sciences 21, no. 21: 7822. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217822