Cocaine Administration and Its Abstinence Conditions Modulate Neuroglia
Abstract
:1. Introduction
2. Results
2.1. Influence of Cocaine on Transcript Levels
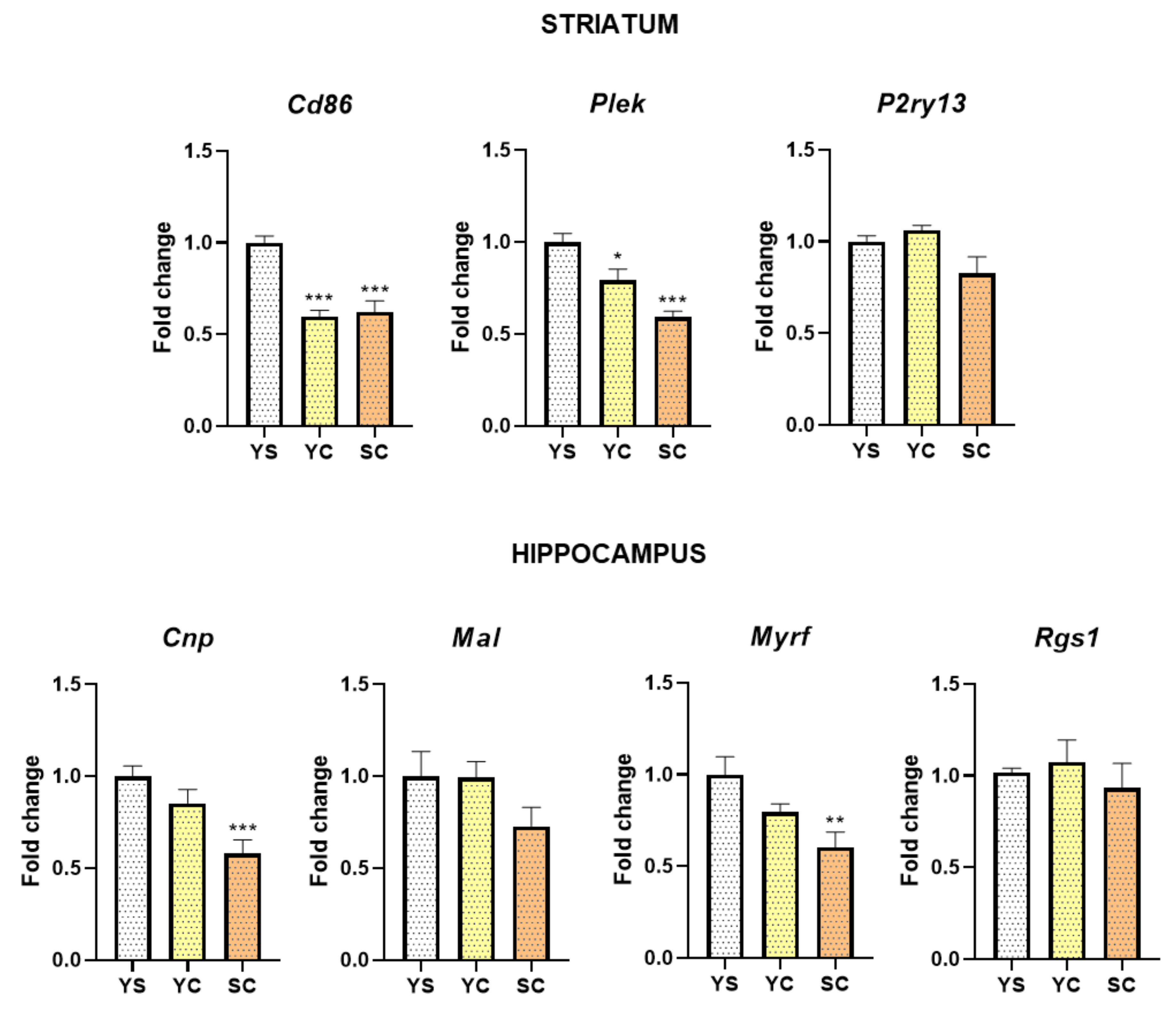
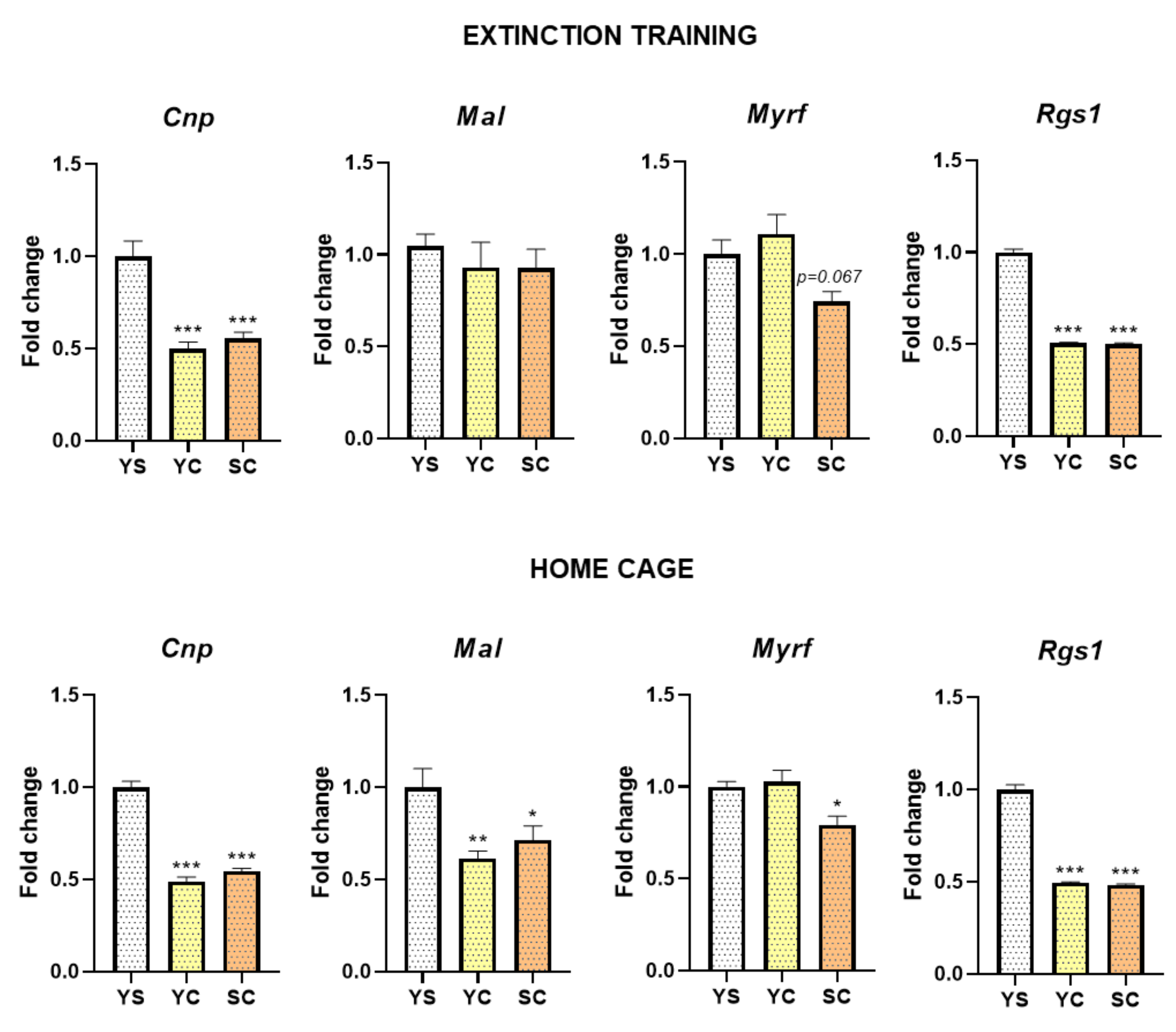
2.2. Alterations in mRNA Expression Induced by Cocaine Self-Administration and Abstinence Conditions
2.3. Alterations in MYRF and PLEK Protein Levels Induced by Cocaine Self-Administration and Abstinence Conditions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cocaine Self-Administration and Extinction Training
4.3. Brain Isolation and RNA Extraction
4.4. Microarray Analyses
4.5. Quantitative Real-Time PCR
4.6. Determination of MYRF and PLEK Protein Concentration
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CD86 | cluster of differentiation 86 |
| CNP | cyclic nucleotide phosphodiesterase |
| CUD | cocaine use disorder |
| MAL | myelin and lymphocyte protein |
| MYRF | myelin regulatory factor |
| PLEK | pleckstrin |
| P2RY13 | P2Y purinoceptor 13 |
| SUD | substance use disorder |
| RGS1 | regulator of G protein signaling 1 |
References
- Forray, A.; Sofuoglu, M. Future pharmacological treatments for substance use disorders. Br. J. Clin. Pharm. 2014, 77, 382–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLellan, A.T. Substance Misuse and Substance use Disorders: Why do they Matter in Healthcare? Trans. Am. Clin. Clim. Assoc. 2017, 128, 112–130. [Google Scholar]
- Jarvis, R.; Tamashiro-Orrego, A.; Promes, V.; Tu, L.; Shi, J.; Yang, Y. Cocaine Self-administration and Extinction Inversely Alter Neuron to Glia Exosomal Dynamics in the Nucleus Accumbens. Front. Cell. Neurosci. 2020, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- A World Drug Report. 2020. Available online: https://wdr.unodc.org/wdr2020/ (accessed on 1 September 2020).
- Walker, D.M.; Cates, H.M.; Loh, Y.H.E.; Purushothaman, I.; Ramakrishnan, A.; Cahill, K.M.; Lardner, C.K.; Godino, A.; Kronman, H.G.; Rabkin, J.; et al. Cocaine Self-administration Alters Transcriptome-wide Responses in the Brain’s Reward Circuitry. Biol. Psychiatry 2018, 84, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Cotto, B.; Li, H.; Tuma, R.F.; Ward, S.J.; Langford, D. Cocaine-mediated activation of microglia and microglial MeCP2 and BDNF production. Neurobiol. Dis. 2018, 117, 28–41. [Google Scholar] [CrossRef]
- Kenny, P.J. Epigenetics, microRNA, and addiction. Dialogues Clin. Neurosci. 2014, 16, 335–344. [Google Scholar]
- Miguel-Hidalgo, J.J. The Role of Glial Cells in Drug Abuse. Curr. Drug Abus. Rev. 2009, 2, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, B.P. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int. J. Neuropsychopharmacol. 2007, 10, 547. [Google Scholar] [CrossRef]
- Burnstock, G. Historical review: ATP as a neurotransmitter. Trends Pharmacol. Sci. 2006, 27, 166–176. [Google Scholar] [CrossRef]
- Noda, M.; Nakanishi, H.; Nabekura, J.; Akaike, N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J. Neurosci. 2000, 20, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Periyasamy, P.; Liao, K.; Kook, Y.H.; Niu, F.; Callen, S.E.; Guo, M.L.; Buch, S. Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Mol. Neurobiol. 2018, 55, 3196–3210. [Google Scholar] [CrossRef]
- Little, K.Y.; Ramssen, E.; Welchko, R.; Volberg, V.; Roland, C.J.; Cassin, B. Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res. 2009, 168, 173–180. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.T.; Wang, M.; Hauberg, M.E.; Fullard, J.F.; Kozlenkov, A.; Keenan, A.; Hurd, Y.L.; Dracheva, S.; Casaccia, P.; Roussos, P.; et al. Brain Cell Type Specific Gene Expression and Co-expression Network Architectures. Sci. Rep. 2018, 8, 8868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuchero, J.B.; Barres, B.A. Between the sheets: A molecular sieve makes myelin membranes. Dev. Cell 2011, 21, 385–386. [Google Scholar] [CrossRef] [Green Version]
- Duncan, G.J.; Plemel, J.R.; Assinck, P.; Manesh, S.B.; Muir, F.G.W.; Hirata, R.; Berson, M.; Liu, J.; Wegner, M.; Emery, B.; et al. Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol. 2017, 134, 403–422. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; De Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef]
- Frank, M. MAL, a proteolipid in glycosphingolipid enriched domains: Functional implications in myelin and beyond. Prog. Neurobiol. 2000, 60, 531–544. [Google Scholar] [CrossRef]
- Cahill, M.E.; Walker, D.M.; Gancarz, A.M.; Wang, Z.J.; Lardner, C.K.; Bagot, R.C.; Neve, R.L.; Dietz, D.M.; Nestler, E.J. The dendritic spine morphogenic effects of repeated cocaine use occur through the regulation of serum response factor signaling. Mol. Psychiatry 2018, 23, 1474–1486. [Google Scholar] [CrossRef]
- Evenden, J. Impulsivity: A discussion of clinical and experimental findings. J. Psychopharmacol. 1999, 13, 180–192. [Google Scholar] [CrossRef]
- Moloney, G.M.; van Oeffelen, W.E.P.A.; Ryan, F.J.; van de Wouw, M.; Cowan, C.; Claesson, M.J.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Differential gene expression in the mesocorticolimbic system of innately high- and low-impulsive rats. Behav. Brain Res. 2019, 364, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kursula, P. Structural properties of proteins specific to the myelin sheath. Amino Acids 2008, 34, 175–185. [Google Scholar] [CrossRef]
- Albertson, D.N.; Pruetz, B.; Schmidt, C.J.; Kuhn, D.M.; Kapatos, G.; Bannon, M.J. Gene expression profile of the nucleus accumbens of human cocaine abusers: Evidence for dysregulation of myelin. J. Neurochem. 2004, 88, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Bannon, M.J.; Kapatos, G.; Albertson, D.N. Gene expression profiling in the brains of human cocaine abusers. Addict. Biol. 2005, 10, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristiansen, L.V.; Bannon, M.J.; Meador-Woodruff, J.H. Expression of transcripts for myelin related genes in postmortem brain from cocaine abusers. Neurochem. Res. 2009, 34, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewohl, J.M.; Wixey, J.; Harper, C.G.; Dodd, P.R. Expression of MBP, PLP, MAG, CNP, and GFAP in the human alcoholic brain. Alcohol. Clin. Exp. Res. 2005, 29, 1698–1705. [Google Scholar] [CrossRef]
- George, O.; Mandyam, C.D.; Wee, S.; Koob, G.F. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 2008, 33, 2474–2482. [Google Scholar] [CrossRef]
- Kovalevich, J.; Corley, G.; Yen, W.; Rawls, S.M.; Langford, D. Cocaine-induced loss of white matter proteins in the adult mouse nucleus accumbens is attenuated by administration of a β-lactam antibiotic during cocaine withdrawal. Am. J. Pathol. 2012, 181, 1921–1927. [Google Scholar] [CrossRef] [Green Version]
- Roy, K.; Murtie, J.C.; El-Khodor, B.F.; Edgar, N.; Sardi, S.P.; Hooks, B.M.; Benoit-Marand, M.; Chen, C.; Moore, H.; O’Donnell, P.; et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl. Acad. Sci. USA 2007, 104, 8131–8136. [Google Scholar] [CrossRef] [Green Version]
- Albouy, G.; King, B.R.; Maquet, P.; Doyon, J. Hippocampus and striatum: Dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus 2013, 23, 985–1004. [Google Scholar] [CrossRef]
- Grafton, S.T.; Hazeltine, E.; Ivry, R. Functional mapping of sequence learning in normal humans. J. Cogn. Neurosci. 1995, 7, 497–510. [Google Scholar] [CrossRef]
- Schendan, H.E.; Searl, M.M.; Melrose, R.J.; Stern, C.E. Sequence? What Sequence?: The human medial temporal lobe and sequence learning. Mol. Psychiatry 2003, 8, 896–897. [Google Scholar] [CrossRef]
- Curran, T. Effects of aging on implicit sequence learning: Accounting for sequence structure and explicit knowledge. Psychol. Res. 1997, 60, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Poldrack, R.A.; Gabrieli, J.D.E. Characterizing the neural mechanisms of skill learning and repetition priming evidence from mirror reading. Brain 2001, 124, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.S.; Benedict, E.; Wu, O.; Rubin, E.; Gluck, M.A.; Foltin, R.W.; Myers, C.E.; Vadhan, N.P. Learning functions in short-term cocaine users. Addict. Behav. Rep. 2019, 9, 100169. [Google Scholar] [CrossRef]
- Du, Y.; Dreyfus, C.F. Oligodendrocytes as providers of growth factors. J. Neurosci. Res. 2002, 68, 647–654. [Google Scholar] [CrossRef]
- Niciu, M.J.; Henter, I.D.; Sanacora, G.; Zarate, C.A. Glial abnormalities in substance use disorders and depression: Does shared glutamatergic dysfunction contribute to comorbidity? World J. Biol. Psychiatry 2014, 15, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.L.; Liao, K.; Periyasamy, P.; Yang, L.; Cai, Y.; Callen, S.E.; Buch, S. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 2015, 11, 995–1009. [Google Scholar] [CrossRef] [Green Version]
- Polazzi, E.; Contestabile, A. Reciprocal interactions between microglia and neurons: From survival to neuropathology. Rev. Neurosci. 2002, 13, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Bidlack, J.M. Detection and function of opioid receptors on cells from the immune system. Clin. Diagn. Lab. Immunol. 2000, 7, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.H.; Kitchen, I.; Bailey, A. The endogenous opioid system in cocaine addiction: What lessons have opioid peptide and receptor knockout mice taught us? Br. J. Pharmacol. 2012, 166, 1993–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Sheng, W.S.; Lokensgard, J.R.; Peterson, P.K. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology 2002, 42, 829–836. [Google Scholar] [CrossRef]
- Lewitus, G.M.; Konefal, S.C.; Greenhalgh, A.D.; Pribiag, H.; Augereau, K.; Stellwagen, D. Microglial TNF-α Suppresses Cocaine-Induced Plasticity and Behavioral Sensitization. Neuron 2016, 90, 483–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.J.; Martin, J.A.; Gancarz, A.M.; Adank, D.N.; Sim, F.J.; Dietz, D.M. Activin A is increased in the nucleus accumbens following a cocaine binge. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hoppmann, N.; Graetz, C.; Paterka, M.; Poisa-Beiro, L.; Larochelle, C.; Hasan, M.; Lill, C.M.; Zipp, F.; Siffrin, V. New candidates for CD4 T cell pathogenicity in experimental neuroinflammation and multiple sclerosis. Brain 2015, 138, 902–917. [Google Scholar] [CrossRef] [Green Version]
- Puchałowicz, K.; Tarnowski, M.; Baranowska-Bosiacka, I.; Chlubek, D.; Dziedziejko, V. P2X and P2Y receptors—Role in the pathophysiology of the nervous system. Int. J. Mol. Sci. 2014, 15, 23672–23704. [Google Scholar] [CrossRef] [Green Version]
- Von Kügelgen, I. Pharmacology of P2Y receptors. Brain Res. Bull. 2019, 151, 12–24. [Google Scholar]
- Twining, R.C.; Bolan, M.; Grigson, P.S. Yoked Delivery of Cocaine Is Aversive and Protects Against the Motivation for Drug in Rats. Behav. Neurosci. 2009, 123, 913–925. [Google Scholar] [CrossRef] [Green Version]
- Pinto, B.F.; Medeiros, N.I.; Teixeira-Carvalho, A.; Eloi-Santos, S.M.; Fontes-Cal, T.C.M.; Rocha, D.A.; Dutra, W.O.; Correa-Oliveira, R.; Gomes, J.A.S. CD86 expression by monocytes influence an immunomodulatory profile in asymptomatic patients with chronic chagas disease. Front. Immunol. 2018, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- Frankowska, M.; Gołda, A.; Wydra, K.; Gruca, P.; Papp, M.; Filip, M. Effects of imipramine or GABAB receptor ligands on the immobility, swimming and climbing in the forced swim test in rats following discontinuation of cocaine self-administration. Eur. J. Pharm. 2010, 627, 142–149. [Google Scholar] [CrossRef]
- Hettema, J.M.; An, S.S.; Van Den Oord, E.J.C.G.; Neale, M.C.; Kendler, K.S.; Chen, X. Genetic association between RGS1 and internalizing disorders. Psychiatr. Genet. 2013, 23, 56–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trossbach, S.V.; Hecher, L.; Schafflick, D.; Deenen, R.; Popa, O.; Lautwein, T.; Tschirner, S.; Köhrer, K.; Fehsel, K.; Papazova, I.; et al. Dysregulation of a specific immune-related network of genes biologically defines a subset of schizophrenia. Transl. Psychiatry 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawliński, D.; Gawlińska, K.; Frankowska, M.; Filip, M. Maternal high-sugar diet changes offspring vulnerability to reinstatement of cocaine-seeking behavior: Role of melanocortin-4 receptors. Faseb J. 2020, 34, 9192–9206. [Google Scholar] [CrossRef] [PubMed]
- Frankowska, M.; Miszkiel, J.; Pomierny-Chamioło, L.; Pomierny, B.; Borelli, A.C.; Suder, A.; Filip, M. Extinction training following cocaine or MDMA self-administration produces discrete changes in D2-like and mGlu5 receptor density in the rat brain. Pharm. Rep. 2019, 71, 870–878. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A.; Frankowska, M.; Wydra, K.; Jastrzębska, J.; Miszkiel, J.; Filip, M. Increased 5-hydroxymethylation levels in the hippocampus of rat extinguished from cocaine self-administration. Hippocampus 2017, 27, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlińska, K.; Frankowska, M.; Gawliński, D.; Piechota, M.; Korostyński, M.; Filip, M. Cocaine Administration and Its Abstinence Conditions Modulate Neuroglia. Int. J. Mol. Sci. 2020, 21, 7970. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217970
Gawlińska K, Frankowska M, Gawliński D, Piechota M, Korostyński M, Filip M. Cocaine Administration and Its Abstinence Conditions Modulate Neuroglia. International Journal of Molecular Sciences. 2020; 21(21):7970. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217970
Chicago/Turabian StyleGawlińska, Kinga, Małgorzata Frankowska, Dawid Gawliński, Marcin Piechota, Michał Korostyński, and Małgorzata Filip. 2020. "Cocaine Administration and Its Abstinence Conditions Modulate Neuroglia" International Journal of Molecular Sciences 21, no. 21: 7970. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217970





